Abstract
Keratoconus (KC) is likely to be more aggressive in the pediatric population, with a higher risk of progression and visual loss. Several techniques have been proposed for corneal crosslinking (CXL) so far. The standard CXL (SCXL) technique, or the Dresden Protocol, originally developed by Wollensak et al., has been shown to be safe and effective in the pediatric KC group. With similar efficacy to the conventional method, the accelerated CXL (ACXL) protocols proposed a reduced UVA exposure time by increasing the intensity of UVA irradiation. Transepithelial CXL (TCXL), considered an “epithelium-on” method, emerged as a strategy to improve safety and reduce postoperative complications and discomfort. For thinner corneas, we can highlight the use of hypoosmolar riboflavin and new studies, such as contact lens-assisted CXL (CACXL), the epithelial-island CXL (EI-CXL), and the Sub400 protocol. In addition to the different protocols used, another factor that changes CXL results is the type of carrier used: dextran-based or hydroxypropyl methylcellulose-based (HPMC) riboflavin solutions. There are several ways to perform a CXL surgery, and it is still unclear which method is the safest and most effective in the pediatric group. This review of the literature in English, available in PubMed, provides an update on corneal CXL in the pediatric KC group, exploring the data on the techniques currently used and under investigation, including their advantages, efficacy, safety profiles, risks, and cost analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Studies of various corneal crosslinking (CXL) techniques in pediatric keratoconus have been developed and continue to be produced with the ultimate goal of improving the procedure’s safety and efficacy, and a consensus on the best technique has not been reached, especially in children |
Standard CXL and accelerated CXL protocols can be considered effective and safe in the management of pediatric keratoconus (KC). Transepithelial CXL, although safe, has not been shown to be as efficient as other existing techniques to this date, but it can be considered in some cases. For thinner corneas, the use of hypoosmolar riboflavin and new studies, such as contact lens-assisted CXL (CACXL), epithelial-island CXL (EI-CXL), and Sub400 protocol, can be highlighted. Studies have confirmed the benefit of hydroxypropyl methylcellulose-based (HPMC-based) riboflavin in maintaining corneal thickness throughout the treatment and reducing soaking time with a good result in accelerated CXL (ACXL) |
In terms of long-term outcomes and safety of corneal CXL in children, we consider using the standard CXL protocol with dextran-based riboflavin or the use of the accelerated CXL protocol with 9 mW/cm2 for 10 min with HPMC-based riboflavin and soaking time of 10–15 min |
Further reports should be carried out, especially long-term prospective studies, to assess the progression of KC after CXL in the pediatric group as well as the need for retreatment to allow for a better definition of the optimal method of CXL. In the future, the use of multimodal propaedeutics and artificial intelligence could provide better therapeutic treatment for this group |
Introduction
Keratoconus (KC) is a progressive, asymmetric, and bilateral degenerative disease of the cornea, associated with structural changes in the organization of corneal collagen; it results in corneal thinning and protrusion [1,2,3], leading to irregular astigmatism, with or without higher-order aberrations, and consequent visual impairment, which is usually not corrected with glasses [2].
The prevalence of KC varies between populations and different ethnicities [4, 5]. In the pediatric group, it is estimated at 0.16% [6]. An increase in the incidence and the prevalence of the disease has been observed, which has proved to be related to the greater diagnostic sensitivity resulting from recent improvements in corneal imaging exams [7, 8] as well as other environmental and/or genetic factors [8,9,10]. In a recent study conducted in Saudi Arabia, the prevalence of KC among patients between 6 and 21 years old using Scheimpflug tomography was 4.79%, which is significantly higher than that reported in all previous studies on KC prevalence [11]. The etiopathogenesis of KC remains controversial and involves various causal factors, namely environmental, genetic, enzymatic, inflammatory, and hormonal factors, as well as the role of oxidative stress [4]. Studies have demonstrated an altered ratio between pro- and anti-inflammatory cytokines in the tears of patients with KC and an increase in the number of matrix metalloproteinases (MMP) (MMP-1 and MMP-13) and inflammatory mediators (IL-6 and TNF-a), which can also occur in eye-rubbers. This imbalance can cause corneal structure modification and function, induce keratocyte apoptosis, and therefore contribute to KC development and progression [3, 12,13,14,15].
