Abstract
Introduction
The Polish National AMD Therapeutic Program offered us a unique opportunity to determine the need for treatment of neovascular age-related macular degeneration (nAMD).
Methods
A search, extraction, and analysis of data from the monitoring system of the Therapeutic Program of the National Health Fund was performed. Demographic data from the Central Statistical Office were also obtained and analyzed. All national data, and from the Silesian Voivodeship specifically, from patients who had received treatment prior to January 14, 2022 (57,876 eyes) were analyzed.
Results
Approximately 0.1% of the Polish population requires treatment for nAMD when the best-corrected visual acuity (BCVA) criteria exclude irreversible severe changes in the fovea (0.2–0.8 by Snellen). There were 30,771 eyes in the therapeutic program in January 2022, and 4898 (15.9%) of them were in Silesia, which contains 11.7% of the total population and 12.4% of the elderly population (65 years of age and older). However, as a result of the COVID-19 pandemic, the average number of monthly enrollments in the therapeutic program decreased from 717 in the first quarter of 2020 to 505 in the second quarter (with a low of 407 in April). Moreover, in 2020, a negative balance was recorded between included and excluded patients.
Conclusion
The need for nAMD treatment in the elderly community (65 years of age and older) is estimated to be 0.55–0.66%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The burden of neovascular age-related macular degeneration (nAMD) treatment on healthcare systems is significant. |
Accurate determination of health needs is difficult because of the many factors that influence such an assessment. |
By excluding patients with advanced cases manifested by macular scarring/atrophy and low visual acuity (at most 0.1 by Snellen), health needs are estimated at 0.1% of the population. |
“0.65 over 65”—as a result of the dominant role that age plays in the development of the disease, a better indicator of health needs is the percentage of the population over 65 years of age who require treatment, which is estimated at 0.65%. |
Introduction
The key to reliably determining health needs in a given area is the ability to use data from extensive sources, preferably ones covering entire populations and that are not burdened with a significant number of errors and omissions. Along with the computerization of medicine, the number of electronic registries that can be employed for this purpose has increased. Replacing paper documentation with electronic health records (EHRs) is becoming a standard in medicine, and although the integration of various systems can be difficult, such medical data sources will contribute to the progress of medicine in the coming years. So far, an excellent example of one of these databases in ophthalmology is the IRIS (Intelligent Research in Sight) Registry [1]. However, the reliability of data from such registries may be undermined because the quality of EHRs is usually not as controlled as in clinical trials, diagnoses are not verified by reading centers, and the number of unknown factors influencing the data can be significant. To some extent, the database described below helps to reduce the risk of poor data quality.
Although the healthcare system in Poland is public, patients co-finance health services in many areas. Treatment of the neovascular form of age-related macular degeneration (AMD) was largely covered by patients’ funds almost until the end of 2015. The Polish National AMD Treatment Program was introduced in October 2015 to reimburse the costs of intravitreal anti-VEGF injections (e.g., ranibizumab, aflibercept, and, recently, brolucizumab). The current inclusion and exclusion criteria for the injections are presented in Table 1, and the first outcomes have already been published [2].
The first full year of the therapeutic program in Poland was 2016. The number of patients participating in the study gradually increased. Access to treatment in this mode was initially hampered by the need to suddenly enroll thousands of patients for treatment in a limited number of centers. Moreover, keeping extensive medical records was an additional limiting factor. The required documentation was simplified in the course of the program, but the changes did not apply to the same extent in all regions of the country.
The AMD program uses a dedicated system for monitoring therapeutic programs—a registry updated via the Internet. Given the limited access to epidemiological data in Central and Eastern Europe, the use of data from the national registry is a valuable supplement to existing data sources.
The aims of this study were primarily to determine the treatment need for nAMD based on data covering the entire population of the country and to compare the national data with the data from a region that had a higher percentage of patients treated.
Methods
Data from the monitoring registry of all the therapeutic program patients (n = 57,876) treated until January 14, 2021 were used and analyzed.
