Abstract
Introduction
This study evaluated the outcomes of laser in situ keratomileusis (LASIK) combined with prophylactic corneal collagen cross-linking (CXL) in correcting myopia in cases with increased estimated risk of postoperative corneal ectasia, detected by regional analysis of corneal morphology.
Methods
The retrospective study included 180 eyes of 99 patients. Group 1 (94 eyes of 49 patients) with increased risk of postoperative corneal ectasia, as detected by “Ectasia Risk Factor Score System for LASIK”, underwent femtosecond laser-assisted LASIK (FS-LASIK) combined with prophylactic CXL, using short riboflavin soaking time and low UV energy. Group 2 (86 eyes of 50 patients) with normal corneal topography, who underwent FS-LASIK alone, were used as controls. Refractive and visual outcomes and Scheimpflug topo/tomography were analyzed preoperatively and 1 week, 1 month, and 12 months postoperatively. Mean regional corneal curvature (M) values for three subregions (the central 3.0 mm region, the paracentral 3.0–6.0 mm region, and the peripheral 6.0–9.0 mm region) of both anterior and posterior surfaces were calculated.
Results
An increase in flattening of the peripheral anterior region and more steepening of the posterior paracentral region were shown at 12 months compared to 1 month postoperatively in group 1. The findings were significantly more pronounced than in group 2 (P < 0.001 and P = 0.035, respectively). The refractive and visual outcomes were comparable in the two groups.
Conclusions
Prophylactic CXL seems to influence corneal regional reshaping after surgery, while not affecting the 1-year visual and refractive results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Reports regarding LASIK combined with prophylactic CXL are mostly focused on visual and refractive outcomes, revealing that the prophylactic CXL may increase stability in vision and refraction. There is a lack of data about the regional analysis of corneal morphology in patients with relatively poor preoperative symmetry in corneal topography, with increased risk of post-LASIK corneal ectasia. |
We found that the magnitude of steepening in the posterior paracentral region and the magnitude of flattening in the anterior peripheral region were more pronounced in patients treated with prophylactic CXL, in the period from 1 to 12 months postoperatively. |
Prophylactic CXL seemed to influence corneal regional reshaping after surgery, while not affecting the 1-year visual and refractive results. |
Further follow-up and true biomechanical assessment are needed to draw definitive conclusions regarding the ability to reduce the risk of ectasia in patients with relatively poor corneal morphology and topography. |
Introduction
The creation of the flap and removal of tissue required in laser in situ keratomileusis (LASIK) negatively affects the corneal biomechanical properties [1] that may lead to refractive regression [2] and in the worst case corneal ectasia [3]. Patients to be treated for high myopia, with thin corneas and/or poor topographic symmetry, have increased risk of post-LASIK corneal ectasia [4,5,6,7]. Therapeutic corneal collagen cross-linking (CXL), using UV-activated riboflavin, has been adopted as standard and effective treatment for corneal ectasia and keratoconus [8], while the prophylactic CXL, using less UVA energy [9], was proposed by Kanellopoulos in 2012 [10]. Several reports regarding LASIK combined with prophylactic CXL are mostly focused on visual and refractive outcomes, revealing that the prophylactic CXL may increase stability in vision and refraction, and reduce refractive regression [5, 11, 12]. Others showed no positive effect of prophylactic CXL [13]. A few reports that analyzed corneal morphological changes measured individual elevation data points on corneal topography as a representation of the whole corneal morphology [14]. Moreover, these studies involve very few patients with poor preoperative symmetry in corneal topography, with increased risk of post-LASIK corneal ectasia [13].
In this study, we evaluated the myopic eyes with relatively poor preoperative topographic symmetry that underwent femtosecond laser-assisted LASIK (FS-LASIK) combined with prophylactic CXL, with the aim to reduce the possibility of postoperative corneal ectasia. Eyes with preoperative myopia with normal corneal topography undergoing FS-LASIK alone were used as the control group. We compared regional corneal morphological changes and visual and refractive outcomes between the two groups.
Methods
This is a comparative nonrandomized retrospective study. We created a form called the “Ectasia Risk Factor Score System for LASIK” to determine patients’ risk of ectasia with refractive surgery by aggregating the existing parameters in preoperative evaluation (Table 1). It considers Chinese patients undergoing myopic refractive surgeries at a young age. The score system contains seven parameters, of which the first three are related to corneal topography, while the last four are the same as in the Ectasia Risk Factor Score System (ERSS) proposed by Randleman et al. [15]. The new system emphasizes the important role of the abnormality of the posterior corneal surface.
