Abstract
Introduction
The assessment of the corneal nerve fibre plexus with corneal confocal microscopy (CCM) is an upcoming but still experimental method in the diagnosis of early stage diabetic peripheral neuropathy (DPN). Using an innovative imaging technique—Heidelberg Retina Tomograph equipped with the Rostock Cornea Module (HRT-RCM) and EyeGuidance module (EG)—we were able to look at greater areas of subbasal nerve plexus (SNP) in order to increase the diagnostic accuracy. The aim of our study was to evaluate the usefulness of EG instead of single image analysis in diagnosis of early stage DPN.
Methods
This prospective study was performed on 60 patients with type 2 diabetes mellitus, classified equally into two subgroups based on neuropathy deficient score (NDS): patients without DPN (group 1) or with mild DPN (group 2). The following parameters were analysed in the two subgroups: corneal nerve fibre length (CNFL; mm/mm2), corneal nerve fibre density (CNFD; no./mm2), corneal nerve branch density (CNBD; no./mm2). Furthermore, we compared the data calculated with the novel mosaic, EG-based method with those received from single image analysis using different quantification tools.
Results
Using EG we did not find a significant difference between group 1 and group 2: CNFL (16.81 ± 5.87 mm/mm2 vs. 17.19 ± 7.19 mm/mm2, p = 0.895), CNFD (254.05 ± 115.36 no./mm2 vs. 265.91 ± 161.63 no./mm2, p = 0.732) and CNBD (102.68 ± 62.28 no./mm2 vs. 115.38 ± 96.91 no./mm2, p = 0.541). No significant difference between the EG method of analysing the SNP and the single image analysis of 10 images per patient was detected.
Conclusion
On the basis of our results it was not possible to differentiate between early stages of large nerve fibre DPN in patients with type 2 diabetes mellitus via SNP analysis. To improve sensitivity and specificity of this method newer technologies are under current evaluation.
Trial Registration
ClinicalTrials.gov Identifier NCT05326958.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In vivo corneal confocal microscopy (CCM) allows quantification of corneal nerve fibre abnormalities in diabetic peripheral neuropathy (DPN). |
The major limitation of CCM is the small size of the single image; with an automated technique—EyeGuidance—it is possible to visualise and analyse an extended area of the subbasal nerve plexus. |
In our study, no significant differences were detected between the calculation of morphometric nerve fibre parameters from large-area images or from a set of single images. |
Additionally, with our data, neither of the two methods delivered sufficient results to differentiate between mild and no large nerve fibre DPN in patients with type 2 diabetes mellitus. |
Introduction
The prevalence of diabetes mellitus is still increasing and it is one of the most frequent metabolic diseases worldwide [1]. More than 90% of the diabetes cases are type 2 diabetes mellitus. It must be assumed that the prevalence of DM and thus the importance of secondary complications will increase significantly worldwide. Diabetic peripheral neuropathy (DPN) appears as a clinically apparent or subclinical disease of the peripheral nerves that occurs as a result of diabetes mellitus without any other cause. As a common complication of diabetes mellitus its prevalence increases with age and duration of the disease. It affects more than 50% of patients and is strongly correlated to the quality of glucose control [2,3,4]. It is a major cause of morbidity and increased mortality [5]. Complications such as neuropathy, loss of sensation and diabetic foot are linked to decreased quality of life and lead to major treatment challenges for physicians [6, 7].
The cornea is the most densely innervated superficial tissue in the human body and thus reflects an optimal investigational target to mimic the body’s nervous system in its entirety [8]. The subbasal nerve plexus (SNP) is a dense network of thin, unmyelinated nerve fibres lying between the basal epithelium and Bowman’s membrane [9, 10]. Because the earliest nerve injury takes place at the level of the small nerve fibres, corneal confocal microscopy (CCM) is a powerful and non-invasive method with great potential to detect and characterise DPN in vivo [11]. Many studies and meta-analyses have proven the role of CCM in quantifying nerve fibre abnormalities in diabetic neuropathy [6, 12,13,14,15,16,17,18]. The major limitation of CCM is the size of the single image (0.16 mm2). With an automated technique—EyeGuidance (EG)—it is possible to analyse an extended area of the SNP [19, 20].
Our hypothesis was that with increasing DPN, pathological changes in nerve morphology in terms of fibre length, fibre density and branch density occur. Therefore, we evaluated these parameters in patients with diabetes without or with mild DPN using CCM with EG and conventional single image analysis of 10 non-overlapping, artefact-free, nerve-fibre-rich images per patient.
