Abstract
Introduction
This study aimed to assess the safety and efficacy of a novel extended-depth-of-focus (EDOF) soft contact lens for myopia control in children.
Methods
A prospective, multicenter, randomized, double-masked, placebo-controlled, contralateral-eye comparison clinical trial was conducted in 72 children (40 male and 32 female) aged 9 to 14 years, with each eye randomly selected to wear either an experimental EDOF contact lens or a single-vision control lens at least 8 h per day, 5 days a week, for 52 weeks. Each contact lens was worn and then replaced daily. Measurements including best-corrected visual acuity, spherical equivalent refractive error (SER), axial length (AXL), and keratometry were performed at weeks 1, 4, and 13, and every 13 weeks thereafter for 52 weeks. The primary outcome measure was the change in SER, measured using cycloplegic auto-refraction. The secondary outcome measure was the change in AXL.
Results
At week 52, the mean change in SER was significantly lower with the experimental lens (−0.70 ± 0.49 D) than with the control lens (−0.88 ± 0.51 D; P < .001). The mean AXL elongation was significantly lower with the experimental lens (0.34 ± 0.19 mm) than with the control lens (0.38 ± 0.19 mm; P < .001). The EDOF lens reduced AXL and myopia progression by 10.5% and 20.5%, respectively. The change in SER, but no AXL, was significantly associated with EDOF lens wear in adjusted multivariate regression analysis. Reported adverse events did not differ significantly between the two lens types.
Conclusions
The results of this 1-year clinical trial demonstrate that the experimental EDOF soft contact lens slows myopia progression and reduces AXL elongation in children compared with a single-vision contact lens. (This study was retrospectively registered with ClinicalTrials.gov; identifier: NCT04238897; date of registration: January 23, 2020.)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The prevalence of myopia has increased significantly worldwide, which may lead to increased risk of sight-threatening complications in the population. |
Time spent on near-work activities is one of the major risk factors for myopia development. |
On- and off-axis hyperopia defocus has been suggested as the impetus for the increase in the axial length of the eye resulting in myopia development. |
This prospective, randomized 1-year study found that a center-for-near extended-depth-of-focus contact lens reduced axial length elongation and myopia progression by 10.5% and 20.5%, respectively, in 72 children aged 9 to 14 years. |
Introduction
The prevalence of myopia has increased significantly in the past few decades [1, 2]. Over 22% of the global population has myopia, with approximately one fifth of myopes having high myopia [3]. In Taiwan, the prevalence of myopia in children aged 7 years was 5.37% in 1983 but had increased to 25.4% by 2017 [1]. The prevalence of myopia also increased to > 70% in 12-year-olds and > 90% in 18-year-olds over the same period. Time spent on near-work activities is cited as a major risk factor for myopia development [1]. High myopia is associated with an increased risk of sight-threatening complications, such as cataracts, glaucoma, chorioretinal degeneration, and retinal detachment [4]. Therefore, controlling the progression of myopia is of paramount importance to prevent vision loss in these children and adolescents.
Accommodation allows the eye to focus on objects at different distances. However, sustained accommodation at near distances may be associated with the development and progression of myopia. Children with myopia were found to have reduced accommodative power or increased accommodative lag during reading [5]. It has been inferred that the decline in the amplitude of accommodation results in the failure to focus an image on the retina. Instead, the image falls behind the retina, causing hyperopic defocus that stimulates excessive eye globe elongation [6]. Nowadays, either on-axis or off-axis hyperopic defocus has been suggested as the impetus for the increase in the axial length of the eye which results in myopia development [7].
Besides pharmacologic intervention, optical methods have demonstrated effective myopia control [7,8,9,10,11,12,13,14,15]. Some treatment modalities were developed based on how accommodative lag plays a role in the progression of myopia. For example, progressive addition lenses (PAL) were designed to modulate accommodative response, with full corrective power in the distant viewing zone and added power in the near zone. Studies using PAL spectacles observed a reduction in myopia progression by approximately 0.20–0.28 D (with variable percentage from 11 to 51%) [8,9,10,11, 16]. The design of prismatic bifocal spectacles incorporating near base-in prisms along with near-addition lenses has also been demonstrated to slow myopia progression in children by reducing convergence and the accommodation induced by convergence [12].