For the purpose of determining the need for nAMD treatment, each eye enrolled in the program was treated as a single patient and we know from published data that over 90% of patients receive treatment in the program in only one eye [2].
This article focuses on one region of Poland for several reasons. The Silesian Voivodeship is an industrialized area with the largest population density in Poland (4.2 million; 379 people per square kilometer), many ophthalmic departments and clinics, and a large number of ophthalmologists. Ease of access to the AMD therapeutic program is exceptionally good, and the distance to the nearest program center does not exceed 50 km for most patients. In fact, for the majority of patients, the distance is much lower, as a significant percentage of residents live in the Silesian agglomeration, within which there are eight treatment centers for almost 2.3 million inhabitants. There are five more centers outside the conurbation. Each center enrolled over 300 patients, and four enrolled over 1000 throughout 2015–2022. One facility that treated a small number of patients was converted into a COVID-19 hospital at the start of the pandemic (patients were transferred to other sites) and was not included.
Patients treated in the program were qualified by the coordination committee, who evaluated the quantitative parameters and imaging results (fundus photo, OCT cross-sections, and OCTA and/or fluorescein angiography) of each person enrolled. The qualification of all patients in Silesia was performed by the authors (Teper and Figurska, listed in order according to the number of qualified), who also co-carried out program treatment in two Silesian centers (Teper and Nowińska), which allowed for an in-depth assessment.
Data from available statistical registers and the monitoring system of the therapeutic program were used and analyzed, as well as supplemented with additional, more detailed statistical information from the two Silesian centers. Information on individual patients was not used. This research adhered to the tenets of the Declaration of Helsinki.
The following formulas were used to calculate the demand for treatment in Poland based on the data from the Silesian region; in the latter, an adjustment was made to account for the age group typical of developing the most cases of nAMD [3]:
The percentage calculated in this way should also be supplemented with the number of patients who self-finance anti-VEGF therapy. To do this, we analyzed the qualification data of the last 100 patients from two Silesian centers—one from the Silesian conurbation and the other located on the periphery of the region.
At the time of writing this article, the authors did not have access to obtain additional data from the registry. Such access has been requested, and further work has been planned to analyze the effectiveness and prognostic factors.
Results
On December 31, 2021, 29,507 patients remained in the program and 27,450 completed their participation. The causes of termination are most often reduced visual acuity (via macular scar, extensive hemorrhage, or retinal atrophy), long-term inactivity of the disease, missed checkups, and death.
The average waiting times for the qualification appointment and the first injection were 15.9 and 30.4 days, respectively. The decision of a committee member was made, on average, in 1.22 days.
Data from 2020 reported 38,265,013 Polish citizens, 4,492,330 (11.7%) of whom lived in the Silesian Voivodship. Of note, the last demographic data available at the time of this writing were collected by the Central Statistical Office in June 2021. As a result of the coronavirus pandemic, the population decreased, the data of which served as the basis for further calculations. The epidemic also slightly changed the country’s expected age structure, which is important when investigating a disease for which age is a major risk component. However, during the pandemic, the population of Silesia (4,472,703) decreased proportionally to the changes in the whole country (38,162,224). Regardless, the average age of the population in Silesia is higher than the national average. In 2021, 12.4% of Polish population aged 65 years and older and 12.3% of those aged 45 years and older lived in Silesia. In 2019, the percentages were 12.5% and 12.4%, respectively.
Detailed results and the flowchart for patients with nAMD are presented in Tables 2, 3, 4, and 5 and Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10.