KC generally begins in adolescence, although the disease can manifest at any age [4, 16]. Due to structural differences, KC in children has numerous unique clinical features, such as faster disease progression, progression in 88% of cases, systemic syndromic association, positive family history, associated vernal keratoconjunctivitis (VKC), ocular allergy, atopy associated with significant ocular friction, and severe visual impairment at the time of diagnosis, creating a negative impact on quality of life and requiring early follow-up and more frequent interventions [1,2,3, 17,18,19,20,21].
KC management poses a big challenge among pediatric patients: biomechanical stiffness of the cornea is inversely correlated with age, making younger ocular rubbers more susceptible to disease progression; corneal changes and visual impairment in childhood can lead to amblyopia; children are less compliant with hard contact lens fitting; and the corneal transplant has a higher risk of graft rejection, with less favorable outcomes in pediatric patients [21,22,23,24,25].
Corneal crosslinking (CXL), first described by Wollensak et al. in 2003, is a safe and effective method to stop the progression of ectasia by strengthening the corneal stroma [2, 18,19,20,21]. However, it has also been documented to be less effective in the pediatric cornea [3]. Studies of various CXL techniques, such as “epithelium-on” or “epithelium-off” methods, changes in ultraviolet light parameters, and riboflavin composition, stay focused on the ultimate goal of improving the safety and efficacy of the procedure. However, no consensus has been reached over which method is more suitable for the pediatric group.
This review provides an update on the current literature about CXL in the pediatric group to treat corneal ectasia, exploring the data on the techniques currently used and under investigation, including their advantages, efficacy, safety profiles, risks, and cost analyses. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
CXL Protocols
CXL is a technique that induces cross-links in the extracellular matrix of the corneal stroma by photosensitizing it with riboflavin and exposing it to ultraviolet-A (UVA, 315–400 nm) light, resulting in increased biomechanical rigidity of the corneal stroma [18, 26]. Riboflavin is a non-toxic precursor of several co-enzymes, which enhances the absorption of UV-A by the corneal stroma. The photosensitization of riboflavin generates reactive oxygen producing anion radicals and superoxide, which react with available groups to generate additional chemical bonds between amino acid residues. This will lead to increased bonds between collagen and proteoglycans, culminating in increased biomechanical and biochemical resistance of the cornea, which reduces disease progression and effectively stabilizes the cornea [2, 4, 20].
Notably, CXL has evolved in the past years to become a procedure that stabilizes the cornea biomechanically and biochemically [4, 20]. The use of the standard CXL (SCXL) was approved for patients between 14 and 65 years of age by the Food and Drug Administration (FDA) in 2016 [2, 4].
The first treatment proposed was the SCXL—or the Dresden Protocol. It was developed by Wollensak et al. and involves the mechanical debridement of the central 9 mm of the corneal epithelium under topical anesthesia. One drop of 0.1% riboflavin solution is then administered every 5 min for 30 min, with exposure to UV-A light, and then the riboflavin solution is administered every 5 min for an additional 30 min [18, 23, 27].
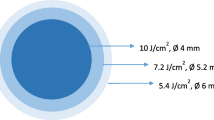
Accelerated CXL (ACXL) protocols, which reduce treatment time by increasing total irradiance, are also considered as “epithelium-off” methods [25, 28,29,30,31]. They were based on the Bunsen-Roscoe reciprocity law, which states that the photochemical effect of ultraviolet light is proportional to the total amount of energy supplied, regardless of the time and intensity of irradiation for each protocol, meaning that 3-min irradiation at 30 mW/cm2, 5-min irradiation at 18.0 mW/cm2, and 10-min irradiation at 9.0 mW/cm2 should provide the same effect as obtained with 30-min irradiation at 3.0 mW/cm2, all delivering 5.4 J/cm2 of energy. Because 1 J = 1 W × second, 3 min of irradiation (180 s) at 30 mW/cm2 (0.03 W/cm2) corresponds to 5.4 J/cm2 (180 × 0.03 = 5.4 J) [32].