The shape of elevation map in Pentacam reflects the difference between corneal original elevation and the best fitting surface (BFS). BFS is set according to data within the central 8-mm diameters whatever the actual corneal diameter is. So, if the corneal is too small, more peripheral data will be involved to set BFS. BFS will become flatter then, and the shape of the elevation map tends to steepen, and vice versa. That is why the corresponding score is reduced if the corneal diameter is less than 11.5 mm, as mentioned in the footnotes of Table 1.
There is a greater intereye asymmetry in corneal thickness and posterior corneal elevation variables in keratoconic eyes compared to corneas [16, 17]. That is why we set such rules in the footnotes of Table 1: if the total score of parameters 1, 2, and 3 is 0 in one eye, but at least 1 point in the fellow eye, the final score of the former will be increased by 1 point for the first three parameters. If the two eyes of one patient show good symmetry in the shape of the posterior corneal surface elevation map, the score for the eye with higher scores for the first three parameters will be reduced by 1 point.
Patients
Patients who underwent FS-LASIK between October 2015 and August 2018 were analyzed in the study. Preoperatively, their thinnest corneal point was thicker than 480 µm, manifest refraction spherical equivalent (MRSE) greater than − 12.00 D, spherical power greater than − 12.00 D, and cylindrical power greater than − 6.00 D. Postoperatively, their residual bed thickness was greater than 300 µm. The participants treated with prophylactic CXL were all strongly motivated to perform laser refractive surgery, mostly because of their occupational requirements (e.g., soldier or policeman), or intolerability for contact lenses. The patients and their guardians were fully informed of the surgical risks and they signed an informed consent. As shown in Table 3, group 1 comprised 49 patients (94 eyes) who underwent FS-LASIK combined with prophylactic CXL, who scored 1–4 points for the first three parameters, 0–2 points for the last four parameters, and total score of 1–6, while 50 patients (86 eyes) in group 2, who underwent FS-LASIK alone, scored 0 points for the first three parameters and 0–2 points for the last four parameters. Percentage tissue alteration (PTA) was at most 40% for all patients. Target refraction was emmetropia in all cases. All patients in the study completed a follow-up that included three time points, namely 1 week, 1 month, and 12 months after surgery. The study was performed in accordance with the ethical standards described in the 2000 revision of the 1964 Declaration of Helsinki. The protocol was reviewed and approved by the ethics committee of the Eye Hospital, Wenzhou Medical University, China.
Surgical Technique and Postoperative Treatment
All surgeries were performed by the same surgeon. Both groups were treated using standard FS-LASIK procedure, with femtosecond laser (iFS150, Abbott Medical Optics, Inc) to create the flap and excimer laser (Amaris 750, Schwind eye-tech-solutions GmbH & Co. KG) to perform ablation [18]. In group 1, the procedure was combined with prophylactic CXL. After excimer laser ablation and before the flap was folded back, the interface was floated with aqueous 0.22% riboflavin solution (Avedro Inc, USA) without dextran, followed by application of 1–2 drops every 10 s for 90–120 s. After that the riboflavin was rinsed from the stroma using balanced salt solution. The corneal stromal bed was then irradiated with 365-nm ultraviolet A (UVA) light (Avedro, MA, USA), delivering a total energy ranging from 1.8 to 2.6 J/cm2 at an area of 9 mm in diameter, with 30 mW/cm2 power. Both the riboflavin soaking time and total irradiation energy were determined by the score obtained from our scoring system (Table 2). The rationale is that the poorer corneal morphology and topography is, the higher total irradiation energy is needed to compensate biomechanical deterioration caused by LASIK. Our animal experiments [19] showed that both 1.8 and 2.7 J/cm2 could improve postoperative corneal stiffness but the latter led to greater improvement. But in the clinic, CXL with energy of 2.7 J/cm2 usually caused more inflammatory response and edema and delayed the visual recovery. So, we customized the energy range from 1.8 to 2.6 J/cm2 according to the score obtained from Table 1. Once the irradiation was finished, balanced salt solution was used to wash the corneal stroma again and the flap was repositioned. The stromal bed was exposed during the UV treatment. At the end of the procedure in both groups, one drop of tobramycin/dexamethasone (Tobradex; Alcon, TX, USA) was instilled and a bandage contact lens (Acuvue Oasys; Johnson & Johnson, FL, USA) was then applied and kept for 1 day.