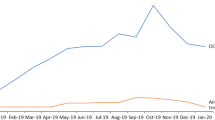
Number of patients starting treatment in the program. Of note: The initial high number of patients and subsequent significant decrease caused by the first wave of the pandemic in Poland. Re-enrolled patients and fewer COVID-19-related impediments then caused an increase in participants at the beginning of 2021
Number of patients ending treatment in the program. Standout period: The second half of 2020, as the COVID-19 pandemic coincided with the program’s decision to exclude patients who had had no treatment for more than 4 months. The change was implemented in November 2020. As of July 2021, the situation has normalized, but the number of patients being excluded has remained twice as high as the number pre-2020, due to the automatic exclusion of patients who lapse in their treatment. However, the opportunity to re-enroll after a return to activity increased the number of people enrolling in the program
Number of patients starting treatment in the program. Graph showing the number of patients enrolling per year, including those returning from relapsed activity (i.e., ≥ 4 months of non-treatment after November 1, 2020). In April 2020, because of the pandemic, a sudden decrease is apparent in the number of patients eligible for treatment
Percentage of patients enrolled in the program at any given time—treated continuously since then. Six years after the start of treatment, 20% of patients remained in the program. After 2.5 years, 50% of participants still receive treatment. This statistic does not include the re-enrollment of patients who were excluded because of relapsed activity
Ratio of patients starting and ending the program over the years. After the program began, the number of treated patients quickly increased, and exclusions were infrequent. In 2017, the visual acuity criterion was narrowed, and the growth dynamics decreased. The pandemic and the new exclusions led to a negative balance in 2020, which returned to a positive balance in the following year
Number of patients (≥ 65 years old) in the program per million citizens over the years. The calculation to determine the demand for treatment must be adjusted when age is the primary risk factor for a disease. Because the population in Silesia is older than that of the entire country, the difference is lower
In the group of the last 100 patients enrolled in the therapeutic program in the two centers studied in detail, 77 were treatment naïve. Of the 23 previously treated patients, 18 had participated in the treatment program, from which they were excluded mostly because of not administering intravitreal injections for over 4 months. Five patients were treated privately and, on average, received 3.2 injections (i.e., 1–7). Although there is easy access to the therapeutic program in Silesia, low average pensions (around 550 EUR) and high costs of anti-VEGF treatments significantly limit private injections. On the basis of the data from the centers, we expected that the demand for anti-VEGF treatments from those with a best-corrected visual acuity (BCVA) of 0.2–0.8 would increase by no more than 10%—and another 10% could be applied to cover people who cannot undergo treatment because of health reasons or infirmity in the absence of proper care. Thus, the calculated TN65 value was multiplied by 1.2, as follows:
The demand (total and by age group) was then calculated as a percentage of the population, where TN = 41,863, TN65 = 39,500, and TN65 (corrected) = 47,400. Thus, the percentage of the population requiring nAMD treatment is 0.124%, the percentage of the population in the at least 45-year-old group requiring nAMD treatment is 0.277%, and the percentage of the population in the at least 65-year-old group requiring nAMD treatment is 0.661% (or 0.551% if the original TN65 equation is used).
Discussion
Estimating treatment needs can be a challenge—population heterogeneity, lack of complete data or limited reliability of data, interrupted access to treatment, non-compliance, and/or the presence of other confounding factors contribute to potential bias. Relying on large epidemiological studies does not always solve a problem, and projected results may vary significantly. Even very precise meta-analyses estimate disease incidence and prevalence rather than reflecting the need for treatment [4].
The treatment of nAMD is a challenge. Regardless of the treatment regimen chosen, visits to a doctor are frequent [5]. Patients often require the care of an attendant and their quality of life is significantly reduced [6]. The studies that have been conducted so far have indicated that patients often give up treatment [7]. However, retinal specialists do not always schedule a sufficient number of injections to maintain the effects of the first months of therapy [8]. Practice patterns in anti-VEGF therapy for patients with nAMD do not reflect optimal treatment strategies suggested by clinical trials’ evidence [9]. Thus, program monitoring in Poland aims to reduce these negative phenomena.
The factors that distinguish the database used in this study are as follows:
-
1.
Coverage of the entire country’s nAMD population
-
2.
Two-step qualification by the coordinating committee and an ophthalmologist with proven experience with AMD
-
3.
Assessment of treatment applications from all over the country by the coordinating committee comprising eight ophthalmologists, all with substantial practical and scientific experience in the field of AMD
-
4.
Use of imaging data for qualification and monitoring—OCT, fundus photos, and OCTA (and FA/ICG, if deemed necessary by the committee)
-
5.
Standardized schedule of follow-up visits (at least every 2 months)
-
6.
