Abstract
Introduction
The purpose of this study is to report the outcomes and complications of ultrasound cyclo plasty (UCP) after failed glaucoma surgery.
Methods
A retrospective case series included patients with previously failed glaucoma surgery who underwent UCP at King Abdul Aziz University Hospital, Riyadh, Saudi Arabia, between 2016 and 2021. The main outcome measures were: intraocular pressure (IOP), number of antiglaucoma medications and presence of vision-threatening complications. The surgical outcome of each eye was based on the main outcome measures.
Results
Seventy eyes of 70 patients were included in the study. The mean follow-up period was 31.89 months (± 17.5). The IOP and the number of antiglaucoma medications decreased significantly from a mean of 23.91 mmHg (± 6.3) and 3.43 (± 0.8) to 17.88 mmHg (± 8.1) and 2.48 (± 1.3) and of 16.74 (± 7.9) and 2.11 (± 1.3) at the 12th and 24th months postoperatively, respectively (p < 0.01 for both). The success rates were 77.1% (54/70) and 48.6% (34/70), while the failure rates were 22.9% (16/70) and 2.9% (2/70) at the 12th and 24th months postoperatively, respectively. The cumulative probabilities of success were 70.0% (± 5.5%) and 47.1% (± 6.0%) at the 12th and 24th months postoperatively, respectively. The most common complications were anterior chamber reaction (24.3%), cataract development/progression (18.6%), hypotony/choroidal detachment (4.3%), phthisis bulbi (1.4%) and aqueous misdirection (1.4%).
Conclusions
UCP is an effective treatment modality to control IOP and decrease the burden of antiglaucoma medications in eyes with previously failed glaucoma surgery. Monitoring and counseling of possible postoperative complications are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
What was known? |
Ultrasound cyclo plasty is safe and effective in the short term as glaucoma treatment. |
The outcome of ultrasound cyclo plasty is unknown after failed glaucoma surgery. |
What this paper adds? |
Ultrasound cyclo plasty is safe and effective after failed glaucoma surgery. |
Ultrasound cyclo plasty can result in controlled intraocular pressure and a decrease in the burden of medications after failed glaucoma surgery. |
Introduction
Glaucoma is one of the leading causes of irreversible vision loss worldwide [1]. Intraocular pressure (IOP) is still the most important risk factor that can halt glaucoma progression and vision loss [2]. However, maximum-tolerated medical therapy and surgical approaches, such as trabeculectomy and tube surgery, might not reach a target IOP deemed safe for the eye; ultimately, further intervention is required [3]. Moreover, the success rate of repeat filtering surgery is usually lower than that performed initially, and an old filtering surgery is considered a risk factor for failure [4].
A cyclo-destructive procedure has been advocated as the next step after failed glaucoma surgery. Laser cyclo-ablation had been shown to be reasonably effective in lowering the IOP, but the non-selective destruction of target tissue and the unpredictable dose-effect relationship might result in a significant risk of hypotony, inflammation and retinal detachment [5]. Ultrasound cyclo plasty (UCP) is a cyclo-ablative procedure that delivers high-intensity focused ultrasound (HIFU) through a circular-shaped probe and therefore correctly ablates the target tissue [6]. Few studies reported the outcomes of UCP in eyes with previous failed glaucoma surgery and, so far, there is no consensus on the influence of old procedures on the efficacy and safety of UCP [7,8,9]. Therefore, the aim of this study was to evaluate the outcomes of UCP after failed glaucoma surgery.
Methods
Patients
In this retrospective case series, we reviewed the medical records of patients who had undergone UCP after previous failed glaucoma surgery between May 2016 and May 2021 at King Abdul Aziz University Hospital, Riyadh, Saudi Arabia. Inclusion criteria were: (1) medically uncontrolled intraocular pressure (IOP) of ≥ 21 mmHg despite maximum tolerated antiglaucoma medications; (2) the presence of glaucomatous optic nerve head damage; (3) history of failed glaucoma surgery; (4) a minimum follow-up period of 6 months. Exclusion criteria were: pregnancy; the use of systemic medications that could affect IOP; a history of refractive surgery, retinal detachment or ocular tumor; and ocular infection 2 weeks prior to UCP. The study was approved by the institutional review board (E-22–6738) of King Saud University, and all procedures adhered to the tenets of the Declaration of Helsinki.