After surgery, patients in group 1 used tobramycin/dexamethasone (Tobradex; Alcon, TX, USA) four times per day for 3 days and then used fluorometholone 0.1% (Flumetholon; Santen, Osaka, Japan) four times per day for 4 days, the dosage was tapered over 1 month. Patients in group 2 used topical levofloxacin 0.5% (Cravit; Santen, Osaka, Japan) four times per day for 3 days, and fluorometholone 0.1% (Flumetholon; Santen, Osaka, Japan) four times per day for 1 week and the dosage was also tapered over 1 month.
Data Acquisition
All patients underwent complete ophthalmic examinations, consisting of central corneal thickness (CCT), intraocular pressure (IOP cc), manifest refraction spherical equivalent (MRSE), spherical power, cylindrical power, uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), preoperatively and at all follow-up visits. The corneal thickness and elevation were acquired using a high-resolution Scheimpflug tomography (Pentacam HR, software version 6.02r23, OCULUS Optikgerate GmbH). Only the examinations with instrument-generated quality factor of at least 95% and 90%, for the anterior and posterior surfaces, respectively, were accepted. Original corneal elevation data were exported from the Pentacam and used to calculate the mean regional corneal curvature (M) for three subregions of both anterior and posterior surfaces, as described in our previous study [18]. The three areas were the central region (0–3.0 mm diameter), the paracentral region (3.0–6.0 mm diameter), and the peripheral region (6.0–9.0 mm diameter). The vector changes in astigmatism from preoperatively to 12 months postoperatively were analyzed by the ASSORT group analysis calculator (©Copyright ASSORT Pty. Ltd. 2012–2020 [Version 1.1.4]) using the Alpins method. Five main parameters, target-induced astigmatism vector (TIA), surgically induced astigmatism vector (SIA), difference vector (DV), angle of error (AE), and correction index (CI), were generated. Only eyes with astigmatism no greater than − 0.25 D (89 eyes in group 1 and 77 eyes in group 2) were contained in the analysis. Endothelial cells of the corneas with residual bed thickness less than 350 µm in group 1 (n = 19) were evaluated preoperatively and 12 months postoperatively using specular microscopy (NSP—9900, Konan Medical, Honshu, Japan).
- Target-induced astigmatism vector (TIA):
-
The astigmatic change (magnitude and axis) that the surgery was intended to induce.
- Surgically induced astigmatism vector (SIA):
-
The astigmatic change (magnitude and axis) the surgery actually induced.
- Difference vector (DV):
-
The astigmatic change (magnitude and axis) that would enable the initial surgery to achieve its intended target. The DV magnitude is an absolute measure of success and is ideally zero.
- Angle of error (AE):
-
The difference in axes between the vectors of the achieved (SIA) and the intended (TIA) astigmatic changes. The AE is positive if the SIA is counterclockwise (CCW) to the TIA and negative if the SIA is clockwise (CW) to the TIA.
- Correction index (CI):
-
Calculated by dividing the SIA magnitude by the TIA magnitude. The CI is preferably 1.0. It is greater than 1.0 if an overcorrection occurs and less than 1.0 if there is an undercorrection.
Statistical Analysis
Statistical analysis was performed using SPSS software (version 22.0; IBM, Inc). Data conforming to a normal distribution was expressed as mean ± standard error (range) and non-normal data was expressed as median (percentile 25, percentile 75). We performed generalized estimating equations (GEE) to analyze the curvature, thickness, refraction, and version data. Preoperative and surgical parameters in the two groups, such as age, gender, CCT, IOP cc, MRSE, spherical power, cylindrical power or TIA, UDVA, CDVA, ablation depth, thickness of corneal flap, residual bed, and diameter of optical zone, were corrected through GEE when analyzing the results. It should be noted that there were no P values when correcting baseline parameters with GEE. The effect of mirror symmetry caused by bilateral corneas [20] was also weakened through GEE analysis, because the two eyes of one patient were both numbered and input into variables when analyzing data. We performed paired-samples t test to evaluate the changes of counts of endothelial cells in group 1. In the result, a P value of less than 0.05 was considered statistically significant.