To solve the problems associated with spectacles, orthokeratology and specially designed disposable soft contact lenses were developed for myopia control in children [7, 13,14,15]. The mainstream designs for myopia control soft contacts are currently the center-for-distance peripheral myopic defocus and concentric multifocal lenses [6, 15, 17, 18]. In the current study, we used a novel extended-depth-of-focus (EDOF) soft contact lens with a center-for-near design that differs from the optic design of the soft contact lenses used in previous studies. The central under-correction design was intended to reduce on-axis hyperopia defocus during near work with surrounding over-correction and on-correction in the outer zone for distant images. This prospective randomized study, which was designed as a superiority trial, aimed to evaluate the safety and efficacy of this newly designed EDOF soft contact lens for reducing the progression of myopia in children.
Methods
Ethical Considerations
The study protocol was approved by the Taiwan Food and Drug Administration (TFDA) and the institutional review boards of National Taiwan University Hospital and Taipei Tzu Chi Hospital; it was conducted in accordance with the tenets of the Declaration of Helsinki for experimentation on humans, the International Conference on Harmonization, and the Good Clinical Practice guidelines. Informed consent was obtained from each participant and one of their parents/guardians following a thorough explanation of the study purpose and required examinations.
Study Design
This prospective, multicenter, randomized, double-masked, placebo-controlled, contralateral-eye comparison clinical trial was conducted in children with myopia aged 9–14 years. Children were recruited from the outpatient clinics of two hospitals, the National Taiwan University Hospital and Taipei Tzu Chi Hospital. Following enrollment, each participant’s eye was allocated the randomization codes “lens X” or “lens Y” by a randomly generated computer list, to wear either the experimental EDOF soft daily disposable contact lens (App Vision Care Co., Ltd., Taipei City, Taiwan) or the single-focus control lens (Ticon Daily Disposable Aspherical Soft Contact Lens, St. Shine Optical Co., Ltd., New Taipei City, Taiwan). The contact lenses were wrapped in identical packaging, labeled with only the laterality of the eye and degree of myopia. Additionally, sealed envelopes of subject randomization were prepared and stored in case emergency unblinding was needed. The participants, care providers, and those assessing outcomes were blinded to the assigned interventions. The randomization data were kept strictly confidential and were not disclosed until completion of the trial.
The participants were asked to wear the lens for at least 8 h daily and 5 days weekly for 52 weeks. Each contact lens was worn and then replaced daily. Compliance was monitored by telephone and by maintenance of a daily diary by each participant. Follow-up visits were scheduled at 1, 4, 13, 26, 39, and 52 weeks for clinical assessment at the same facility using the same equipment and methods to avoid bias.
Participants
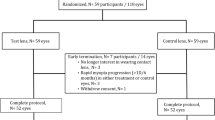
The participants were considered eligible for inclusion if they had a myopic spherical equivalent ranging from −1.00 D to −8.00 D, astigmatism ≤ 1.75 D, myopia progression of at least 0.75 D within the past 12 months with or without pharmacologic intervention, best-corrected visual acuity (BCVA) of 0.1 logMAR or better, and follow-up availability for at least 1 year. The exclusion criteria were as follows: anisometropia > 2.00 D, ocular disease preventing contact lens wear, severe ocular or systemic allergies, the use of any medications that might interfere with contact lens wear or ocular refraction, ocular or systemic conditions that might affect refractive development, use of atropine or pirenzepine treatment for myopia control within the past month, prior use of orthokeratology lenses, dry eye (Schirmer’s test < 5 mm/5 min), or other physical conditions that prevented the use of contact lenses. Figure 1 demonstrates the flowchart for study participants from screening to study completion.
