Abstract
Introduction
To evaluate the outcomes of intravitreal aflibercept injections for retinal angiomatous proliferation (RAP) according to disease stage.
Methods
This retrospective chart review included 68 eyes of 53 individuals diagnosed as having RAP and 109 neovascular age-related macular degeneration (nAMD) eyes of 109 patients as controls. All patients received intravitreal injections of aflibercept in a real-world setting. The main outcome measures were the changes in the mean of best-corrected visual acuity (BCVA) and central retinal thickness (CRT) as well as the total number of injections received during the 3-year follow-up period.
Results
The average BCVA and CRT changes in eyes affected by RAP and the controls at 3 years were non-significant. Both populations received a similar number of injections. After 3 years of treatment, patients with RAP had visual decline despite stable anatomical outcomes. Approximately 50% of the eyes with stage II RAP exhibited significant BCVA decline at the end of the third year. Among those eyes that had deteriorated BCVA, persistently worsening BCVA and thinning CRT were observed from year 2 to year 3.
Conclusion
Similar to treating nAMD, intensive injections or aggressive treatment strategies are required to treat RAP to achieve optimal visual outcomes in a real-world setting. The response to aflibercept treatment at the second year is associated with the final visual outcome of eyes with stage II RAP lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Intravitreal injection of anti-VEGF has been widely used in treating RAP. The influence of RAP stage on treatment outcomes needs further investigation. |
Eyes with stage I or III RAP were able to achieve stabilized visual function throughout the study period. |
Approximately 50% of the eyes with stage II RAP were unable to maintain their visual acuity. |
The response to aflibercept treatment at the second year is associated with the final visual outcome of eyes with stage II RAP lesions. |
Introduction
Retinal angiomatous proliferation (RAP), also known as type 3 choroidal neovascularization (CNV), is a subtype of neovascular age-related macular degeneration (nAMD) that is characterized by intraretinal neovascularization that eventually leads to retinal-choroidal anastomosis [1]. Up to 15% of patients with nAMD are estimated to have RAP, and it accounts for 4–11% of CNV in Asian populations [2] and 13–15% in Caucasian populations [3].
Patients with RAP are predominantly female; they have an average age of approximately 80 years and have a higher prevalence of bilateral eye involvement than patients with type 1 or type 2 CNV [4, 5]. Clinical findings frequently associated with RAP include reticular pseudodrusen, superficial intraretinal hemorrhage, and the presence of pigment epithelial detachments [6, 7]. Yannuzzi et al. [4] classified RAP lesions into three stages based on the evolution of the neovascular process. This staging method remains the most widely used classification in clinical studies [1]. The initial visual acuity among patients with RAP varies widely, and studies have suggested that the stage of the disease at presentation may be a key factor in prognosis and the effectiveness of treatment [1, 8, 9].
In earlier reports, RAP was thought to be relatively treatment resistant and to have worse functional outcomes compared with other types of CNV [10, 11]. The intravitreal injection of vascular endothelial growth factor inhibitor (anti-VEGF) has been widely used in treating RAP and has been reported by many studies to be effective [12,13,14,15]. Analyses from the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) revealed that eyes with RAP lesions had visual outcomes no worse than those with other types of CNV under anti-VEGF treatment over a 2-year period [16]. However, whether visual and anatomical outcomes of RAP lesions treated with anti-VEGF are comparable to those of non-RAP CNV lesions remains controversial [1]. Furthermore, the influence of RAP stage on treatment outcomes has been highlighted in many investigations, which demonstrated a trend of better prognosis when early-stage RAP is treated relative to trends when more advanced lesions are treated [8, 12, 17,18,19,20,21]. Nevertheless, differences in the anti-VEGF agents used and re-treatment criteria in studies may have affected the reported outcomes, and the RAP lesion stage was often not clearly detailed [1].
The aim of this study was to compare real-world outcomes for different stages of RAP treated with intravitreal aflibercept injections and those for other nAMD subtypes.
Methods
Study Design, Patient Selection, and Treatment Intervention
This retrospective study was approved by the Institutional Review Board of Taipei Veterans General Hospital (IRB-TPEVGH No. 2020-01-016BC), and all research steps followed the tenets of the Declaration of Helsinki. We reviewed the medical records of all patients who visited Taipei Veterans General Hospital during 2013–2020 with a diagnosis of nAMD. If both eyes were diagnosed as having nAMD, we enrolled them as a single case.