Results
Table 3 summarizes the patient demographics, preoperative clinical characteristics, and surgical parameters.
Preoperative Absolute Value Versus 1-Week Postoperative Absolute Value
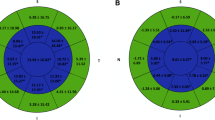
In the central region of the anterior cornea, M was reduced by 5.355 D in group 1 (P < 0.001) and 5.373 D in group 2 (P < 0.001). In the paracentral region of the anterior cornea, M was reduced by 2.317 D in group 1 (P < 0.001) and 2.576 D in group 2 (P < 0.001). In the peripheral region of the anterior cornea, M was increased by 3.457 D in group 1 (P < 0.001) and 3.283 D in group 2 (P < 0.001). The between-group differences were all not statistically significant (P = 0.958, 0.11, 0.351 respectively) (Fig. 1).
In the central region of the posterior cornea, M was reduced by 0.003 D in group 1 (P = 0.507) and 0.026 D in group 2 (P < 0.001); the between-group difference was statistically significant (P = 0.001). In the paracentral region of the posterior cornea, M was increased by 0.022 D in group 1 (P = 0.007) and 0.012 D in group 2 (P = 0.005); the between-group difference was not statistically significant (P = 0.244). In the peripheral region of the posterior cornea, M was increased by 0.05 D in group 1 (P < 0.001) and 0.055 D in group 2 (P < 0.001); the between-group difference was not statistically significant (P = 0.794) (Fig. 2).
1-Month Postoperative Absolute Value Versus 12-Month Postoperative Absolute Value
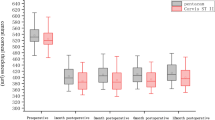
In the peripheral region of the anterior cornea, M was reduced by 0.736 D in group 1 (P < 0.001) and 0.319 D in group 2 (P < 0.001); the between-group difference was statistically significant (P < 0.001).
In the paracentral region of the posterior cornea, M was increased by 0.022 D in group 1 (P < 0.001) and 0.009 D in group 2 (P = 0.019); the between-group difference was statistically significant (P = 0.035).
Visual and Refractive Outcomes
At 1 month postoperatively, the mean UDVA (logMAR) was − 0.01 in group 1 and − 0.06 in group 2 (P < 0.001) (Table 4). At 12 months postoperatively, the mean UDVA was − 0.04 in group 1 and − 0.05 in group 2 (P = 0.658).
Vector Changes in Astigmatism from Preoperatively to 12 Months Postoperatively
There were no statistically significant differences between the two groups (Table 5, Figs. 3, 4).
Change in Best Spherical Equivalent (BSE)/Change in CCT Ratio 12 Months Postoperatively
There were no statistically significant differences between the two groups (Table 6).
Changes of Counts of Endothelial Cells in Group 1
The mean counts of endothelial cells of the corneas with residual bed thickness less than 350 µm in group 1 were 3005.00 ± 74.881/mm2 preoperatively and 3037.32 ± 64.358/mm2 at 12 months postoperatively (P = 0.177).
Discussion
LASIK combined with prophylactic CXL aims to correct the refractive error while keeping the corneal strength and increasing the stability in visual outcomes [9], which should be beneficial for all cases with increased risk of post-LASIK corneal ectasia [4, 7, 21]. The patients with suspect corneal tomography and topography were usually excluded in the previous studies [5,6,7] on the subject. However, this study included patients with myopia at increased estimated risk of postoperative corneal ectasia defined by the “Ectasia Risk Factor Score System for LASIK” (Table 1). Both the magnitude of steepening in the posterior paracentral region and the magnitude of flattening in the anterior peripheral region were more pronounced in group 1 than in group 2, in the period from 1 to 12 months postoperatively. Refractive outcomes show that there was a hyperopic shift in group 1 from 1 week to 1 month postoperatively, which is consistent with the corneal reshaping in this period, where both the anterior peripheral region and the posterior central region flattened. Visual rehabilitation was delayed in group 1, probably as a result of the inflammatory response and more edema caused by CXL, but it reached a similar level to group 2 at the final follow-up. Vector analysis of astigmatism suggests that prophylactic CXL induced no unexpected astigmatism 12 months postoperatively. The change in BSE/change in CCT ratio 12 months postoperatively shows that the additional CXL treatment did not affect the pachymetry significantly, which might because the energy was much lower than standard CXL.