Contact Lens Design
The study EDOF soft contact lens is made of 2-hydroxyethyl methacrylate and methacrylic acid [8.6-mm base curve, 14.2-mm diameter, 0.05–0.3-mm central thickness, 55% water content, oxygen permeability value of 19.5 × 10−11 (cm2/s) (mL O2/(mL × mmHg)]. The refractive power profile across a single lens varies above and below the mean power, resulting in a non-monotonic and aperiodic variation across the optic zone diameter (Fig. 2a). The contact lens comprises a central under-correction zone surrounded by an over-correction and on-correction outer zone. The central zone is the primary viewing zone when the pupil constricts during near work. The under-correction power of the central zone can help focus the image on the retina and reduce the amount of accommodation required during near work (Fig. 2b). When viewing a distant target (Fig. 2c), the pupil dilates to allow more light to pass through the different refractive zones of the experimental contact lens. The aspherical single-focus control lens is made of 2-hydroxyethyl methacrylate and methacrylic acid [8.6-mm base curve, 14.2-mm diameter, 0.07–0.25-mm central thickness, 58% water content, oxygen permeability value of 22 × 10−11 (cm2/s) (mL O2/(mL × mmHg)].
Design of the experimental EDOF soft contact lens. a Two-dimensional plot depicting the power profile design of the contact lens. The color intensity represents the power amplitude. b During near viewing, light passes through the constricted pupil, and the central under-corrected refractive power decreases the amplitude of the required accommodation. c During distance viewing, light passes through the refractive power profile of the lens across the optic zone, making points at, anterior to, and posterior to the retina, ultimately producing a simultaneous image for distance viewing. EDOF extended depth of focus
Study Procedures and Measurements
The participants were screened according to the inclusion and exclusion criteria on the screening visit. Visual acuity was measured using the logarithm of the minimum angle of resolution chart viewed at 6 m. Slit-lamp biomicroscopy was also performed to assess contact lens fit and anterior segment condition. Contact lenses were then dispensed to the participants according to their randomization assignment. Over-refraction with spherical lenses was performed on both eyes during the wearing of the assigned lenses to confirm BCVA and appropriateness of the lens power.
Outcome measurements including BCVA, spherical equivalent refractive error (SER), axial length (AXL), and keratometry were performed at weeks 1, 4, and 13, and every 13 weeks thereafter for 52 weeks. Cycloplegic refraction was performed 30 min after application of one drop of a 0.5% tropicamide and 0.5% phenylephrine hydrochloride solution (Mydrin-P®, Santen Pharmaceutical Co., Ltd., Osaka, Japan) three times at 5-min intervals using the Topcon KR-8800 autorefractor (Topcon, Tokyo, Japan). AXL was examined using partial coherence interferometry (LENSTAR LS 900 all-in-one biometer, Haag-Streit AG, Köniz, Switzerland). The average of three measurements was recorded. Contact lens fit, near and distance visual acuity, slit-lamp biomicroscopy, and safety were also assessed at each visit. Any symptom or complaint was recorded using a subjective comfort questionnaire. The compliance and lens wearing time were verified with the participants’ diaries.
During the trial, the contact lens prescription could be changed based on the investigator’s judgment or when the BCVA decreased by one or more lines or below 0.1 logMAR.
The primary outcome was the change in SER, measured using cycloplegic autorefraction over 52 weeks. The secondary outcome was the change in AXL over 52 weeks. The safety outcome was the presence of corneal abnormalities above grade 2 on the Efron Grading Scale assessed by slit-lamp examination. Events of interest included corneal edema, epithelial microcysts, corneal staining, limbal/bulbar injection, conjunctival abnormalities, and corneal neovascularization/infiltrates.
Statistical Analysis
This clinical study was designed as a superiority trial, and we hypothesized that at 52 weeks, the mean SER change in the experimental eye would be less than that in the control eye. A minimum sample size of 46 was estimated for a within-subject comparison study with a power of 90%, a significance level of 5%, a difference in means of 0.25 D, and an intra-subject standard deviation (SD) of 0.35 D for cycloplegic autorefraction. To comply with the regulatory requirements of the TFDA, the sample size was increased to 72 with an estimated dropout rate of 15% to target a minimum of 60 participants [19].