The diagnostic criteria for RAP lesions were based on clinical and angiographic findings, including (1) age of 55 years or over; (2) nAMD with characteristics of intraretinal lesions when observed on optical coherence tomography (OCT), including associated intraretinal edema with or without subretinal pigment epithelium fluid; (3) focal hyperfluorescent lesions and late leakage on fluorescein angiography at the site of an intraretinal lesion; and (4) a corresponding “hot spot” on indocyanine green angiography. Eyes with RAP lesions were classified as stage I, II, or III according to the classification system proposed by Yannuzzi et al. [4]: stage I, intraretinal neovascularization; stage II, subretinal neovascularization; stage II,: choroidal neovascularization with vascularized pigment epithelium detachment and retinal-choroidal anastomosis. Controls were selected from the nAMD population diagnosed as having CNV other than RAP. All enrolled patients had best-corrected visual acuity (BCVA) between 20/40 and 20/400, as tested by Snellen equivalent, due to the reimbursement regulation of the National Health Insurance program in Taiwan. Patients who had BCVA better than 20/40 or worse than 20/400 were not eligible to receive intravitreal injections of aflibercept. Patients who withdrew or were followed up for less than 1 year were excluded. All enrolled patients were followed up for 3 years after the first aflibercept injection. The data of patients who were followed up for less than 3 years were recorded but excluded from the final analysis.
A complete ophthalmologic examination was performed at baseline and during all follow-up visits, including BCVA, slit-lamp biomicroscopy, noncontact tonometry, dilated fundus evaluation and photography, and OCT (Avanti RTVue XR, OptoVue, Fremont, CA, USA). Fluorescein angiography and indocyanine green angiography were performed at baseline and if any signs indicating relapse were observed on fundoscopy during the study period.
Treatment Protocol
Treatment decisions, such as the frequency and timing of treatment, were entirely at the discretion of the practitioner in consultation with the patient, reflecting real-world practice. Most patients received three consecutive monthly injections during the initial loading, followed by a pro re nata regimen; in the latter, patients received injections when their visual acuity dropped more than two lines in a Snellen chart compared with the previous visit without developing other ocular diseases, or if intraretinal or subretinal fluid was shown on OCT exams.
Outcome Measurement
In all patients, the primary outcome was the final BCVA at 3 years after treatment initiation. The BCVA was converted to a logarithm of the minimum angle of resolution (logMAR). Secondary outcomes were the change of central retinal thickness (CRT) and the number of injections.
Statistical Analysis
Statistical analysis was performed using SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA). Because the distributions of most quantitative variables were greatly different from normal ones, nonparametric tests were used. The Mann–Whitney U test and the Kruskal–Wallis test were used to analyze quantitative variables such as BCVA, CRT, and the number of injections between or within groups. The chi-squared test was used to compare binomial variables. Within-group changes in BCVA and CRT were analyzed using the Wilcoxon signed-rank test. A P value less than 0.05 was considered statistically significant in all analyses.
Results
A total of 68 eyes of 53 patients diagnosed as having RAP were initially included; 16 (24%) and 18 (26%) patients were lost to follow-up before the second and third year, respectively. Among all cases, 18, 29, and 21 eyes were classified as stage I, II, or III RAP, respectively. The control group included 109 nAMD eyes of 109 patients with type 1 or type 2 CNV lesions; 24 (28%) and 12 (11%) patients were lost to follow-up before the second and third year, respectively.
RAP Versus Controls
The demographic characteristics of all patients are presented in Table 1. The initial BCVA, CRT, and age of the two populations did not differ. The group affected by RAP had a higher proportion of female cases compared with the controls (47% vs. 29%, P = 0.017). Gradual decline of BCVA was noted in both populations but without significant difference during the 3-year follow-up. The average CRT measurement of the two populations was similar during the study period. Numbers of injections in the first, second, and third year also exhibited no difference between the RAP and control groups.
In eyes diagnosed as having RAP, the average follow-up times were 17.4 ± 3.7 months and 29.2 ± 8.1 months for those lost to follow-up before the second or third year, respectively. Patients who were lost to follow-up in the second year had a steeper drop in vision than those lost in the first year. By contrast, a better baseline BCVA and a relatively flatter decline slope were noted in those who completed 2 or 3 years of follow-up, as demonstrated in Fig. 1.