A similar treatment regimen from late 2020; that is, performance of an anti-VEGF injection at least every 4 months in subsequent years of treatment
-
7.
BCVA excluding patients with predominant scarring and atrophy (but having the option of reimbursed treatment with bevacizumab)
In Poland, patients with mild to moderate AMD are admitted for routine follow-up visits, usually at intervals of several months, by district ophthalmologists. These patients also receive the Amsler test for self-monitoring. Patients with reduced visual acuity due to exudative AMD can be diagnosed if:
-
1.
They accelerate routine checkups at their eye clinic.
-
2.
They are admitted to hospital emergency departments.
-
3.
They are referred to an ophthalmologist by their GP.
-
4.
They make an appointment with an ophthalmologist privately.
Regardless of the diagnostic method, patients are generally referred to a center that has a treatment reimbursement option by a primary ophthalmologist, usually because of macular OCT results (OCT devices are available in almost every ophthalmology clinic). Patients may choose any treatment facility. The results of the imaging tests are sent for evaluation by the coordination team. Usually, applications are processed within a few days. In about 80–90% of the submitted applications, OCT, OCTA, and fundus photography are included. In the remaining 10–20%, FA results, instead of OCTA, are sent. Applications may be accepted, rejected, or returned with requests for additional imaging results. Centers are rated according to certain parameters so that patients can choose which is best for them, e.g., the time a patient starts treatment after diagnosis is evaluated and available online.
From the time of qualification and treatment initiation, visits are required at least every 2 months. The number of injections given in the first and subsequent years of treatment in each center is also documented. Patients can also see this information online.
A loading dose regimen is used during the first year. Aflibercept is often used with a fixed regimen throughout the first year of treatment; ranibizumab and brolucizumab are usually administered pro re nata after the loading dose.
If an injection has not been given for 4 months, the patient requires a new request to be sent to the coordination committee if/when disease activity resumes. Visit intervals after exclusion from the program are at the discretion of the treating physician. Most patients return to the drug program if their exclusion was caused by exceeding the 4-month no injection threshold. Control visits and treatments are both fully reimbursed by the National Health Fund, regardless of the treatment drug.
The provisions of the drug program have significantly changed twice during its duration because of the emergence of undesirable trends. The first change was largely related to the overqualification of patients with end-stage disease, so the minimum BCVA was changed (beginning January 1, 2017) from 0.1 to 0.2, according to Snellen. Continuous observation of results allowed us to quickly notice that, especially in some centers, visits during which the doctor performed only the OCT were common, and injections were administered much less frequently than in clinical trials. Consequently, another change was introduced (starting November 1, 2020): patients who do not receive an injection in 4 months are automatically excluded from further participation in the program. This change does not prevent re-treatment if disease activity returns, however; patients can be requalified for the program by both the attending physician and the coordinating committee.
Initially, concerns were raised about the need for a two-stage verification of access to nAMD treatment, because the time from diagnosis to the start of therapy should be as short as possible [10]. However, the waiting time for both a screening appointment and the first injection is much longer than the time needed for the committee’s assessment.
We do not have satisfactory data from private centers, but the population entering the program has gradually changed. Currently, the majority of previously treated people are patients who have already participated in the therapeutic program. In addition, the number of private injections prior to participation in the program has decreased. This type of treatment is sometimes associated with the desire for immediate therapy, so it is then continued as reimbursed treatment.
Similar results have been reported in a Norwegian study—private injections are rare. In Norway, however, data are calculated on the basis of patient visits. In addition, the number of simultaneous binocular administrations is unknown. In Poland, each eye is treated as a separate patient by the system, and reimbursement can be given for both eyes of a patient separately on the same day. This method gives a more complete picture of the need for treatment [11].
The demographic data presented require some comment because the size of the elderly population determines the need for AMD treatment. Smoking is a major environmental factor that influences the incidence of AMD [12]. If, despite the aging of the population, the percentage of smokers decreases (which seems likely in the future), the upward trend in the number of nAMD cases will be reduced significantly. However, as the main risk factor—genetic polymorphisms, also studied in the Silesian population [13]—is not modifiable, the disease will certainly remain one of the main causes of blindness.