Methods
Subjects
All patients with type 2 diabetes mellitus (T2DM) were recruited from the diabetes centre at the Herz- und Diabeteszentrum NRW, Ruhr Universität Bochum, Bad Oeynhausen, Germany. The ethics committee of the Ruhr University of Bochum located in Bad Oeynhausen approved the local study (Application number AZ37/2016, dated 30 August 2016). All study procedures followed the rules of the Declaration of Helsinki, and written informed consent was obtained from all subjects before enrolment.
Sixty patients with a minimum age of 18 years and clinical diagnosis of T2DM without diabetic retinopathy (HbA1c ranging between 6.5% and 9.5%) were included and divided into two subgroups according to large nerve fibre assessment with validated neuropathy deficit score (NDS). The score takes the assessment of presence or absence of ankle reflexes, vibration perception, pin-prick and temperature sensation into account [2]. Group 1 consists of 30 patients without coexisting diabetic neuropathy (NDS score ≤ 2) and group 2 comprises 30 patients with mild diabetic neuropathy (NDS 3–5).
Individuals with symptomatic dry eyes, contact lens wear (soft and rigid), conjunctivitis at the time of examination, previous corneal surgery, history of neuronal diseases except diabetic neuropathy, and mental disabilities were excluded. Study examinations included the gathering of demographic and clinical data for the characterisation of the disease (duration of the disease, mode of therapy, mean HbA1c, blood pressure) by interview and/or medical chart review. Ophthalmological slit lamp examination and ophthalmoscopy were performed to confirm the absence of diabetic retinopathy. This was followed by measurements with Heidelberg Retina Tomograph II equipped with the Rostock Cornea Module (HRT-RCM) and EyeGuidance (EG) module. The selection of the eye for the measurement was prespecified by randomisation protocol.
HRT-RCM with EG
A confocal microscope HRT-RCM (Heidelberg Engineering GmbH, Heidelberg, Germany) was used for CCM and combined with EG (Institute for Automation and Applied Informatics, Karlsruhe Institute of Technology KIT, Karlsruhe, Germany) [19]. The non-invasive procedures of HRT-RCM with EG were explained to the patient prior to examination. Before the procedure, a disposable sterile cap (TomoCap, Heidelberg Engineering GmbH, Germany) was filled with carbomer eye gel (Carbomer 980, Vidisic, Bausch&Lomb, Germany) and put on the RCM objective. Topical anaesthetics (proxymetacaine hydrochloride, Proparakain 0.5%, Ursapharm, Germany) and carbomer eye gel were applied to the eye randomised for analysis. After the measurement, a slit lamp assessment of the subject’s eye was performed to rule out any epithelial lesions.
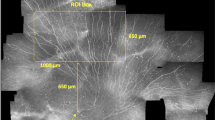
Standard CCM is limited by a relatively small image area (400 µm × 400 µm = 0.16 mm2) by image resolution of 384 × 384 pixels. Furthermore, there is a major inhomogeneity in the distribution of the nerve fibres in the SNP over the corneal area [21]. This can lead to insufficiency for reliable assessment of corneal nerve fibre morphology if too few images are taken into consideration. To enlarge the area of analysis, a computer-controlled moving fixation target is arranged on a display in front of the non-examined eye. The target is moving on an expanding spiral pattern while the guided and predictable movement of the eye leads to a continuously increased scanning area. Specialised software algorithms subsequently assemble a mosaic image from the acquired CCM image data. With this setting called EyeGuidance it is possible to achieve mosaic images of up to 18 mm2 in size within a short image recording time [10, 19, 20]. A minimum size of the mosaic images of 1.5 mm2 is recommended for a reliable morphological characterisation of the SNP [22]. In our study, the intended area of analysis was 2 mm2.
Image Processing and Mosaic Image Analysis
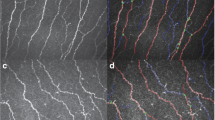
Image processing was previously described by Winter et al. [9]. In brief, each recorded HRT-RCM data set was split into the individual single images (on average approximately 1250 single images per data set). Single images underwent automatic tissue classification for the main type of tissue visible to identify subbasal nerve plexus images. Mosaic image reconstruction was based only on images classified as SNP. A 2 mm2 circle, centred at the first image of the data set, was cropped from the mosaic in order to obtain a similar size of analysed SNP region across all data sets. Non-SNP tissue patterns were recognised and masked in the circular image to eliminate artefacts. This was followed by image preparation for nerve fibre recognition, skeletonisation and pruning. Nerve fibre segmentation and pixel classification was performed to obtain corneal nerve fibre length (CNFL; mm/mm2), corneal nerve fibre density (CNFD; no./mm2) and corneal nerve branch density (CNBD; no./mm2) (Fig. 1).