Comparison of Stages of RAP
The baseline characteristics and 3-year clinical outcomes for the three stages are presented in Table 2. The average baseline BCVA was 0.61 ± 0.39, 0.84 ± 0.45, and 0.80 ± 0.39 in logMAR and the mean final BCVA at the third year was 0.84 ± 0.53, 1.21 ± 0.53, and 0.73 ± 0.41 in eyes with stage I, II, and III RAP, respectively. No significant difference in average BCVA was observed among the three stages at baseline and at the end of the first and third year. In the second year, the mean BCVA was significantly poorer in stage II (1.08 ± 0.43 logMAR, P = 0.023) than that in stage I and stage III RAP (0.60 ± 0.42 logMAR and 0.85 ± 0.59 logMAR).
Among those with 3 years of follow-up, 18 eyes with stage II RAP had significantly declined BCVA (from 0.77 ± 0.43 to 1.24 ± 0.55 in logMAR, P = 0.027) compared with those with stage I or III RAP lesions. The CRT values and number of total injections among the three stages exhibited no significant difference during the study period. Representative cases of stage I and III RAP lesions before and after 3 years of follow-up are demonstrated in Figs. 2 and 3.
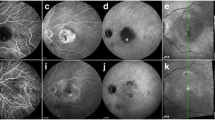
Multimodal imaging of stage I RAP. a Fundus photograph showing drusens and intraretinal hemorrhage. b Early phase FA shows blocked fluorescence. c Late phase shows indistinct leakage. d Late phase ICGA shows a hot spot. e Fundus photograph showing resolution of intraretinal hemorrhage at year 3 follow-up. f OCT image before treatment shows RPE elevation and intraretinal thickening and suggestion of a communicating vessel (arrow). g OCT image taken at year 3 follow-up shows some RPE bumps. FA, fluorescein angiography; ICGA, indocyanine green angiography; OCT, optical coherence tomography; RAP, retinal angiomatous proliferation; RPE, retinal pigment epithelium
Multimodal imaging of stage III RAP. a Fundus photograph showing a circumscribed area of PED with a focal area of retinal hemorrhage. b Early phase of ICGA showing a vertically diving retinal vessel with retinal-retinal anastomosis. c Late phase ICGA shows hot spots. d Late phase FA shows choroidal neovascularization. e Fundus photograph showing resolution of the circumscribed PED and the retinal hemorrhage at year 3 follow-up. f OCT image before treatment shows exudative maculopathy with PED, subretinal and intraretinal fluid. g OCT image taken at year 3 follow-up shows resolution of exudative maculopathy. FA, fluorescein angiography; ICGA, indocyanine green angiography; OCT, optical coherence tomography; PED, pigment epithelial detachment; RAP, retinal angiomatous proliferation
Predictive Factors for Visual Outcome in Stage II RAP
We further divided the eyes with stage II RAP lesions into two groups for analysis (Table 3). Group 1 consisted of eight eyes with stabilization or improvement in BCVA; group 2 consisted of 10 eyes with a decline in BCVA of more than two lines in the Snellen chart compared with baseline at the end of the third year. Representative cases of group 1 and group 2 are demonstrated in Figs. 4 and 5. The baseline BCVA and CRT of the two groups were similar. Starting from the end of the second year, the average BCVA of eyes in group 2 were significantly poorer than those of group 1 (1.27 ± 0.37 vs. 0.85 ± 0.38 in logMAR, P = 0.036) at 2 years and (1.50 ± 0.35 vs. 0.86 ± 0.47 in logMAR, P = 0.034) at 3 years. When further analyzing the change in BCVA, we noted that in group 1, no eyes had declines in BCVA during the 3-year follow-up. In group 2, declined BCVA was observed in approximately 40% of the eyes at 3 and 6 months, and in 60% of eyes at the first year, and finally in 100% of eyes at the second year. These eyes were then observed to have persistently worsening BCVA from year 2 to year 3. The average CRT was thinner in the eyes of those in group 2 over the 3-year period, and the difference reached statistical significance at the second year compared with in group 1 (210.2 ± 37.32 μm vs. 279.1 ± 79.32 μm, P = 0.04). The number of injections given to the two groups did not differ.