Surgical Methods
Second-generation EyeOP1 probes were used for all treatments. Simply stated, the automated UCP device (EyeOP1, Eye Tech Care, Rillieux-la-Pape, France) consists of a single-use sterile pack including a coupling cone and treatment probe of three sizes and a compact operator console. The probe size is determined based on the patient’s eye biometric readings of the axial length and white-to-white diameter. All procedures were performed by five glaucoma specialists credentialed for the procedure under peribulbar anesthesia. All procedures were carried out under adequate peribulbar anesthesia. The coupling cone was adjusted on the center of the patient’s eye by visualizing an equal white scleral ring surrounding the cornea and kept in place via 70 mmHg vacuum suction activated with a foot pedal. The six piezoelectric transducers were then automatically activated using a frequency of 21 MHz, acoustic power of 2.45 W, 8-s insonification duration for each sector, and 20-s pause between each treatment to allow complete evacuation of heat. The cone was filled with balanced salt solution to facilitate ultrasound transmission. After the surgery, all patients were treated with topical prednisolone drops. Antiglaucoma drops were resumed postoperatively based on the surgeon’s preference according to the case status.
Data Analysis
The postoperative visits considered for this study were those made at the 1st postoperative day; 2–4 weeks; 3, 6 and 12 months; 18 and 24 months. Pre- and postoperative data were collected whenever available and applicable for the following variables: age at time of surgery, gender, etiologic diagnosis, IOP, number of antiglaucoma medications, best corrected visual acuity (BCVA) converted into logarithm of minimal angle of resolution (logMAR) format, time to failure, postoperative complications and the need for subsequent pressure-lowering procedures to control IOP. The anterior chamber reaction was graded from 0 to 4 according to the standardization of the uveitis nomenclature scheme [10]. Significant early, that is, within the first 2 weeks, or late-recurring reaction after that of ≥ 2 was considered one of the postoperative complications.
Variables were evaluated using Student’s t-test and Wilcoxon rank test. Variables were presented as mean and standard deviation (SD), and the p value was considered statistically significant when < 0.05. Surgical success was classified as: (1) success (IOP reduction of ≥ 20% from baseline level and IOP between 6 and 21 mmHg with or without antiglaucoma medications, no loss of vision due to glaucoma progression, no postoperative vision-threatening complications and no need for further glaucoma procedure to control the IOP); (2) failure (if any of the following develops: IOP reduction < 20% from baseline level and IOP > 21 mmHg despite maximum tolerated antiglaucoma medications on two visits, persistent hypotony [IOP ≤ 5 mmHg] on two visits causing hypotony maculopathy, loss of vision due to glaucoma progression, postoperative vision threatening complications or the need of further glaucoma procedures to control the IOP). The severity of glaucoma and glaucoma progression were based on clinical assessments of glaucomatous disc changes and visual field loss involving one or both hemifields and following the Hodapp-Anderson-Parrish system. Repeated UCP cases were considered failures. The cumulative probabilities of overall success, presented as a percentage ± standard error (SE), were determined by Kaplan-Meier life table analysis. Statistical analysis was carried out using SPSS version 23 (SPSS Inc., Chicago, IL, USA).
Results
Patient Characteristics
Seventy eyes of 70 patients were included in this study. The mean follow-up time was 31.89 (± 17.5) months. Fifty-nine eyes (84.3%) had undergone one previous glaucoma surgery, nine eyes had two glaucoma surgeries (12.9%), and two eyes had three glaucoma surgeries (2.9%). The most common diagnosis was primary open-angle glaucoma (POAG), followed by primary angle closure glaucoma (PACG) and uveitic glaucoma. Most eyes had advanced glaucomatous disc damage (82.9%). Thirty-five eyes (50.0%) had previous non-glaucoma surgery, mainly cataract surgery (38.6%), followed by keratoplasty (5.7%). Fifty eyes were pseudophakic (71.4%), while the remaining 20 eyes (28.6%) were phakic (Table 1).