Segmentation of nerve fibres from CCM image. Example of three steps from the initial confocal corneal microscopy (CCM) image (from Winter et al. [9]). a Via the segmented nerve fibre network, b to pixel classification, c as a prerequisite to calculate nerve fibre parameters
Single Image Analysis
We also selected 10 non-overlapping, nerve-fibre-rich and artefact-free single images from the original recorded image set for each patient to perform a single image analysis. Most study protocols use a selection of maximum five single images [16, 23,24,25], a some even fewer than three [14, 26,27,28]. In this study we used a higher image number of 10 to reduce the uncertainty induced by the inhomogeneous distribution of subbasal nerve fibres. As demonstrated by Vagenas et al., one must assess at least eight corneal images to reduce inhomogeneity [29].
Ten single images (0.16 mm2 each) selected from each patient were analysed using purpose designed fully automated nerve analysis software (ACCMetrics) made freely available by the University of Manchester (Manchester, UK) [30,31,32,33]. ACCMetrics is an image analysis software which allows automatic quantification of corneal nerve fibre measures from single or multiple corneal confocal microscopy (CCM) images.
The selected CCM images with a field of view of 400 × 400 µm2 were automatically analysed and the SNP parameters were quantified using the following definitions: CNFL—total length of all nerve structures per square millimetre (mm/mm2), CNFDACC—total number of main nerves per square millimetre (no./mm2), CNBDACC—total number of main nerve branches per square millimetre (no./mm2).
The same 10 images were analysed in parallel with the procedure published by Winter et al. [9] and comparison between the methods was performed. We quantified corneal nerve fibre length CNFL—defined as the total length of all nerve fibres (mm/mm2), corneal nerve fibre density CNFDWIN—defined as the number of all nerve fibres per mm2, and corneal nerve branch density CNBDWIN—defined as the number of all nerve branches per mm2 per single image analysis.
Statistical Analysis
Descriptive statistics were performed to analyse group specificities and the Mann–Whitney test was applied. Univariate analysis was performed including patient age as covariate to determine group-to-group variabilities in targeted parameters (CNFD, CNBD, CNFL). Results are given as mean ± SD; p values less than 0.05 are regarded as statistically significant.
Results
Clinical Assessment
Grouping of patients was done according to NDS score. Patients with no DPN presented with a score of 0.6 ± 0.9 (group 1) while patients with mild DPN had a score of 4.2 ± 0.6 (group 2). Patients without DPN were younger than patients with mild DPN (58.1 ± 13.4 vs. 65.2 ± 9.1 years, p = 0.02). There was no significant difference in BMI (33.5 ± 6.5 kg/m2 vs. 33.4 ± 6.3 kg/m2), diabetes duration (11.2 ± 11.1 vs. 14.7 ± 10.6 years, p = 0.225), HbA1c (8.3 ± 0.9 vs. 8.2 ± 0.8%, p = 0.604) and blood pressure, both systolic (132.4 ± 14.7 vs. 134.3 ± 19.5 mmHg, p = 0.677) and diastolic (77.1 ± 9.0 vs. 78.0 ± 11.9 mmHg, p = 0.743) between group 1 and group 2 (Table 1). For this reason, age was included in the analysis model for comparison of CNFL, CNFD and CNBD.
Corneal Confocal Microscopy
The comparison of main SNP parameters between group 1 and group 2 was performed by applying three different methods: EyeGuidance (EG), and single image analysis (according to Winter et al. and ACCMetrics, respectively). A representative image is given in Fig. 2.
There was no significant difference between group 1 and group 2 in the EG method: corneal nerve fibre length CNFL (mm/mm2) (16.81 ± 5.87 vs. 17.19 ± 7.19, p = 0.895), corneal nerve fibre density CNFDWIN (no./mm2) (254.05 ± 115.36 vs. 265.91 ± 161.63, p = 0.732) and corneal nerve branch density CNBDWIN (no./mm2) (102.68 ± 62.28 vs. 115.38 ± 96.91, p = 0.541).
Additionally, by applying the single image analysis algorithm from Winter et al., we could not detect any significant difference between group 1 and 2 in corneal nerve fibre length CNFL (mm/mm2) (15.83 ± 4.48 vs. 15.72 ± 6.16, p = 0.843), corneal nerve fibre density CNFDWIN (no./mm2) (225.76 ± 82.26 vs. 243.00 ± 154.82, p = 0.340) and corneal nerve branch density CNBDWIN (no./mm2) (102.29 ± 51.12 vs. 116.46 ± 95.11, p = 0.280).