Multimodal imaging of stage II RAP in group 1 without reported BCVA decline. a Fundus photograph showing drusens and intraretinal hemorrhage. b Early phase ICGA demonstrating a neovascularized lesion in a macula with retino-retinal vessel anastomosis. c Late phase ICGA revealing a hot spot corresponding to a neovascularized macular lesion. d Late phase of FA showing retinal-retinal anastomosis and progressive pooling within the PED. e Fundus photograph showing drusens and a PED at year 3 follow-up. f OCT image before treatment showing a PED with intraretinal fluid accumulation. g OCT image taken at year 3 follow-up. BCVA, best corrected visual acuity; FA, fluorescein angiography; ICGA, indocyanine green angiography; OCT, optical coherence tomography; PED, pigment epithelial detachment; RAP, retinal angiomatous proliferation
Multimodal imaging of stage II RAP in group 2 with reported BCVA decline. a Fundus photograph of a PED with a diving retinal vessel (arrow). b Early phase ICGA showing a vertically diving retinal vessel with retinal-retinal anastomosis (arrow). c Early phase FA shows a diving vessel with possible intraretinal neovascularization. d Late phase of FA showing progressive pooling within the PED. e Fundus photograph showing geographic atrophy at year 3 follow-up. f OCT image before treatment showing a PED with intraretinal fluid accumulation. g OCT image taken at year 3 follow-up demonstrating a thin CRT. BCVA, best corrected visual acuity; CRT, central retinal thickness; FA, fluorescein angiography; ICGA, indocyanine green angiography; OCT, optical coherence tomography; PED, pigment epithelial detachment; RAP, retinal angiomatous proliferation
Discussion
The natural course of RAP causing visual impairment is believed to be less favorable than that of other types of nAMD. Viola et al. [10] reported that in stage I and stage II RAP-affected eyes, progression to poor vision was common within 3 months in rapidly progressing cases and within 1 year in cases with a slower progression. Several studies have reported many options for treating RAP lesions [22,23,24,25]. At present, anti-VEGF therapy is the first-line therapy for RAP, and it has been reported to be efficacious for these lesions [1, 12,13,14,15,16]. Aflibercept was demonstrated to be as effective as ranibizumab for treating nAMD but with fewer injections required [26]. Although optimal outcomes with aflibercept have been reported [27, 28], most studies have been limited by small numbers of included individuals and varied follow-up durations. Questions remain regarding optimal treatment regimens, treatment responses depending on the disease stage, and long-term outcomes.
In our study, the patients enrolled with either RAP lesions or other types of nAMD had similar baseline characteristics including mean age, BCVA, and CRT. The only difference noted was the higher proportion of female patients in the RAP group, which was consistent with other investigations indicating that RAP is a female-predominant disease [4, 5]. The 3-year visual and anatomical outcomes indicated that aflibercept was equally efficacious in both groups. Eyes affected by RAP and controls received an average of 9.2 ± 3.7 and 8.3 ± 4.7 injections, respectively.
In our previous work, we described the real-world outcomes of patients with nAMD treated with aflibercept with an average total of 8.16 ± 4.57 injections over 3 years [29]. Despite good anatomical outcomes, the average BCVA at 3-year follow-up had declined to a level below the baseline one. We observed a similar trend of BCVA deterioration in the current study from year 1 to year 3. The suboptimal visual outcome was assumed to be related to relatively fewer injections being administered compared with in other studies [15, 27]. Invernizzi et al. reported 2-year real-world outcomes of eyes with RAP treated with anti-VEGF. They administered a median of 8 injections in the first year, and 13 injections at the end of the second year [15]. In another study that had a subgroup of 10 eyes with RAP which showed vision gains at 1 year, the dosing regimen for aflibercept was 3 monthly injections followed by 4 bimonthly injections [30]. Browning et al. reported that patients with RAP treated prospectively with aflibercept showed a similar degree of visual improvement to those with nAMD. The treatment strategy of this study was 3 initial monthly injections of aflibercept followed by bimonthly injections up to week 48. During weeks 52 to 96, patients then received injections at least every 12 weeks [27]. Thus, intensive injections or aggressive treatment strategies such as fixed-dose or treat-and-extend regimen other than a pro re nata regimen result in better visual outcomes.