Efficacy
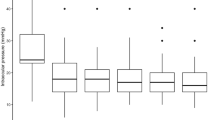
The IOP decreased from a preoperative baseline of 23.91 (± 6.3) mmHg to 17.88 (± 8.1), 16.68 (± 4.9) mmHg and 16.74 (± 7.9) mmHg, while the number of antiglaucoma medications decreased from a preoperative baseline of 3.43 (± 0.8) to 2.48 (± 1.3), 2.27 (± 1.2) and 2.11 (± 1.3) at the 12th, 18th and 24th months postoperatively, respectively (Table 2). The success rates were 77.1% (54/70), 52.9% (37/70) and 48.6% (34/70), while the failure rates were 22.9% (16/70), 8.6% (6/70) and 2.9% (2/70) at the 12th, 18th and 24th months postoperatively, respectively. The cumulative probabilities of overall success were 70.0% (± 5.5%), 57.1% (± 5.9%) and 47.1% (± 6.0%) at the 12th, 18th and 24th months postoperatively, respectively (Fig. 1). Nineteen eyes (27.1%) failed because of uncontrolled IOP requiring further glaucoma surgery: 11 eyes underwent cyclo-ablative procedures (15.7%), 3 eyes underwent Ahmed implant (4.3%), 3 eyes underwent Express shunt with MMC (4.3%) and 2 eyes underwent trabeculectomy with MMC (2.9%).
Safety
There was a significant change between the mean preoperative logMAR (0.89 [± 1.0]) and the logMAR at the 12th month (1.13 [± 1.2], p < 0.01), but none at the 18th month (0.95 [± 1.0], p = 0.06) and the 24th month (0.99 [± 1.2], p = 0.27) of follow-up. Twelve eyes (17.1%) lost more than two lines of vision at the 12th month, while five eyes (7.1%) lost more than two lines at the 24th month postoperatively. The most common causes of changes in vision were cataract development and progression and glaucoma progression. The most common postoperative complication was anterior chamber reaction in 17 eyes (24.3%)—early (17.1%) and recurrent (7.2%)—followed by cataract development or progression in 13 eyes (18.6%), out of which 11 eyes needed cataract surgery; hypotony/choroidal detachment in three eyes (4.3%), phthisis bulbi in one eye (1.4%) and aqueous misdirection in one eye (1.4%). The patient who developed phthisis bulbi had advanced PACG and underwent endoscopic cyclophotocoagulation combined with anterior vitrectomy, followed by transscleral cyclophotocoagulation, while the patient who developed aqueous misdirection had advanced PACG, for which he underwent a single trabeculectomy before UCP. The attack was broken after pars plana vitrectomy (Table 3).
Discussion
Lowering the IOP is the only proven approach to halt or reduce the rate of glaucoma progression and visual loss [11]. In some cases, even surgical approaches do not reach a low IOP deemed safe for the eye [12]. In such a condition, more aggressive methods are required, such as cyclo-destructive procedures. UCP is a modern ciliary body-dependent procedure that utilizes HIFU to thermally ablate the distal part of the ciliary body in selected areas, while being independent of tissue pigmentation and, therefore, likely to cause limited collateral damage to neighboring tissue [13]. The current study included 70 eyes of 70 patients with a mean follow-up of 31.89 months. Thirty-six eyes completed 24 months of follow-up. The IOP number of antiglaucoma medications decreased significantly from 23.91 mmHg and 3.43, to 16.74 mmHg and 2.11 at the 24th month after UCP, while complications were mostly anterior chamber inflammation and cataract development/progression. Our study suggests that UCP is effective in challenging cases with previously failed glaucoma surgery.
Few studies have evaluated the outcomes of UCP after failed glaucoma surgery. In a study that included 52 eyes, Denis et al. [8] reported an IOP reduction from 29.7 and 29.0 mmHg to 20.1 mmHg (32.2% IOP reduction) and 18.5 mmHg (36.0% IOP reduction) after 12 months in two groups subjected to 4-s and 6-s insonification exposure, while achieving 57.1% and 48.0% success rates for both groups, respectively. The study excluded eyes with previous ciliary body intervention or drainage implants. UCP was repeated in eight eyes because the IOP remained > 28 mmHg or did not have 20% reduction. In another study that included 20 patients, Melamed et al. [7] reported an IOP reduction of 39%, from 36.4 to 22.5 mmHg at 12 months, in a group of eyes that had 6-s insonification exposure. The reported success rate was 65% at the last follow-up. In a further study, De Gregorio et al. [9] reported IOP reduction of 26.12%, from 32.4 to 23.9 mmHg, at 12 months in 17 eyes with previous glaucoma surgery, while having 8-s insonification exposure. The success rate was 45% at 12 months in 18 eyes after a single UCP procedure and increased to 85% in 34 eyes after a maximum of three UCP procedures. The 12-month IOP reduction in our study was 25.2%, while the success rate was 77.1% after a single UCP, with a cumulative probability of success of 70.0%. Three main factors differentiate the IOP reduction and success rates achieved in our study from those achieved in the previous three studies. The first is the difference in defining success. Denis et al. and Melamed et al. considered success an IOP reduction of ≥ 20% and IOP > 5 mmHg, with or without