Furthermore, there was no significant difference between group 1 and 2 in corneal nerve fibre length CNFL (mm/mm2) (13.76 ± 3.43 vs. 13.37 ± 4.25, p = 0.911), corneal nerve fibre density CNFDACC (no./mm2) (23.09 ± 6.71 vs. 22.23 ± 7.28, p = 0.894) and corneal nerve branch density CNBDACC (no./mm2) (27.29 ± 14.30 vs. 30.99 ± 22.62, p = 0.261) using the ACCMetrics algorithm (Table 2).
Discussion
In our study analysis of the SNP of patients with T2DM with mild DPN or without DPN, CCM failed to distinguish between the analysed groups, irrespective of the algorithm used for quantification of results. Heterogeneous interindividual results were obtained for the parameters analysed, resulting in increased standard deviations per parameter. Nevertheless, many studies, including large reviews and meta-analyses have proven the applicability of CCM in quantifying nerve fibre abnormalities in diabetic neuropathy [16,17,18, 25, 34,35,36,37,38,39,40,41,42,43]. Moreover, there are studies showing that CCM is able to detect early regeneration of corneal nerve fibres before changes in neurophysiology occur. Azmi et al. showed that in patients with type 1 diabetes mellitus (T1DM) after simultaneous pancreas and kidney transplantation, regeneration of small corneal nerve fibre occurs after 6 months compared to intraepidermal nerve fibre regeneration after 12 months and changes in neuropathic symptoms and neurophysiology by 36 months [44].
Ponirakis et al. demonstrated that in patients with T2DM after treatment with exenatide and pioglitazone or basal-bolus insulin, regeneration of corneal nerve fibre occurs but neuropathic symptoms and sudomotor function stay unchanged [45].
Reproducibility of the CCM measurements with the fully automated software ACCMetrics was proven by Kalteniece et al. [34]. In that publication, the following parameter values were obtained on two occasions in a cohort of 35 patients with diabetes mellitus and NDS 2.12 ± 2.78: CNFL (14.97 ± 2.91; 15.20 ± 2.88), CNFDACC (25.25 ± 6.25; 25.34 ± 6.58), CNBDACC (35.27 ± 16.05; 36.12 ± 17.23). In our study, the image analysis with the same software (ACCMetrics) delivered comparable results for CNFL (13.76 ± 3.43 vs. 14.97 ± 2.91), CNFDACC (23.09 ± 6.71 vs. 25.25 ± 6.25) and CNBDACC (27.29 ± 14.30 vs. 35.27 ± 16.05). The analysis conducted according to Winter et al. [9] exhibits results in other dimensions for CNFDWIN (10 × higher) and CNBDWIN (3 × higher): CNFL (16.81 ± 5.87), CNFDWIN (254.05 ± 115.36), CNBDWIN (102.68 ± 62.28). This shows that not only the quality of the CCM sample but also the analysis method may have an impact on the results [22, 46]. In contrast to other studies [46,47,48], we did not perform controlled focus adjustment. This might contribute to slight differences in results. In addition, it was difficult to compare the nerve fibre parameters CNFD and CNBD with those in other studies because of different definitions of the parameters. The only parameter defined identically across all publications is CNFL, as it is the sum of the length of all nerve fibres or nerve fibre segments detected. This difference in definition of the parameters may have at least in part contributed to the mismatch with the published data. In our study we followed the parameter definitions proposed by Ziegler et al. [13]: CNFL—defined as the total length of all nerve fibres (mm/mm2), CNFDWIN—defined as the number of all nerve fibres per mm2, CNBDWIN—defined as the number of all branches per mm2. In contrast, many others study groups use the following ACCMetrics definitions of the SNP parameters: CNFL—the total length of all nerve fibres per square millimetre (mm/mm2), CNFDACC—the total number of main nerves per square millimetre (no./mm2), CNBDACC—the total number of main nerve branches per square millimetre (no./mm2).
Comparable results were delivered by the study from Alam et al. [37] which involved 30 subjects with T1DM without diabetic peripheral neuropathy (DPN) compared to 31 with DPN. This study revealed a significant difference between CNFL, CNFD, CNBD in patients with and without DPN: CNFL (21.5 ± 4.8 vs. 15.8 ± 7.0, p = 0.001), CNFDACC (30.1 ± 6.7 vs. 19.8 ± 9.2, p < 0.0001), CNBDACC (60.7 ± 27.9 vs. 45.4 ± 32.0, p = 0.02), the strongest correlation was found by CNFDACC. Our measurements of CNFDACC were comparable (23.09 ± 6.71 vs. 22.23 ± 7.28, p = 0.894) but without statistical significance between DPN groups.