Although transepithelial photorefractive keratectomy (Trans-PRK) combined with prophylactic CXL seems much safer for patients with risk of ectasia, haze and longer recovery are not accepted by particular young people in China who are eager to pass an eyesight examination in a short time. Moreover, a phakic intraocular lens (PIOL) is disapproved in enlistment requirements. Many patients hesitate to accept PIOL because of its higher cost and potential risks of intraocular complications. So, LASIK combined with prophylactic CXL is worth considering as an alternative surgery in China with higher incidence of myopia, under the premise of reasonable indications.
The Ectasia Risk Factor Score System (ERSS), proposed by Randleman et al. [15], consists of five parameters, namely anterior curvature topography pattern, residual stromal bed thickness, age, corneal thickness, and MRSE. Originating in 2008, ERSS was based on the analysis of the anterior corneal surface obtained by Placido topography. However, it became clear that corneas with normal astigmatism could also show abnormal patterns on Placido topography, when the corneal apex is not on the visual axis [22,23,24,25]. Moreover, keratoconus shows abnormalities on the posterior corneal surface earlier than on the anterior [26, 27]. With the development of Scheimpflug and OCT-based systems, topography of the posterior cornea started to play a greater role in early detection of keratoconus. Therefore, the assessment of the posterior cornea was added to our “Ectasia Risk Factor Score System for LASIK”. Belin/Ambrosio Enhanced Ectasia Display (BAD) involves both anterior and posterior factors [28]. In our score system, it counts only in cases where posterior abnormalities exist or the anterior surface displays red in Belin/Ambrosio. According to Table 1, the first three parameters were related to corneal morphology, representing the main differences between the two groups. Without assessment of the posterior surface, patients with poor topography trends would have been evaluated with lower score by ERSS, and either excluded from prophylactic CXL or too low UV energy would have been used in CXL, while the patients with only imperfect anterior corneas would have been overtreated by CXL. Although all the parameters in the form are indeed considered clinically in the preoperative screening, the limitation is that it has not been validated independently or tested in big data.
Previous studies evaluated corneal morphological changes using elevation data directly from the corneal elevation map [26, 27], ignoring the change in the corneal apex location after surgery, which may have introduced changes in the coordinate system. In this study, local and mean regional curvatures (M) were calculated from the original elevation data to analyze corneal morphology. This may make it a more reliable method, because it depends on the relative position of the adjacent points, representing true curvature of the surface that is not influenced by the postoperative shift of the corneal apex location [29,30,31,32,33].
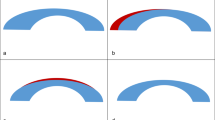
Changes of M in this study showed that the two groups demonstrated a similar trend during 12 months follow-up after surgery. During the first week postoperatively, the anterior cornea flattened in the central and paracentral regions and steepened in the peripheral region, while the posterior cornea flattened in the central region and steepened in the paracentral and peripheral regions. This finding is consistent with the conceptual model proposed by Roberts [34]. According to this model, a series of lamellae are severed and obliterated centrally after myopic laser refractive surgery. The remaining peripheral segments relaxed and the distance between lamellae expanded [35]. Then the outward expansion force in the periphery pulled laterally, and further flattened the center. Thus, the cornea tends to flatten centrally and steepen peripherally after laser ablation, but if the cornea is biomechanically compromised beyond a certain level, then IOP may cause its central steepening [36, 37], and corneal ectasia may develop. From 1 to 12 months after surgery, the anterior cornea normally steepens in the central and paracentral regions and flattens in the peripheral region [18], causing a reversal of the short-term overcorrection. Flap separation and tissue ablation, reducing corneal biomechanical strength, are responsible for the immediate changes in corneal shape shortly after LASIK [34]. But the later reshaping of the anterior cornea observed from 1 to 12 months postoperatively is mainly influenced by wound healing [18, 38]. Wound healing, taking effect in the anterior stroma, is expected to result in tissue stiffening [39] and cause reverse changes [18]. A similar previous study showed that the whole posterior surface remained stable during the periods between 1 week and 6 months after LASIK [18], but in our study, posterior paracentral regions became steeper from 1 to 12 months postoperatively in both group 1 and group 2. Posterior corneal steepening is consistent with the relative backward movement of peripheral stroma in response to the differential swelling [34, 35, 40], representing a stable remodelling, normally not an ectasia event [40, 41]. Posterior stroma, with a higher degree of swelling than the anterior third [42], is expected to cause the peripheral cornea to move backward continually, even after 1 month, possibly explaining why the posterior paracentral region remained steepened in our cases. Additionally, following the decrease of swelling of the anterior cornea in wound healing, the backward movement of the anterior peripheral cornea may drive the posterior peripheral cornea backwards further.