medications [7, 8], while Gregorio et al. considered success an IOP > 5 and ≤ 21 mmHg. [9] All previous studies considered repeating UCP when the IOP began to increase as part of the initial procedure and where there had been multiple UCP treatments for such cases. Ciliary body epithelium can regenerate, resulting in restoration of aqueous humor production. Some studies reported that multiple UCP treatments can have a cumulative effect on IOP reduction and a success rate with a good safety profile [9, 14]. The second differentiating factor is the difference in insonification exposure, which was 8 s in our study. It is well known that a longer insonification can result in greater IOP reduction [5, 6, 15]. In addition, most eyes in our current study had advanced cupping, and we did not exclude eyes with previous cyclo-destructive and Ahmed implant surgeries. Therefore, UCP might have been used in more refractory and advanced cases, which highlights the possible limited IOP control in such conditions. The third differentiating factor is the difference in antiglaucoma medications. Melamed et al. reported a decrease of antiglaucoma medications from 4.6 to 4.0 at 12 months, while De Gregorio et al. reported a decrease in the number of antiglaucoma medications from 3.6 to 2.4 after 12 months in 40 eyes, only 17 of which had had previous glaucoma surgery. In the Denis et al.’s study, the number of antiglaucoma medications was almost the same after 12 months, reaching 3.2 and 3.5, compared with 3.5 and 3.3 at baseline in the 4- and 6-s insonification exposure groups, respectively. In our current study, the number of antiglaucoma medications decreased significantly from 3.43 to 2.48 (p < 0.01), with a corresponding decrease in the IOP. Therefore, maintaining antiglaucoma medications could play a role in having more IOP reduction [7,8,9].
The IOP and the number of antiglaucoma medications both significantly decreased within the same range after UCP overall during the follow-up period. Patients who underwent UCP after previous filtering surgery could have scleral architecture rearrangement and scleral scarring involving the flap itself, resulting in fiber delamination that allowed aqueous drainage to the suprachoroidal and transscleral space as well enhancing flap filtration. The subsequent separation of the ciliary body from the sclera will enhance the suprachoroidal outflow and decrease aqueous humor production by localized and circumferentially distributed coagulative necrosis of the ciliary body that involves areas previously spared by old cyclodestructive procedures.
The most common complications in our study were anterior chamber inflammation, both early and recurrent (24.3%), cataract development/progression (18.6%) and hypotony with choroidal detachment (4.3%). The insonification of the ciliary body epithelium will disrupt the tight junction which forms the blood-aqueous barrier; therefore, inflammatory mediators, including prostaglandins, will egress into the anterior chamber. Such an inflammatory process would lead to the uveoscleral pathway and result in further IOP reduction [15, 16]. The additional hyposecretion of aqueous liquid might also lead to hypotony and choroidal detachments. The release of inflammatory mediators and the possible thermal injury induced by UCP could cause cataract. Denis et al. reported anterior chamber reaction in 13 eyes out of 52 (25.0%), and hypotony/choroidal detachment in one eye only (1.9%), but no cases of cataract. Melamed et al. reported flare in 13 eyes (65.0%), but no cases of hypotony or cataract. De Gregorio et al. did not report any of the previous complications, although 42.5% of eyes had previous filtering surgery. Interestingly, one eye developed phthisis bulbi after UCP, which had never previously been reported in the literature. The eye underwent both endoscopic and transscleral cyclophotocoagulation. The coagulative necrosis of the ciliary epithelium induced by UCP on previously spared areas of the ciliary body and the circumferential coagulation necrosis on the regenerated ciliary epithelium that partially recovered after previous cyclodesctructive procedures can result in profound reduction in aqueous production and stimulation of the uveoscleral pathway. We believe that ultrasound biomicroscopy should have been carried out before UCP to assess the status of the ciliary body and hence to rule out the possibility of atrophy. Another eye with an old history of trabeculectomy developed aqueous misdirection, which had also never been previously reported after UCP. Such complications can result from hypotony post-UCP due to scleral scarring that could involve the flap itself and cause contraction, the stimulation of more transconjunctival filtration and the separation of the ciliary body from the sclera, all of which are potentially contributing factors. Baseline visual acuity was poor because of the advanced glaucomatous damage, while the development/progression of cataract and the subsequent cataract surgery contributed to changes in vision.