Another study by Petropoulos et al. [15] compared 111 patients with T1DM and T2DM and stratified according to NDS in no, mild, moderate and severe DPN groups and showed that all three parameters—CNFL, CNFD and CNBD—were connected to the severity of DPN. In comparison to our study, CNFL in that publication was 20.4 ± 5.9 for no DPN vs. 13.76 ± 3.43 in our study and 17.5 ± 8.1 for mild DPN vs. 13.37 ± 4.25, CNFDACC for no DPN 27.4 ± 8.9 vs. 23.09 ± 6.71 and for mild DPN 22.9 ± 10.5 vs. 22.23 ± 7.28, CNBDACC for no DPN 56.4 ± 35.7 vs. 27.29 ± 14.30 and for mild DPN 50.5 ± 43.3 vs. 30.99 ± 22.62 which seems of comparable magnitude.
In contrast with other studies Quattrini et al. [6] showed statistically significant reduction of CNFD and CNBD but not CNFL between controls and patients with T1DM and T2DM. Interestingly, the range of the CNFL results differs highly from our results (e.g. 3.87 ± 0.62 in patients with mild DPN vs. 13.37 ± 4.25 in the comparable group in our study). This study was conducted using a different device (Confoscan corneal confocal microscope model P4 Tomey, Erlangen, Germany) and different image analysis software (Scion Image for Windows; Scion, Frederick, MD).
Furthermore the study group of Stem et al. [49] used CCM to examine the relationship between CNFL and DPN status in patients with T1DM or T2DM. Their results show that patients with T2DM and mild DPN did not have lower CNFL compared to controls. Patients with T2DM and severe DPN had lower CNFL (12.5 ± 5.1) compared to controls (20.7 ± 1.7) (p = 0.009), patients with T1DM without DPN also had lower CNFL (15.1 ± 1.9) relative to controls (p = 0.033), but those with mild DPN did not exhibit significantly lower CNFL compared to controls (p = 1.000). We can assume that not only duration but also the type of diabetes may influence the CCM results.
This hypothesis was proved by Ferdousi et al. [50], whose study showed greater corneal nerve fibre loss in individuals with T1DM compared to T2DM, and also strong association with other risk factors. Moreover a study from Kheirkhah et al. [51] compared CNFD between standard vs. wide-field images of SNP and found no significant difference between them (17.10 ± 6.10 vs. 17.17 ± 5.60). According to that study there is no benefit using wide-field composite imaging. Unfortunately, that study does not mention the other SNP parameters CNFL and CNBD.
In the study by Ziegler et al. [13] a comparison of CCM parameters between 86 patients with recently diagnosed T2DM and mild DPN and 48 control subjects showed significant reduction in all main parameters of the SNP. The image analysis was performed with the same algorithm as in our study but imaging was without the use of EG (Winter et al. for single images). According to this study, and in contrast with previous reports, CNFDWIN was the most sensitive among the SNP parameters. In comparison with our results there is a similarity between the parameters CNFL (19.7 ± 7.5 vs. 15.72 ± 6.16), CNFDWIN (299.2 ± 52.8 vs. 243.00 ± 154.82) and CNBDWIN (165.2 ± 96.4 vs. 116.46 ± 95.11).
Conclusion
According to our data, interpretation of SNP with 2D HRT-RCM with the EG module does not differ significantly from single image analysis and provides no technical improvement. Additionally, with our data, both methods did not deliver sufficient results to differentiate between mild and no DPN in patients with T2DM. Our study has some strengths and limitations. The main strength was a highly selected, well-matched study cohort which underwent detailed clinical assessment of disease duration and neuropathic severity provided by experienced and highly trained physicians from a large diabetes centre (Herz- und Diabeteszentrum NRW, Ruhr Universität Bochum, Bad Oeynhausen, Germany). Moreover, highly trained ophthalmologists performed all examinations for the participants of this study. A major limitation of our study is that the severity of DPN was defined by large nerve fibre assessment using the subjective NDS whereas CCM allows quantification of small nerve fibre morphology. The idea was to avoid any invasive screening methods, e.g. skin biopsy. Despite those well-planned conditions, we could not reproduce all previous published results.
CCM is a non-invasive, easy-to-perform, safe and highly promising method. Up to now, it does not deliver repetitive and reliable data essential to prove its value in daily clinical practice for quantification of DPN stages. Thus, it remains experimental and needs further investigation based on prospective clinical trials. In addition, measuring protocols, data analysis algorithms and procedures should be standardised to further develop this method for routine use and to allow comparison of the data on an international level.