We speculate that the differences in corneal reshaping between the two groups were possibly related to the differences in corneal biomechanics caused by CXL. IOP-mediated strain steepens the cornea, especially its posterior surface. Anterior stromal edema, usually appearing early after CXL due to keratocyte loss [43], may attenuate over a period of 1 month with keratocyte repopulation [44], and may increase corneal stiffness relatively and resist IOP-mediated strain in the posterior surface. So, the posterior central cornea flattened significantly from 1 week to 1 month postoperatively in group 1, not in group 2. While in the anterior surface, the increase of curvature in the central and paracentral regions in group 1 was not statistically significant from 1 week to 1 month postoperatively. We speculate that this limited improvement of corneal biomechanical properties resulting from stromal edema and the aforementioned mechanism of tissue stiffening in wound healing may be responsible for it.
Prophylactic CXL may have improved corneal biomechanical properties in group 1 as expected with therapeutic CXL. The magnitude of flattening in the anterior peripheral region was bigger in group 1 than in group 2 from 1 to 12 months postoperatively. Moreover, the magnitude of steepening in the posterior paracentral region was bigger in group 1 than in group 2 during this period, which may be caused by the decrease of anterior stromal swelling in wound healing, as described earlier. The compaction of stromal collagen, combined with increased hydrostatic and osmotic resistance to fluid accumulation after CXL [44], may have caused swelling in our group1 to decline more significantly, leading to a more obvious steepening of the posterior paracentral cornea. Corneal stiffening caused by CXL redirected the IOP-mediated strain away from the hardened area and toward the limbus [45], and may have caused the posterior periphery to move backward more significantly in group 1 than in group 2. We speculate that this is another reason for the more obvious steepening trend of the posterior paracentral region in group 1.
A limitation of the study is that Scheimpflug imaging is prone to errors due to any decrease of corneal transparency presented early after CXL, which might have affected the early results in group 1. From a scientific point of view, it would seem more reliable if all patients at risk of corneal ectasia were randomized to undergo different surgeries. But that is infeasible ethically in the clinic, and previous research on the subject has used the same nonrandomized design as the present study [5, 7, 13, 14]. Interestingly, group 1, with relatively poor corneal topography, shows relatively positive results. Further follow-up and true biomechanical assessment are needed to draw definitive conclusions regarding the ability to reduce the risk of ectasia in patients with relatively poor corneal morphology and topography.
Conclusions
Postoperative corneal shape changes were different in different regions in both groups. Prophylactic CXL seems to influence corneal regional reshaping after surgery, while not affecting the 1-year visual and refractive results and not causing endothelial injury.
References
Binder PS. Analysis of ectasia after laser in situ keratomileusis: risk factors. J Cataract Refract Surg. 2007;33(9):1530–8.
Yuen LH, Chan WK, Koh J, et al. A 10-year prospective audit of LASIK outcomes for myopia in 37 932 eyes at a single institution in Asia. Ophthalmology. 2010;117(6):1236-U269.
Randleman JB, Russell B, Ward MA, et al. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003;110(2):267–75.
Tomita M. LASIK Xtra in clinical practice. In: Presented at american academy of Ophthalmology. New Orleans, USA. 2013 Nov 16–19.
Kanellopoulos AJ, Asimellis G, Karabatsas C. Comparison of prophylactic higher fluence corneal cross-linking to control, in myopic LASIK, one year results. Clin Ophthalmol. 2014;8:2373–81.
Mazzotta C, Balestrazzi A, Traversi C, et al. In vivo confocal microscopy report after Lasik with sequential accelerated corneal collagen cross-linking treatment. Case Rep Ophthalmol. 2014;5(1):125–31.