Thus far, the median age in Poland has been gradually but steadily increasing for many years—a measure of an aging society [14]. The pandemic, which increased the mortality rate among the elderly, did not disrupt this trend, but the overall aging of the population was slower than expected.
A reduction in the number of ophthalmic visits due to COVID-19 has been reported. For example, according to the IRIS Registry, the total number of patient visits in the USA decreased by 62% from March through April 2020 (from 2.9 million to 1.1 million), and the number of monthly anti-VEGF injections decreased by approximately 8% (from 330,000 to 304,000). Furthermore, the dropout rate for retina specialists’ visits was approximately twice as low as the other types of ophthalmologists’ visits [15].
The pandemic effect coincided with the November 2020 modification of the therapeutic program’s terms. Our analysis showed a decline in the number of patients enrolled in the program during the same period, although exclusions were postponed by several months—a result of the delay in reporting the missed visits. Therefore, the increase in the number of exclusions from July 2020 to December 2020 should be treated as a result of COVID-19. At the beginning of 2021, the number of program participants increased, reaching a number higher than in the period 2018–2020. This reflects the re-enrollment of those who were excluded from the program for not receiving timely intravitreal injections.
Limitations of the Study
The therapeutic program is characterized by the defined inclusion and exclusion criteria (Table 1). For this reason, the determination of treatment needs must refer to those with characteristics related to the criteria of the program. Among them, the criterion of visual acuity stands out. Patients with low visual acuity are also treated; however, the effectiveness of treatment in a patient with an already significant reduction in visual acuity is limited by the atrophy and scarring of the retina. These patients often discontinue treatment quickly because of a lack of improvement [7]. Thus, it can be presumed that lowering the visual acuity criterion to 0.1 by Snellen would result in no more than a 10% increase in the number of injections. Changing the BCVA criterion from 0.1 to 0.2 provided exceptional insight into the treatment needs of the most visually impaired. When one compares 2017’s data with 2016’s, it can be seen that the number of people starting the program decreased by about 20%—which may be related not only to the narrowing of the visual acuity criterion but also to the “initial boom” period of the therapeutic program in 2016.
Moreover, the study did not take into account those who sought private treatment. However, it can be presumed that the number of patients who do does not exceed 10% of the population in the therapeutic program.
Finally, selection bias cannot be excluded. We do not know the reason why there is a higher percentage of patients treated in Silesia, and investigating it would probably require extensive survey research.
Conclusions
On the basis of only data from the Silesia region, it can be assumed that 9000 more patients should participate in the therapeutic program in Poland.
Approximately 0.1% of the population requires treatment for nAMD, even if the program’s BCVA criterion excludes those with irreversible, severe changes in the fovea (0.2–0.8 by Snellen). As a result of the age group comprising the largest number of patients with AMD (65 years of age and older), we estimate the approximate need for treatment in a given community to be 0.55–0.66%.
References
Chiang MF, Sommer A, Rich WL, Lum F, Parke DW. The 2016 American Academy of Ophthalmology IRIS® Registry (Intelligent Research in Sight) database: characteristics and methods. Ophthalmology. 2018;125:1143–8. https://doi.org/10.1016/J.OPHTHA.2017.12.001.
Figurska M, Matysik-Wożniak A, Adamiec-Mroczek J, et al. One-year outcomes of the Polish treatment program for the wet form of age-related macular degeneration using intravitreal therapy. Eur J Ophthalmol. 2020;30:586–94. https://doi.org/10.1177/1120672119874598.
Colijn JM, Buitendijk GHS, Prokofyeva E, et al. Prevalence of age-related macular degeneration in Europe: the past and the future. Ophthalmology. 2017;124:1753. https://doi.org/10.1016/J.OPHTHA.2017.05.035.
Rudnicka AR, Kapetanakis V, Jarrar Z, et al. Incidence of late-stage age-related macular degeneration in American whites: systematic review and meta-analysis. Am J Ophthalmol. 2015;160:85-93.e3. https://doi.org/10.1016/j.ajo.2015.04.003.