References
Heidemann C, Du Y, Scheidt-Nave C. Diabetes mellitus in Deutschland. 2011. Hrsg. Robert Koch-Institut Berlin GBE kompakt 2(3) www.rki.de/gbe-kompakt (Stand: 06.05.2011). https://doi.org/10.25646/3019
Young M, Boulton AJM, Macleod AF, Williams DRR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36(2):150–4. https://doi.org/10.1007/bf00400697.
Ziegler D, Strom A, Lobmann R, Reiners K, Rett K, Schnell O. High prevalence of diagnosed and undiagnosed polyneuropathy in subjects with and without diabetes participating in a nationwide educational initiative (PROTECT study). J Diabetes Complications. 2015;29(8):998–1002. https://doi.org/10.1016/j.jdiacomp.2015.09.008.
Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–62. https://doi.org/10.2337/diacare.28.4.956.
Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28:8–14. https://doi.org/10.1002/dmrr.2239.
Quattrini C, Tavakoli M, Jeziorska M, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56(8):2148–54. https://doi.org/10.2337/db07-0285.
Tesfaye S, Vileikyte L, Rayman G, et al. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev. 2011;27(7):629–38. https://doi.org/10.1002/dmrr.1225.
Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90(4):478–92. https://doi.org/10.1016/j.exer.2009.12.010.
Winter K, Scheibe P, Guthoff RF, Allgeier S, Stachs O. Morphometrische Charakterisierung des subbasalen Nervenplexus. Ophthalmologe. 2017;114(7):608–16. https://doi.org/10.1007/s00347-017-0465-3.
Köhler B, Allgeier S, Bartschat A, et al. In-vivo-Bildgebung des kornealen Nervenplexus. Ophthalmologe. 2017;114(7):601–7. https://doi.org/10.1007/s00347-017-0464-4.
Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60(1):108–11. https://doi.org/10.1212/wnl.60.1.108.
Kalteniece A, Ferdousi M, Petropoulos I, et al. Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Sci Rep. 2018;8(1):3283. https://doi.org/10.1038/s41598-018-21643-z.
Ziegler D, Papanas N, Zhivov A, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63(7):2454–63. https://doi.org/10.2337/db13-1819.
Ahmed A, Bril V, Orszag A, et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care. 2012;35(4):821–8. https://doi.org/10.2337/dc11-1396.
Petropoulos IN, Alam U, Fadavi H, et al. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care. 2013;36(11):3646–51. https://doi.org/10.2337/dc13-0193.
Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46(5):683–8. https://doi.org/10.1007/s00125-003-1086-8.
De Clerck EE, Schouten JS, Berendschot TT, et al. New ophthalmologic imaging techniques for detection and monitoring of neurodegenerative changes in diabetes: a systematic review. Lancet Diabetes Endocrinol. 2015;3(8):653–63. https://doi.org/10.1016/s2213-8587(15)00136-9.
Jiang MS, Yuan Y, Gu ZX, Zhuang SL. Corneal confocal microscopy for assessment of diabetic peripheral neuropathy: a meta-analysis. Br J Ophthalmol. 2016;100(1):9–14. https://doi.org/10.1136/bjophthalmol-2014-306038
Allgeier S, Maier S, Mikut R, et al. Mosaicking the subbasal nerve plexus by guided eye movements. Invest Ophthalmol Vis Sci. 2014;55(9):6082–9. https://doi.org/10.1167/iovs.14-14698.
Köhler B, Bretthauer G, Guthoff RF, et al. EyeGuidance–a computer controlled system to guide eye movements. Curr Dir Biomed Eng. 2016;2(1):433–6. https://doi.org/10.1515/cdbme-2016-0096.
Winter K, Scheibe P, Köhler B, Allgeier S, Guthoff RF, Stachs O. Local variability of parameters for characterization of the corneal subbasal nerve plexus. Curr Eye Res. 2016;41(2):186–98. https://doi.org/10.3109/02713683.2015.1010686.
Allgeier S, Winter K, Bretthauer G, et al. A novel approach to analyze the progression of measured corneal sub-basal nerve fiber length in continuously expanding mosaic images. Curr Eye Res. 2017;42(4):549–56. https://doi.org/10.1080/02713683.2016.1221977.
Tavakoli M, Kallinikos P, Iqbal A, et al. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med. 2011;28(10):1261–7. https://doi.org/10.1111/j.1464-5491.2011.03372.x.
Tavakoli M, Mitu-Pretorian M, Petropoulos IN, et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes. 2013;62(1):254–60. https://doi.org/10.2337/db12-0574.