Kanellopoulos AJ, Asimellis G. Combined laser in situ keratomileusis and prophylactic high-fluence corneal collagen crosslinking for high myopia: two-year safety and efficacy. J Cataract Refract Surg. 2015;41(7):1426–33.
Gomes JA, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359–69.
Rajpal RK, Wisecarver CB, Williams D, et al. Lasik Xtra(®) provides corneal stability and improved outcomes. Ophthalmol Ther. 2015;4(2):89–102.
Kanellopoulos AJ. Long-term safety and efficacy follow-up of prophylactic higher fluence collagen cross-linking in high myopic laser-assisted in situ keratomileusis. Clin Ophthalmol. 2012;6:1125–30.
Celik HU, Alagoz N, Yildirim Y, et al. Accelerated corneal crosslinking concurrent with laser in situ keratomileusis. J Cataract Refract Surg. 2012;38(8):1424–31.
Tan J, Lytle GE, Marshall J. Consecutive laser in situ keratomileusis and accelerated corneal crosslinking in highly myopic patients: preliminary results. Eur J Ophthalmol. 2015;25(2):101–7.
Seiler TG, Fischinger I, Koller T, et al. Superficial corneal crosslinking during laser in situ keratomileusis. J Cataract Refract Surg. 2015;41(10):2165–70.
Lee H, Kang DS, Ha BJ, et al. Changes in posterior corneal elevations after combined transepithelial photorefractive keratectomy and accelerated corneal collagen cross-linking: retrospective, comparative observational case series. BMC Ophthalmol. 2016;16:139.
Randleman JB, Woodward M, Lynn MJ, et al. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115(1):37–50.
Saad A, Guilbert E, Gatinel D. Corneal enantiomorphism in normal and keratoconic eyes. J Refract Surg. 2014;30(8):542–7.
Henriquez MA, IzquierdoMannis LMJ Jr. Intereye asymmetry detected by Scheimpflug imaging in subjects with normal corneas and keratoconus. Cornea. 2013;32(6):779–82.
Bao F, Cao S, Wang J, et al. Regional changes in corneal shape over a 6-month follow-up after femtosecond-assisted LASIK. J Cataract Refract Surg. 2019;45(6):766–77.
Bao F, Chen W, Zheng X, et al. Changes in corneal biomechanical properties in PRK followed by two accelerated CXL energy doses in rabbit eyes. J Refract Surg. 2021;37(12):853–60.
Bao F, Chen H, Yu Y, et al. Evaluation of the shape symmetry of bilateral normal corneas in a Chinese population. PLoS ONE. 2013;8(8): e73412.
Aslanides IM, Mukherjee AN. Adjuvant corneal crosslinking to prevent hyperopic LASIK regression. Clin Ophthalmol. 2013;7:637–41.
Mandell RB. Everett Kinsey Lecture. The enigma of the corneal contour. CLAO J. 1992;18(4):267–73.
Kremer I, Shochot Y, Kaplan A, et al. Three year results of photoastigmatic refractive keratectomy for mild and atypical keratoconus. J Cataract Refract Surg. 1998;24(12):1581–8.
Sun R, Gimbel HV, Kaye GB. Photorefractive keratectomy in keratoconus suspects. J Cataract Refract Surg. 1999;25(11):1461–6.
Bilgihan K, Ozdek SC, Konuk O, et al. Results of photorefractive keratectomy in keratoconus suspects at 4 years. J Refract Surg. 2000;16(4):438–43.
Ciolino JB, Belin MW. Changes in the posterior cornea after laser in situ keratomileusis and photorefractive keratectomy. J Cataract Refract Surg. 2006;32(9):1426–31.
Ciolino JB, Khachikian SS, Cortese MJ, et al. Long-term stability of the posterior cornea after laser in situ keratomileusis. J Cataract Refract Surg. 2007;33(8):1366–70.
Belin MW, Villavicencio OF, Ambrosio RR Jr. Tomographic parameters for the detection of keratoconus: suggestions for screening and treatment parameters. Eye Contact Lens. 2014;40(6):326–30.
Martin R, Rachidi H. Stability of posterior corneal elevation one year after myopic laser in situ keratomileusis. Clin Exp Optom. 2012;95(2):177–86.