The efficacy analyses were performed on the evaluable population (EP), who had worn contact lenses for a minimum of 9 months, and in the per-protocol population (PP), a subset of the EP population comprising all patients who fulfilled all of the inclusion and exclusion criteria and without major protocol deviations during the study period. Safety evaluations were conducted on the intent-to-treat (ITT) population, which comprised all randomized subjects. Changes in SER and AXL from baseline were computed for each eye and compared between the experimental and control eyes using the Wilcoxon signed-rank test or paired t tests. A linear mixed model was used to determine the correlation between the outcome variables (i.e., changes in SER and AXL) and covariates such as age, sex, and baseline characteristics. Data are expressed as mean ± SD and 95% confidence intervals. McNemar’s chi-square test was performed for categorical variables and a paired t test for continuous variables. All tests were two-sided. The level of statistical significance was set at 5%. Statistical analyses were performed using SAS [Statistical Analysis System] 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
A total of 72 eligible children were enrolled. Five children (6.9%) dropped out during the 52-week study period (two subjects were not interested in wearing contact lens, one had idiopathic uveitis, one was lost to follow-up, and one used tropicamide) (Fig. 1). All 72 participants (ITT population) were included in the safety analysis, while 68 participants who had worn the study lens for a minimum of 9 months were included in the efficacy analysis (EP and PP population) (Fig. 1). Sixty-seven participants completed the 52-week study period. None of the participants requested extra lenses due to lens breakage or loss. The average lens wearing time was 11.2 h/day.
Demographic and Baseline Characteristics of the Study Participants
The mean (± SD) age of participants was 12.36 ± 1.46 years (range 9–14 years). Males accounted for 55.6% of the subjects. There were no significant differences between the experimental and control eyes regarding corrected visual acuity and cycloplegic refraction (Table 1).
Efficacy Assessment
The SER and AXL means over the study period are presented in Table 2 and Fig. 3. At week 52, the mean SER change in the experimental eye was significantly less than that in the control eye (−0.70 ± 0.49 vs. −0.88 ± 0.51 D; P < 0.001), and the mean AXL increase in the experimental eye was also significantly lower than that in the control eye (0.34 ± 0.19 vs. 0.38 ± 0.19 mm; P < 0.001).
The best-corrected logMAR visual acuity for distance and near did not differ significantly between the eyes at baseline and week 52. The change in SER was significantly associated with EDOF lens wear after adjusting for age, sex, lens wearing time, and baseline AXL/SER (P < 0.001). The change in AXL was significantly associated with age (P = 0.02) and baseline SER (P < 0.001), but not with lens type (Table 3).
Safety Assessment
The total number of adverse events (AE) was 207. No serious adverse events or significant AEs happened in the study. In general, most AEs were mild (92.75%) and unrelated to the experimental lens (96.62%). The most common AE by preferred terms (incidence ≥ 5%) was dry eye (37.5%), followed by eye pain (25%), keratitis and conjunctivitis (both 9.7%), eye pruritus (8.3%), and foreign body in the eye (6.9%). There were no cases of severe adverse corneal events during the study period. Eleven events were asymptomatic superficial punctate keratitis: six in eyes wearing the EDOF lens and five in those wearing the control lens. These events were resolved without clinical consequences or a decrease in BCVA. There was no significant difference in AEs between the experimental and control lens groups.
Discussion
This clinical study was designed to assess the effects of a center-for-near EDOF soft contact lens on slowing the progression of pediatric myopia. We found a significant reduction in myopia and AXL progression in eyes wearing the EDOF lens after 1 year compared with that in the contralateral eye wearing a single-focus soft control lens.
Various interventions are currently in use to slow the progression of myopia. Pharmaceutical treatments such as atropine may cause photophobia and affect near visual acuity, and have been shown to be susceptible to post-treatment acceleration, especially in higher concentration [20,21,22]. Optical treatment such as overnight orthokeratology lens has been shown to slow eye globe elongation [13]. Without the need for a cleaning process, the use of daily disposable contact lenses is less time-consuming in handling than orthokeratology and is shown to have a lower risk of bacterial infection [23]. In the past decade, emerging evidence has demonstrated the effectiveness of specially designed disposable soft contact lenses for myopia control in children [14, 15, 17, 18]. The mainstream designs for myopia control soft contacts are currently peripheral hyperopia-reducing and concentric multifocal lenses, with targeting of distance correction in the center [6, 15, 17, 18].