Tavakoli M, Quattrini C, Abbott C, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33(8):1792–7. https://doi.org/10.2337/dc10-0253.
De Cillà S, Ranno S, Carini E, et al. Corneal subbasal nerves changes in patients with diabetic retinopathy: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2009;50(11):5155–8. https://doi.org/10.1167/iovs.09-3384.
Grupcheva CN, Wong T, Riley AF, McGhee CN. Assessing the sub-basal nerve plexus of the living healthy human cornea by in vivo confocal microscopy. Clin Exp Ophthalmol. 2002;30(3):187–90. https://doi.org/10.1046/j.1442-9071.2002.00507.x.
Rosenberg ME, Tervo TM, Immonen IJ, Müller LJ, Grönhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41(10):2915–21.
Vagenas D, Pritchard N, Edwards K, et al. Optimal image sample size for corneal nerve morphometry. Optom Vis Sci. 2012;89(5):812–7. https://doi.org/10.1097/opx.0b013e31824ee8c9.
Dabbah MA, Graham J, Petropoulos IN, Tavakoli M, Malik RA. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal. 2011;15(5):738–47. https://doi.org/10.1016/j.media.2011.05.016.
Dehghani C, Pritchard N, Edwards K, Russell AW, Malik RA, Efron N. Fully automated, semiautomated, and manual morphometric analysis of corneal subbasal nerve plexus in individuals with and without diabetes. Cornea. 2014;33(7):696–702. https://doi.org/10.1097/ico.0000000000000152.
Dabbah MA, Graham J, Petropoulos I, Tavakoli M, Malik RA. Dual-model automatic detection of nerve-fibres in corneal confocal microscopy images. Med Image Comput Comput Assist Interv. 2010;13(Pt 1):300–7. https://doi.org/10.1007/978-3-642-15705-9_37.
Chen X, Graham J, Dabbah MA, Petropoulos IN, Tavakoli M, Malik RA. An automatic tool for quantification of nerve fibers in corneal confocal microscopy images. IEEE Trans Biomed Eng. 2016;64(4):786–94. https://doi.org/10.1109/tbme.2016.2573642.
Kalteniece A, Ferdousi M, Adam S, et al. Corneal confocal microscopy is a rapid reproducible ophthalmic technique for quantifying corneal nerve abnormalities. PLoS ONE. 2017;12(8): e0183040. https://doi.org/10.1371/journal.pone.0183040.
Azmi S, Ferdousi M, Petropoulos IN, et al. Corneal confocal microscopy identifies small-fiber neuropathy in subjects with impaired glucose tolerance who develop type 2 diabetes. Diabetes Care. 2015;38(8):1502–8. https://doi.org/10.2337/dc14-2733.
Efron N. The Glenn A. Fry award lecture 2010: Ophthalmic markers of diabetic neuropathy. Optom Vis Sci. 2011;88(6):661–83. https://doi.org/10.1097/opx.0b013e3182171020.
Alam U, Jeziorska M, Petropoulos IN, et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS ONE. 2017;12(7): e0180175. https://doi.org/10.1371/journal.pone.0180175.
Tavakoli M, Petropoulos IN, Malik RA. Corneal confocal microscopy to assess diabetic neuropathy: an eye on the foot. J Diabetes Sci Technol. 2013;7(5):1179–89. https://doi.org/10.1177/193229681300700509
Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clin Exp Optom. 2012;95(3):348–54. https://doi.org/10.1111/j.1444-0938.2012.00740.x.
Efron N, Edwards K, Roper N, et al. Repeatability of measuring corneal subbasal nerve fiber length in individuals with type 2 diabetes. Eye Contact Lens. 2010;36(5):245–8. https://doi.org/10.1097/icl.0b013e3181eea915.
Hertz P, Bril V, Orszag A, et al. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet Med. 2011;28(10):1253–60. https://doi.org/10.1111/j.1464-5491.2011.03299.x.
Petropoulos IN, Ponirakis G, Khan A, et al. Corneal confocal microscopy: ready for prime time. Clin Exp Optom. 2020;103(3):265–77. https://doi.org/10.1111/cxo.12887.
Kalteniece A, Ferdousi M, Azmi S, et al. Corneal confocal microscopy detects small nerve fibre damage in patients with painful diabetic neuropathy. Sci Rep. 2020;10(1):1–9. https://doi.org/10.1038/s41598-020-60422-7.
Azmi S, Jeziorska M, Ferdousi M, et al. Early nerve fibre regeneration in individuals with type 1 diabetes after simultaneous pancreas and kidney transplantation. Diabetologia. 2019;62(8):1478–87. https://doi.org/10.1007/s00125-019-4897-y.