Smadja D, Santhiago MR, Mello GR, et al. Response of the posterior corneal surface to myopic laser in situ keratomileusis with different ablation depths. J Cataract Refract Surg. 2012;38(7):1222–31.
Chan TC, Liu D, Yu M, et al. Longitudinal evaluation of posterior corneal elevation after laser refractive surgery using swept-source optical coherence tomography. Ophthalmology. 2015;122(4):687–92.
Gyldenkerne A, Ivarsen A, Hjortdal JO. Comparison of corneal shape changes and aberrations induced By FS-LASIK and SMILE for myopia. J Refract Surg. 2015;31(4):223–9.
Wang B, Zhang Z, Naidu RK, et al. Comparison of the change in posterior corneal elevation and corneal biomechanical parameters after small incision lenticule extraction and femtosecond laser-assisted LASIK for high myopia correction. Cont Lens Anterior Eye. 2016;39(3):191–6.
Roberts C. The cornea is not a piece of plastic. J Refract Surg. 2000;16(4):407–13.
Dupps WJ Jr, Roberts C. Effect of acute biomechanical changes on corneal curvature after photokeratectomy. J Refract Surg. 2001;17(6):658–69.
Gilbert ML, Roth AS, Friedlander MH. Corneal flattening by shallow circular trephination in human eye bank eyes. Refract Corneal Surg. 1990;6(2):113–6.
Sinha Roy A, Dupps WJ Jr. Effects of altered corneal stiffness on native and postoperative LASIK corneal biomechanical behavior: a whole-eye finite element analysis. J Refract Surg. 2009;25(10):875–87.
Dupps WJ Jr, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006;83(4):709–20.
Raghunathan VK, Thomasy SM, Strom P, et al. Tissue and cellular biomechanics during corneal wound injury and repair. Acta Biomater. 2017;58:291–301.
Grzybowski DM, Roberts CJ, Mahmoud AM, et al. Model for nonectatic increase in posterior corneal elevation after ablative procedures. J Cataract Refract Surg. 2005;31(1):72–81.
Wang Z, Chen J, Yang B. Posterior corneal surface topographic changes after laser in situ keratomileusis are related to residual corneal bed thickness. Ophthalmology. 1999;106(2):406–9.
Lee D, Wilson G. Non-uniform swelling properties of the corneal stroma. Curr Eye Res. 1981;1(8):457–61.
Mazzotta C, Balestrazzi A, Traversi C, et al. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen: ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea. 2007;26(4):390–7.
Arora R, Manudhane A, Saran RK, et al. Role of corneal collagen cross-linking in pseudophakic bullous keratopathy: a clinicopathological study. Ophthalmology. 2013;120(12):2413–8.
Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–7.
Acknowledgements
Funding
This study was supported by the National Natural Science Foundation of China (31771020). The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Concept and design (CD), data acquisition (DA), data analysis/interpretation (DI), drafting manuscript (DM), statistical analysis (SA), critical revision (CR) of manuscript, supervision (SV). Jia Zhang: CD, DA, DI, DM, CR, SV. Tong Chen: CD, DA, DI, DM, SA. Junjie Wang: DI, CR. Fangjun Bao: DI, CR. Wen Chen: DA. Aleksandar Stojanovic: CR, SV. Qinmei Wang: CR, SV. Shihao Chen: CD, CR, SV. All authors read and approved the final manuscript.
Disclosures
Jia Zhang, Tong Chen, Junjie Wang, Fangjun Bao, Wen Chen, Aleksandar Stojanovic, Qinmei Wang and Shihao Chen declare that they have no conflict of interest.
Compliance with Ethics Guidelines
The Eye Hospital, Wenzhou Medical University Ethics Committee approved this study as an audit study and gave it the following reference number: 2021-070-K-59. The study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All subjects provided informed consent to participate in the study. All participants gave their informed consent for their anonymized data to be submitted for audit and publication.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhang, J., Chen, T., Wang, J. et al. Laser In Situ Keratomileusis (LASIK) Combined with Prophylactic Corneal Cross-Linking for Correction of Myopia: Regional Analysis of Corneal Morphology. Ophthalmol Ther 11, 1423–1439 (2022). https://doi.org/10.1007/s40123-022-00510-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00510-1