The time spent on near-work activities is a well-documented risk factor for myopia [1, 24]. A greater accommodative lag in association with near work is one possible mechanism in the development and progression of myopia [5, 25, 26]. Insufficient accommodation during near-work activities may produce hyperopic defocus that can lead to axial elongation and cause myopia progression [25, 26]. Theoretically, the center-for-near design of the current EDOF soft contact lens may help reduce the amount of accommodation required for near work and thus prevent myopia progression.
EDOF soft contact lenses have recently been investigated for their effect on myopia control in children [7]. A randomized clinical trial by Sankaridurg et al. reported a 24–32% decrease in myopia progression and 22–32% decrease in AXL elongation in children using center and peripheral myopia defocus, or EDOF soft contact lens [7]. Their EDOF lens was specially designed to incorporate and manipulate selected higher-order aberrations to achieve a through-focus global retinal image quality that was optimized for points at and anterior to the retina and degraded for points posterior to the retina [7].
Previous studies using multifocal soft contact lenses for myopia control demonstrated decreased myopia progression of 20.6–77.2% and decreased AXL of 25.0–79.2% [17, 18, 27]. Recently, two rigorous 3-year randomized clinical trials revealed significant efficacy in myopia control using multifocal soft contact lenses. The overall results showed 0.46–0.73 D in reduction of myopia progression and 0.23–0.32 mm in slowing of eye growth [17, 18]. The reduction rate in our study was 20.5% for myopia progression and 10.5% for AXL, which is slightly less than in other studies. These differences might be attributable to the lens design, inclusion criteria and ethnicity of the study subjects, and study design and duration. For example, over-correction in the intermediate zone of the test lens might produce greater relative hyperopic defocus than a single-vision lens and other EDOF lenses [7]. It is notable that the contralateral paired-eye design and the paired t test used in the study, as well as the inclusion of children with fast myopia progression (> 0.75D in the previous year), may inflate the significance of the findings [16]. The results of a clinical trial with a duration of 1 year should be regarded as preliminary. To date, there is currently no consensus on a specific minimum percent reduction in myopia progression for a treatment outcome to be considered clinically meaningful. Any reduction in progression could be considered beneficial [28]. However, the clinical effectiveness of myopia control with the test lens requires further evaluation.
Multivariate regression analysis showed that while the change in SER was associated with the use of the EDOF experimental lens, the change in AXL was more strongly associated with age and baseline SER (Table 3). Although changes in SER are usually positively correlated with changes in AXL, the relationship strength differed by age [28, 29]. Compared with preschoolers, a fixed amount of axial elongation is associated with greater refraction change in schoolchildren [30]. Although lens wearing time was not significantly associated with SER/AXL change in the multivariate regression analysis, soft contact lenses have a recommended maximum wear time, which is a concern for their use as a myopia control device compared with orthokeratology [7, 31].
Regarding the safety profile of the lens, no serious adverse events were reported in either group. Our dropout rate of 6.9% was also relatively low compared with that in similar studies, in which dropout rates ranged from 12.5 to 54% [6, 7, 31,32,33,34].
This study has several limitations. First, the contralateral eye comparison design does not reflect the real-world situation, and the possibility of inter-ocular interactions could not be eliminated. This study design, as requested by the TFDA, had the advantage of reducing confounding factors due to biological and environmental exposure differences between the two groups. The design has also been used in other myopia control studies [35,36,37]. Second, the follow-up period was relatively short. The current study reports the preliminary 1-year results. Further studies with longer follow-up are warranted to substantiate the conclusions of this study. Third, direct measurement of pupil sizes, binocularity, effects on the accommodative amplitude in individual participants, and the rebound phenomenon after cessation of lens wear were not within the scope of this study, but all warrant further investigation.
Conclusion
The novel center-for-near EDOF daily disposable soft contact lens delayed pediatric myopia progression and AXL elongation without serious adverse events in a 1-year clinical trial. Further studies are necessary to evaluate this device’s long-term and rebound effects on myopia control and the optimal design of EDOF myopia control soft contact lenses.