Ponirakis G, Abdul-Ghani MA, Jayyousi A, et al. Effect of treatment with exenatide and pioglitazone or basal-bolus insulin on diabetic neuropathy: a substudy of the Qatar Study. BMJ Open Diabetes Res Care. 2020. https://doi.org/10.1136/bmjdrc-2020-001420.
Lagali NS, Allgeier S, Guimarães P, et al. Wide-field corneal subbasal nerve plexus mosaics in age-controlled healthy and type 2 diabetes populations. Sci Data. 2018;5(1):1–12. https://doi.org/10.1038/sdata.2018.75.
Allgeier S, Bartschat A, Bohn S, et al. 3D confocal laser-scanning microscopy for large-area imaging of the corneal subbasal nerve plexus. Sci Rep. 2018;8(1):1–10. https://doi.org/10.1038/s41598-018-25915-6.
Lagali NS, Allgeier S, Guimarães P, et al. Reduced corneal nerve fiber density in type 2 diabetes by wide-area mosaic analysis. Invest Ophthalmol Vis Sci. 2017;58(14):6318–27. https://doi.org/10.1167/iovs.17-22257.
Stem MS, Hussain M, Lentz SI, et al. Differential reduction in corneal nerve fiber length in patients with type 1 or type 2 diabetes mellitus. J Diabetes Complications. 2014;28(5):658–61. https://doi.org/10.1016/j.jdiacomp.2014.06.007.
Ferdousi M, Kalteniece A, Azmi S, et al. Diagnosis of neuropathy and risk factors for corneal nerve loss in type 1 and type 2 diabetes: a corneal confocal microscopy study. Diabetes Care. 2021;44(1):150–6. https://doi.org/10.2337/dc20-1482.
Kheirkhah A, Muller R, Mikolajczak J, et al. Comparison of standard versus wide-field composite images of the corneal subbasal layer by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2015;56(10):5801–7. https://doi.org/10.1167/iovs.15-17434.
Acknowledgements
Funding
Heidelberg Engineering, Heidelberg, Germany, provided the HRT device as an unrestricted material grant to Herz- und Diabeteszentrum NRW, Bad Oeynhausen, Germany. Financial support was granted to HDZ NRW and Eye Clinic Minden for conductance of the study. Heidelberg Engineering granted financial compensation to KIT for providing the EG module for the duration of the study. Parts of this work were funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – project number 273371152. The journal’s Rapid Service fee was funded by the authors.
Editorial, Medical Writing and Other Assistance
The authors thank Bernd Köhler, Klaus-Martin Reichert and Ralf Mikut at the Institute for Automation and Applied Informatics at KIT for their theoretical and technical contributions to this work, particularly with respect to the EyeGuidance module (BK, KMR), the mosaicking software (BK, KMR) and the tissue classification algorithm (RM).
Author Contributions
Matuszewska-Iwanicka and Stratmann acquired, analysed and interpreted data, and wrote the manuscript. Stratmann participated in conception of the study; Tschoepe and Hettlich participated in design and conception of the trial; contributed to discussion; reviewed and edited the manuscript. Stachs, Allgeier, Bartschat, Winter, and Guthoff analysed final data and reviewed the manuscript. All authors had full access to all data in the study and Hettlich takes full responsibility as a guarantor for the conduct of the study, the integrity of the data and the accuracy of the data analysis.
Disclosures
The authors declared that they have no conflict of interest associated with this manuscript. Heidelberg Engineering provided the HRT and granted financial support to HDZ NRW and Eye Clinic Minden for study conductance. Heidelberg Engineering granted financial compensation to KIT for providing the EG module for the duration of the study. Heidelberg Engineering was involved in the management, analysis, and interpretation of the data; monitored the clinical study, but was not involved in preparation, writing, review, or approval of the manuscript; and did not impose any restrictions on publication.
Compliance with Ethics Guidelines
The ethics committee of the Ruhr University of Bochum located in Bad Oeynhausen approved the local study (Application number AZ37/2016, dated 30 August 2016). All study procedures followed the rules of the Declaration of Helsinki, and written informed consent was obtained from all subjects before enrolment.
Data Availability
The data sets generated and analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Matuszewska-Iwanicka, A., Stratmann, B., Stachs, O. et al. Mosaic vs. Single Image Analysis with Confocal Microscopy of the Corneal Nerve Plexus for Diagnosis of Early Diabetic Peripheral Neuropathy. Ophthalmol Ther 11, 2211–2223 (2022). https://doi.org/10.1007/s40123-022-00574-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00574-z