The current study has limitations owing to its retrospective nature and to the relatively small sample size. Larger comparative studies are needed to explore the difference in outcomes of multiple glaucoma entities and patient characteristics. A further limitation is the lack of a grading system for cataract, such as the lens opacity classification system (LOCS), to assess progression. Nevertheless, the current study represents one of the largest in the literature to evaluate the outcomes of UCP after failed glaucoma surgery, with the longest follow-up period.
Conclusion
In conclusion, the present study has a number of limitations, but it does suggest that UCP can be considered an option after failed glaucoma surgery, giving significant IOP reduction and a decrease in the antiglaucoma medication burden. However, proper patient counseling on possible postoperative complications is needed, and further study is required.
References
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90.
Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(714–20):829–30.
Mastropasqua R, Fasanella V, Mastropasqua A, Ciancaglini M, Agnifili L. High-intensity focused ultrasound circular cyclocoagulation in glaucoma: a step forward for cyclodestruction? J Ophthalmol. 2017;2017:7136275.
Law SK, Shih K, Tran DH, Coleman AL, Caprioli J. Long-term outcomes of repeat vs initial trabeculectomy in open-angle glaucoma. Am J Ophthalmol. 2009;148(685–695): e1.
Giannaccare G, Vagge A, Gizzi C, et al. High-intensity focused ultrasound treatment in patients with refractory glaucoma. Graefes Arch Clin Exp Ophthalmol. 2017;255:599–605.
Aptel F, Charrel T, Lafon C, et al. Miniaturized high-intensity focused ultrasound device in patients with glaucoma: a clinical pilot study. Invest Ophthalmol Vis Sci. 2011;52:8747–53.
Melamed S, Goldenfeld M, Cotlear D, Skaat A, Moroz I. High-intensity focused ultrasound treatment in refractory glaucoma patients: results at 1 year of prospective clinical study. Eur J Ophthalmol. 2015;25:483–9.
Denis P, Aptel F, Rouland JF, et al. Cyclocoagulation of the ciliary bodies by high-intensity focused ultrasound: a 12-month multicenter study. Invest Ophthalmol Vis Sci. 2015;56:1089–96.
De Gregorio A, Pedrotti E, Stevan G, Montali M, Morselli S. Safety and efficacy of multiple cyclocoagulation of ciliary bodies by high-intensity focused ultrasound in patients with glaucoma. Graefes Arch Clin Exp Ophthalmol. 2017;255:2429–35.
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature Working G. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16.
Kim KE, Jeoung JW, Kim DM, Ahn SJ, Park KH, Kim SH. Long-term follow-up in preperimetric open-angle glaucoma: progression rates and associated factors. Am J Ophthalmol. 2015;159(160–8):e1-2.
Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–72.
Aptel F, Charrel T, Palazzi X, Chapelon JY, Denis P, Lafon C. Histologic effects of a new device for high-intensity focused ultrasound cyclocoagulation. Invest Ophthalmol Vis Sci. 2010;51:5092–8.
Aptel F, Tadjine M, Rouland JF. Efficacy and safety of repeated ultrasound cycloplasty procedures in patients with early or delayed failure after a first procedure. J Glaucoma. 2020;29:24–30.
Mastropasqua R, Agnifili L, Fasanella V, et al. Uveo-scleral outflow pathways after ultrasonic cyclocoagulation in refractory glaucoma: an anterior segment optical coherence tomography and in vivo confocal study. Br J Ophthalmol. 2016;100:1668–75.
Hugo J, Matonti F, Beylerian M, Zanin E, Aptel F, Denis D. Safety and efficacy of high-intensity focused ultrasound in severe or refractory glaucoma. Eur J Ophthalmol. 2021;31:130–7.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
All authors contributed to the study conception, design, and data collection. Faisal A. Almobarak wrote the initial draft and undertook the analysis. All authors reviewed the draft and approved the final manuscript.
Disclosures
Faisal A. Almobarak, Ahmed Alrubean, Waleed K. Alsarhani, Abdullah Aljenaidel and Essam Osman declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
The study was approved by the institutional review board (E-22-6738) of King Saud University, and all procedures adhered to the tenets of the Declaration of Helsinki.
Data Availability
All data are available from the corresponding author upon request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Almobarak, F.A., Alrubean, A., Alsarhani, W.K. et al. Ultrasound Cyclo Plasty After Failed Glaucoma Surgery: Outcomes and Complications. Ophthalmol Ther 11, 1601–1610 (2022). https://doi.org/10.1007/s40123-022-00538-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00538-3