Li E, Donati S, Lindsley KB, Krzystolik MG, Virgili G. Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2020. https://doi.org/10.1002/14651858.CD012208.PUB2.
Taylor DJ, Hobby AE, Binns AM, Crabb DP. How does age-related macular degeneration affect real-world visual ability and quality of life? A systematic review. BMJ Open. 2016. https://doi.org/10.1136/BMJOPEN-2016-011504.
Okada M, Mitchell P, Finger RP, et al. Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmology. 2021;128:234–47. https://doi.org/10.1016/j.ophtha.2020.07.060.
Mehta H, Kim LN, Mathis T, et al. Trends in real-world neovascular AMD treatment outcomes in the UK. Clin Ophthalmol. 2020;14:3331–42. https://doi.org/10.2147/OPTH.S275977.
Lad EM, Hammill BG, Qualls LG, Wang F, Cousins SW, Curtis LH. Anti-VEGF treatment patterns for neovascular age-related macular degeneration among Medicare beneficiaries. Am J Ophthalmol. 2014. https://doi.org/10.1016/J.AJO.2014.05.014.
Rasmussen A, Brandi S, Fuchs J, et al. Visual outcomes in relation to time to treatment in neovascular age-related macular degeneration. Acta Ophthalmol. 2015;93:616–20. https://doi.org/10.1111/AOS.12781.
Kristiansen IS, Haugli Bråten R, Jørstad ØK, Moe MC, Sæther EM. Intravitreal therapy for retinal diseases in Norway 2011–2015. Acta Ophthalmol. 2020;98:279–85. https://doi.org/10.1111/AOS.14262.
Velilla S, García-Medina JJ, García-Layana A, et al. Smoking and age-related macular degeneration: review and update. J Ophthalmol. 2013;2013:11. https://doi.org/10.1155/2013/895147.
Teper SJ, Nowińska A, Wylegała E. A69S and R38X ARMS2 and Y402H CFH gene polymorphisms as risk factors for neovascular age-related macular degeneration in Poland— a brief report. Med Sci Monit. 2012. https://doi.org/10.12659/MSM.882447.
Leszko M, Zajac-Lamparska L, Trempala J. Aging in Poland. Gerontologist. 2015;55:707–15. https://doi.org/10.1093/GERONT/GNU171.
Leng T, Gallivan MD, Kras A, et al. Ophthalmology and COVID-19: the impact of the pandemic on patient care and outcomes: an IRIS® Registry study. Ophthalmology. 2021;128:1782–4. https://doi.org/10.1016/j.ophtha.2021.06.011.
Acknowledgements
Funding
This research received no external funding. The journal’s Rapid Service fees were funded by the Medical University of Silesia and Military Institute of Medicine.
Author Contributions
Conceptualization, Sławomir Jan Teper; methodology, Sławomir Jan Teper; investigation, Sławomir Jan Teper, Anna Nowinska, and Malgorzata Figurska; resources, Sławomir Jan Teper, Malgorzata Figurska, and Marek Rekas; original draft preparation, Sławomir Jan Teper; peer review and editing, Sławomir Jan Teper, Anna Nowinska, Malgorzata Figurska, Marek Rekas, and Edward Wylegala; supervision, Marek Rekas and Edward Wylegala. All authors have read and agreed to the published version of the manuscript.
Disclosures
All named authors confirm they have no conflicts of interest to declare.
Compliance with Ethics Guidelines
This study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments. Approval by an ethics committee was not required for the study, as it was based on anonymous statistical registry data.
Data Availability
Demographic statistics can be obtained from https://stat.gov.pl/, while National Health Fund’s data related to therapeutic programs in Poland can be requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Teper, S.J., Nowińska, A., Figurska, M. et al. The Need for Treatment of Neovascular Age-Related Macular Degeneration: A Study Based on the Polish National Registry. Ophthalmol Ther 11, 1805–1816 (2022). https://doi.org/10.1007/s40123-022-00545-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00545-4