Whether retina specialists undertreat patients using anti-VEGF therapy in real-world settings is debated. Undoubtedly, some patients do not receive sufficient anti-VEGF treatment [31]. In previous work, we concluded that in treating nAMD in a real-world setting, intensive treatment with more injections or other strategies would be required for patients that are at risk of having a poor prognosis [29].
The stage of RAP has been proposed as a key factor in the effectiveness of treatment and prognosis. Early-stage lesions (stage I) seem to be associated with better visual and anatomical outcomes, whereas late-stage diseases (stage III) were reported to exhibit limited improvement with treatment by intravitreal injections of ranibizumab and bevacizumab [8, 12, 17,18,19,20,21]. In the current study, the baseline BCVA and CRT were similar among different stages. During the 3-year study period, a significant decrease in BCVA was noted in eyes with stage II RAP at the second year, whereas eyes with stage I or stage III RAP could achieve stabilization in BCVA. Park and Roh [21] reported that three consecutive loading doses of intravitreally administered ranibizumab was an effective treatment for patients with stage I RAP within 1 year, resulting in a significantly lower recurrence rate than in patients in later stages. By contrast, our results indicated that an average of 5.3 ± 1.72 injections in the first year followed by a total of 10.4 ± 3.85 injections were required for patients with stage I RAP to maintain stabilized BCVA after 3 years.
The response to aflibercept in eyes with stage II RAP was unsatisfactory in our study, as BCVA significantly decreased at the second year relative to eyes with stage I or III RAP. The decline in BCVA at the second-year timepoint was consistent with the observation in the CATT study, in which, among patients with nAMD followed for 5 years, average BCVA declined from year 2 to year 5 [16, 31]. Subgroup analysis was performed for the eyes with stage II RAP that achieved improvement or stabilization in BCVA (group 1) and those that had declines in BCVA (group 2) at the end of the third year. The eyes in group 1 were able to maintain BCVA during the 3-year period. In group 2, declines in BCVA were observed in approximately 40% of the eyes at 3 and 6 months, and 60% of eyes at the first year, and finally in 100% of eyes at the second year compared with baseline. The average CRT was thinner in group 2 patients throughout the study period, and CRT was significant different at the second year compared with in group 1. Taken together, we found that in patients with stage II RAP, approximately 50% were unable to maintain their BCVA at the end of the third year. Among them, 60% had deteriorated BCVA at the end of the first year, and eventually all of them had worsened BCVA at year 2. All these eyes were noted to have a thin CRT at year 2, and to have persisted poor BCVA at the end of year 3. In the CATT study, the development of new adverse pathological features was thought to be the reason for BCVA worsening from year 2. Nevertheless, some eyes without any adverse pathology were still observed to have deteriorated BCVA from year 2 to year 5. The authors stated that some factors have not yet been identified to account for this observation [31]. Similarly, Cho et al. reported that in the treatment of patients with RAP, visual acuity improved over the first 2 years but subsequently declined through year 3. They also determined that the improvement in visual acuity was limited by geographic atrophy (GA) that developed in one-third of the patients [12]. In our study, 43% of the eyes in group 2 (data not showed) were noted to have GA and demonstrated a thin CRT on OCT at the end of the second year.
The optimal treatment for stage III RAP remains unclear [1]. Costagliola et al. [17] reported that, despite reduction in macular thickness, visual improvement was not observed in stage III RAP treated with intravitreal bevacizumab injections. However, a larger study demonstrated stabilization of BCVA in stage III RAP [18], which was consistent with our results. It is expected that patients with poor treatment response to anti-VEGF therapy tend to be lost to follow-up [29]. Indeed, we found that there was a relatively higher lost to follow-up rate in patients stage III RAP.
This study has limitations. First, the relatively small number of cases in each stage at the end of the study period may have compromised the data analysis. Second, because the study reflects real-world practice, the frequency and number of injections were determined by different physicians, and this variation may have led to different responses to treatment. Third, the high lost to follow-up rate may have led to bias toward more favorable visual prognosis because these lost patients may have had a poor visual prognosis that was not taken into consideration in determining final outcomes. As demonstrated in Fig. 1, patients with poorer initial vision and fast decline of vision tend to be lost to follow-up. This may raise the possibility that the treatment is not perceived to be efficacious by the patients and hence they tend to skip their follow-up. This reflects real-world problems as these patients do not notice benefit from aggressive treatment.