References
Tsai TH, Liu YL, Ma IH, et al. Evolution of the prevalence of myopia among Taiwanese schoolchildren: a review of survey data from 1983 through 2017. Ophthalmology. 2021;128:290–301.
Ding BY, Shih YF, Lin LLK, Hsiao CK, Wang IJ. Myopia among schoolchildren in East Asia and Singapore. Surv Ophthalmol. 2017;62:677–97.
Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42.
Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:381–91.
Charman WN. Near vision, lags of accommodation and myopia. Ophthalmic Physiol Opt. 1999;19:126–33.
Walline JJ, Greiner KL, McVey ME, Jones-Jordan LA. Multifocal contact lens myopia control. Optom Vis Sci. 2013;90:1207–14.
Sankaridurg P, Bakaraju RC, Naduvilath T, et al. Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: 2 year results from a randomised clinical trial. Ophthalmic Physiol Opt. 2019;39:294–307.
Berntsen DA, Sinnott LT, Mutti DO, Zadnik K. A randomized trial using progressive addition lenses to evaluate theories of myopia progression in children with a high lag of accommodation. Invest Ophthalmol Vis Sci. 2012;53:640–9.
Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–500.
Correction of Myopia Evaluation Trial 2 Study Group for the Pediatric Eye Disease Investigator Group. Progressive-addition lenses versus single-vision lenses for slowing progression of myopia in children with high accommodative lag and near esophoria. Invest Ophthalmol Vis Sci. 2011;52:2749–57.
Hasebe S, Jun J, Varnas SR. Myopia control with positively aspherized progressive addition lenses: a 2-year, multicenter, randomized, controlled trial. Invest Ophthalmol Vis Sci. 2014;55:7177–88.
Cheng D, Woo GC, Drobe B, Schmid KL. Effect of bifocal and prismatic bifocal spectacles on myopia progression in children: three-year results of a randomized clinical trial. JAMA Ophthalmol. 2014;132:258–64.
Lee YC, Wang JH, Chiu CJ. Effect of orthokeratology on myopia progression: twelve-year results of a retrospective cohort study. BMC Ophthalmol. 2017;17:243.
Cheng X, Brennan NA, Toubouti Y, Greenaway NL. Safety of soft contact lenses in children: retrospective review of six randomized controlled trials of myopia control. Acta Ophthalmol. 2020;98:e346–51.
Sankaridurg P. Contact lenses to slow progression of myopia. Clin Exp Optom. 2017;100:432–7.
Brennan NA, Toubouti YM, Cheng X, Bullimore MA. Efficacy in myopia control. Prog Retin Eye Res. 2021;83: 100923.
Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G. A 3-year randomized clinical trial of MiSight lenses for myopia control. Optom Vis Sci. 2019;96(8):556–67.
Walline JJ, Walker MK, Mutti DO, et al. Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: the BLINK randomized clinical trial. JAMA. 2020;324(6):571–80.
Krummenauer F, Dick B, Schwenn O, Pfeiffer N. The determination of sample size in controlled clinical trials in ophthalmology. Br J Ophthalmol. 2002;86:946–7.
Fan DS, Lam DS, Chan CK, Fan AH, Cheung EY, Rao SK. Topical atropine in retarding myopic progression and axial length growth in children with moderate to severe myopia: a pilot study. Jpn J Ophthalmol. 2007;51:27–33.
Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116:572–9.
Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014;157:451-457.e1.
Zimmerman AB, Nixon AD, Rueff EM. Contact lens associated microbial keratitis: practical considerations for the optometrist. Clin Optom (Auckl). 2016;8:1–12.
Bez D, Megreli J, Bez M, Avramovich E, Barak A, Levine H. Association between type of educational system and prevalence and severity of myopia among male adolescents in Israel. JAMA Ophthalmol. 2019;137:887–93.
Gwiazda J, Bauer J, Thorn F, Held R. A dynamic relationship between myopia and blur-driven accommodation in school-aged children. Vis Res. 1995;35:1299–304.