Conclusion
Our study demonstrated that eyes with RAP and other types of nAMD lesions had similar responses to aflibercept over a 3-year follow-up in a real-world setting. More aggressive injections or treatment strategies other than a pro re nata regimen are required to achieve optimal visual outcomes. Eyes with stage I or III RAP were able to achieve stabilized BCVA throughout the study period. However, approximately 50% of the eyes with stage II RAP were unable to maintain their BCVA. The response to aflibercept treatment at the second year is associated with the final visual outcome of eyes with stage II RAP lesions.
References
Tsai ASH, Cheung N, Gan ATL, et al. Retinal angiomatous proliferation. Surv Ophthalmol. 2017;62(4):462–92.
Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007;144(1):15–22.
Cohen SY, Creuzot-Garcher C, Darmon J, et al. Types of choroidal neovascularisation in newly diagnosed exudative age-related macular degeneration. Br J Ophthalmol. 2007;91(9):1173–6.
Yannuzzi LA, Negrão S, Iida T, et al. Retinal angiomatous proliferation in age–related macular degeneration. 2001. Retina. 2012;32(Suppl 1):416–34.
Gross NE, Aizman A, Brucker A, Klancnik JM Jr, Yannuzzi LA. Nature and risk of neovascularization in the fellow eye of patients with unilateral retinal angiomatous proliferation. Retina. 2005;25(6):713–8.
Hunter MA, Dunbar MT, Rosenfeld PJ. Retinal angiomatous proliferation: clinical characteristics and treatment options. Optometry. 2004;75(9):577–88.
Sawa M, Ueno C, Gomi F, Nishida K. Incidence and characteristics of neovascularization in fellow eyes of Japanese patients with unilateral retinal angiomatous proliferation. Retina. 2014;34(4):761–7.
Hemeida TS, Keane PA, Dustin L, Sadda SR, Fawzi AA. Long-term visual and anatomical outcomes following anti-VEGF monotherapy for retinal angiomatous proliferation. Br J Ophthalmol. 2010;94(6):701–5.
Parodi MB, Iacono P, Menchini F, et al. Intravitreal bevacizumab versus ranibizumab for the treatment of retinal angiomatous proliferation. Acta Ophthalmol. 2013;91(3):267–73.
Viola F, Massacesi A, Orzalesi N, Ratiglia R, Staurenghi G. Retinal angiomatous proliferation: natural history and progression of visual loss. Retina. 2009;29(6):732–9.
Scott AW, Bressler SB. Retinal angiomatous proliferation or retinal anastomosis to the lesion. Eye (Lond). 2010;24(3):491–6.
Cho HJ, Lee TG, Han SY, et al. Long-term visual outcome and prognostic factors of intravitreal anti-vascular endothelial growth factor treatment for retinal angiomatous proliferation. Graefes Arch Clin Exper Ophthalmol. 2016;254(1):23–30.
Matsumoto H, Sato T, Morimoto M, et al. Treat-and-extend regimen with aflibercept for retinal angiomatous proliferation. Retina. 2016;36(12):2282–9.
Shin JY, Yu HG. Optical coherence tomography-based ranibizumab monotherapy for retinal angiomatous proliferation in Korean patients. Retina. 2014;34(12):2359–66.
Invernizzi A, Teo K, Nguyen V, et al. Type 3 neovascularisation (retinal angiomatous proliferation) treated with antivascular endothelial growth factor: real-world outcomes at 24 months. Br J Ophthalmol. 2019;103(9):1337–41.
Daniel E, Shaffer J, Ying GS, et al. Outcomes in eyes with retinal angiomatous proliferation in the comparison of age-related macular degeneration treatments trials (CATT). Ophthalmology. 2016;123(3):609–16.
Costagliola C, Romano MR, dell’Omo R, Cipollone U, Polisena P. Intravitreal bevacizumab for the treatment of retinal angiomatous proliferation. Am J Ophthalmol. 2007;144(3):449–51.
Montero JA, Fernandez MI, Gomez-Ulla F, Ruiz-Moreno JM. Efficacy of intravitreal bevacizumab to treat retinal angiomatous proliferation stage II and III. Eur J Ophthalmol. 2009;19(3):448–51.
Hufendiek K, Hufendiek K, Panagakis G, Helbig H, Gamulescu MA. Visual and morphological outcomes of bevacizumab (Avastin®) versus ranibizumab (Lucentis®) treatment for retinal angiomatous proliferation. Int Ophthalmol. 2012;32(3):259–68.