Goss DA. Clinical accommodation and heterophoria findings preceding juvenile onset of myopia. Optom Vis Sci. 1991;68:110–6.
Wildsoet CF, Chia A, Cho P, et al. IMI—Interventions Myopia Institute: interventions for controlling myopia onset and progression report. Invest Ophthalmol Vis Sci. 2019;60:M106–31.
Wolffsohn JS, Kollbaum PS, Berntsen DA, et al. IMI—clinical myopia control trials and instrumentation report. Invest Ophthalmol Vis Sci. 2019;60:M132–60.
Ip JM, Huynh SC, Kifley A, et al. Variation of the contribution from axial length and other oculometric parameters to refraction by age and ethnicity. Invest Ophthalmol Vis Sci. 2007;48:4846–53.
Guo X, Fu M, Ding X, Morgan IG, Zeng Y, He M. Significant axial elongation with minimal change in refraction in 3- to 6-Year-Old Chinese preschoolers: the Shenzhen kindergarten eye study. Ophthalmology. 2017;124:1826–38.
Lam CS, Tang WC, Tse DY, Tang YY, To CH. Defocus incorporated soft contact (DISC) lens slows myopia progression in Hong Kong Chinese schoolchildren: a 2-year randomised clinical trial. Br J Ophthalmol. 2014;98:40–5.
Pauné J, Morales H, Armengol J, Quevedo L, Faria-Ribeiro M, González-Méijome JM. Myopia control with a novel peripheral gradient soft lens and orthokeratology: a 2-year clinical trial. BioMed Res Int. 2015;2015: 507572.
Cheng X, Xu J, Chehab K, Exford J, Brennan N. Soft contact lenses with positive spherical aberration for myopia control. Optom Vis Sci. 2016;93:353–66.
Sankaridurg P, Holden B, Smith E 3rd, et al. Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: one-year results. Invest Ophthalmol Vis Sci. 2011;52:9362–7.
Swarbrick HA, Alharbi A, Watt K, Lum E, Kang P. Myopia control during orthokeratology lens wear in children using a novel study design. Ophthalmology. 2015;122:620–30.
Tsai WS, Wang JH, Lee YC, Chiu CJ. Assessing the change of anisometropia in unilateral myopic children receiving monocular orthokeratology treatment. J Formos Med Assoc. 2019;118:1122–8.
Anstice NS, Phillips JR. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology. 2011;118:1152–61.
Acknowledgements
Funding
This study was funded by grants from App Vision Care Co, Ltd (Jhuanli) in Taiwan (MQ2432 to National Taiwan University Hospital; 03-FS05-33 to Taipei Tzu Chi Hospital). This funding organization had no role in the design or conduct of this research, nor in the decision to submit the manuscript for publication. The journal’s Rapid Service Fee was supported by National Taiwan University Hospital.
Medical Writing, Editorial, and Other Assistance
We thank Formosa Biomedical Technology Corp for their statistical support.
Author Contributions
All authors contributed to the study conception and design, material preparation, data collection and analysis. The first draft of the manuscript was written by Elizabeth P Shen and Tzu-Hsun Tsai and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Elizabeth P Shen, Hsiao-Sang Chu, Han-Chih Cheng, and Tzu-Hsun Tsai declare that they have no competing interests.
Compliance with Ethics Guidelines
This study protocol was approved by the Taiwan Food and Drug Administration (TFDA) and the Institutional Review Boards of National Taiwan University Hospital and Taipei Tzu Chi Hospital; it was conducted in accordance with the tenets of the Declaration of Helsinki for Experimentation on Humans, the International Conference on Harmonization, and the Good Clinical Practice guidelines. Informed consent was obtained from all participants and one of their parents/guardians following a thorough explanation of the study purpose and required examinations.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Shen, E.P., Chu, HS., Cheng, HC. et al. Center-for-Near Extended-Depth-of-Focus Soft Contact Lens for Myopia Control in Children: 1-Year Results of a Randomized Controlled Trial. Ophthalmol Ther 11, 1577–1588 (2022). https://doi.org/10.1007/s40123-022-00536-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00536-5