Maier M, Perz C, Bockmaier J, Feucht N, Lohmann CP. [Therapy of stage III retinal angiomatous proliferation. Intravitreal ranibizumab injections]. Der Ophthalmologe. 2013;110(12):1171–8.
Park YG, Roh YJ. One year results of intravitreal ranibizumab monotherapy for retinal angiomatous proliferation: a comparative analysis based on disease stages. BMC Ophthalmol. 2015;15:182.
Johnson TM, Glaser BM. Focal laser ablation of retinal angiomatous proliferation. Retina. 2006;26(7):765–72.
Sakimoto S, Gomi F, Sakaguchi H, Tano Y. Recurrent retinal angiomatous proliferation after surgical ablation. Am J Ophthalmol. 2005;139(5):917–8.
Kuroiwa S, Arai J, Gaun S, Iida T, Yoshimura N. Rapidly progressive scar formation after transpupillary thermotherapy in retinal angiomatous proliferation. Retina. 2003;23(3):417–20.
Boscia F, Furino C, Sborgia L, Reibaldi M, Sborgia C. Photodynamic therapy for retinal angiomatous proliferations and pigment epithelium detachment. Am J Ophthalmol. 2004;138(6):1077–9.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201.
Browning AC, O’Brien JM, Vieira RV, Gupta R, Nenova K. Intravitreal aflibercept for retinal angiomatous proliferation: results of a prospective case series at 96 weeks. Ophthalmologica. 2019;242(4):239–46.
Rezar-Dreindl S, Eibenberger K, Buehl W, et al. Clinical outcomes of different subtypes of neovascular age-related macular degeneration during aflibercept treatment. Retina. 2021;41(1):103–10.
Lo KJ, Chang JY, Chang HY, Chiou SH, Hwang DK, Chen SJ. Three-year outcomes of patients with neovascular age-related macular degeneration treated with aflibercept under the national health insurance program in Taiwan. J Ophthalmol. 2020;2020:4538135.
Oishi A, Tsujikawa A, Yamashiro K, et al. One-year result of aflibercept treatment on age-related macular degeneration and predictive factors for visual outcome. Am J Ophthalmol. 2015;159(5):853-60.e1.
Jaffe GJ, Ying GS, Toth CA, et al. Macular morphology and visual acuity in year five of the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2019;126(2):252–60.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were supported by Taipei Veterans General Hospital (V111C-150).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Conceptualization: Wen-Jung Lo, Yu-Bai Chou, Tai-Chi Lin. Data curation: Ya-Yun Huang, Tai-Chi Lin. Formal analysis: Ya-Yun Huang, Wen-Jung Lo, Hsin-Yi Chang. Investigation: Ya-Yun Huang, Yu-Bai Chou, Tai-Chi Lin. Methodology: Wen-Jung Lo, Yu-Bai Chou, Tai-Chi Lin. Project administration: Yu-Bai Chou, Tai-Chi Lin. Resources: Yu-Bai Chou, Tai-Chi Lin. Supervision: Yu-Bai Chou, Tai-Chi Lin. Validation: Yu-Bai Chou, Tai-Chi Lin. Visualization: Yu-Bai Chou, Tai-Chi Lin. Writing of original draft: Ya-Yun Huang, Wen-Jung Lo, Hsin-Yi Chang. Review & editing: Ya-Yun Huang, Wen-Jung Lo, Hsin-Yi Chang, Yu-Bai Chou, Tai-Chi Lin.
Compliance with Ethics Guidelines
This retrospective study was approved by the Institutional Review Board of Taipei Veterans General Hospital (IRB-TPEVGH No. 2020-01-016BC), and all research steps followed the tenets of the Declaration of Helsinki of 1964 and its later amendments.
Disclosures
Ya-Yun Huang, Wen-Jung Lo, Hsin-Yi Chang, Yu-Bai Chou and Tai-Chi Lin have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Huang, YY., Lo, WJ., Chang, HY. et al. Three-Year Outcomes of Intravitreal Aflibercept Injections for Retinal Angiomatous Proliferation According to Disease Stage. Ophthalmol Ther 11, 1503–1516 (2022). https://doi.org/10.1007/s40123-022-00521-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00521-y