Transepithelial CXL (TCXL) emerged as a strategy to improve safety and reduce postoperative complications and discomfort. It is considered an “epithelium-on” method, which intends to bypass the epithelial barrier by modifying riboflavin Ricrolin TE (Sooft, Italia SpA) with the addition of two agents to increase penetration through the intact epithelium (trometamol and sodium ethylenediaminetetraacetic acid—EDTA) [33,34,35,36]. However, some studies have shown reduced penetration of riboflavin into the corneal stroma when the epithelium remained intact [23, 27, 37, 38]. Due to the unsatisfactory penetration of TCXL, the iontophoretic transepithelial CXL (I-CXL) strategy has been developed, which involves the application of a small electric current prior to the instillation of riboflavin to enhance its penetration into the corneal stroma. This strategy, however, was shown to be less efficient than SCXL [39, 40]. One of the limitations of the transepithelial treatment is the higher oxygen consumption by the corneal epithelium, which reduces the effectiveness of the CXL treatment [41,42,43]. A novel I-CXL associated with pulsed light, the enhanced-fluence I-CXL (EF I-CXL) protocol, seems to partially compensate the consumption of epithelial oxygen, optimizing fluence by 30% (7 J/cm2) and UV-A power set at 18 mW/cm2 × 6.28 min of exposure time, pulsing the light 1 s on/1 s off with a total prolonged UV-A irradiation time of 12.56 min [39].
In all of the protocols described above, the corneal thickness limit of 400 mm is crucial to perform CXL, and many KC patients with thinner corneas, who may benefit from CXL-induced strengthening, are excluded from treatment. Since 2009, hypoosmolar riboflavin has yielded good results to inflate a thin cornea to a thickness of > 400 mm, but this is often not achieved when the corneas are thinner [44]. Therefore, other techniques have emerged, such as contact lens-assisted CXL (CACXL), in which the stroma is artificially “thickened” by placing a contact lens over the cornea [45], and epithelial-island CXL (EI-CXL), an approach in which islands of epithelium are left over the thinnest areas of the corneal stroma [46]. Recently, the novel Sub400 protocol has proposed the use of 3 mW/cm2 with individualized irradiation time and customization for each corneal thickness, so that the corneal endothelium remains protected from damaging amounts of UV-A irradiation [47].
In addition to the different forms of CXL protocols, a wide range of riboflavin formulations is commercially available. These formulations differ in composition and can include additional components designed to increase the penetration of riboflavin; they can also change CXL results by modifying the thickness of the corneal stroma by the chemical composition and affect the 400-mm treatment threshold that is classically required to safely perform CXL [48]. Among these formulations, two substances stand out: riboflavin with dextran and hydroxypropyl methylcellulose (HPMC). The use of dextran has been the gold standard in CXL methods; however, due to its hyperosmolar effects, it can cause corneal thinning and harm the endothelium in the thinnest corneas [49,50,51]. HPMC is a well-known agent in ophthalmology, and recently, it has been introduced as a solution in CXL with the advantage of shortening riboflavin diffusion time without corneal thinning [49, 52, 53].
CXL Results and Discussion
Soeters et al. were the first to report CXL results in progressive KC in a pediatric group (10–16 years). In this case series, five eyes from four patients were submitted to SCXL; the procedure successfully stabilized KC and demonstrated significant visual and topographical improvement [29]. Since then, prospective, retrospective, and meta-analyses studies have been performed to assess the safety and efficacy of CXL in the pediatric group. However, studies are still scarce, especially with longer follow-up periods and a significant number of patients (Table 1) [19, 20, 54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85]. So far, no randomized clinical trials comparing the different CXL protocols for a pediatric group have been published.
Mazzotta et al. reported the most extended follow-up after SCXL (10-year follow-up) in patients aged ≤ 18 years, stabilizing the disease process in nearly 80% [20]. Arora et al., Vinciguerra et al., and Caparossi et al. determined that the SCXL procedure stabilized the disease, showed improvement in uncorrected visual acuity (UCVA), corrected distance visual acuity (CDVA), and produced changes in the reading of the keratometry values (K) [54,55,56]. Zotta et al. obtained similar results from 20 eyes during an 89-month follow-up, noting that flat K, steep K, and the topographic cylinder remained stable at 7.5 years [64]. McAnena also reported a reduction in maximum K (K max) in a KC pediatric group analysis at the end of 24 months of follow-up [73]. In this sense, stabilization and a decrease in K measurements are expected with SCXL, as demonstrated in a recent meta-analysis. Also, there was a significant postoperative improvement in all K parameters in the period of 12–24 months in the conventional protocol group, with K readings continuing to improve over the following 10 years [2].
By contrast, Chatzis and Hafezi pointed out that the effect of CXL might not last as long as in adults [86]. Also, Godefrooij et al. reported, at a 5-year follow-up, that progression occurred in 22% of the treated eyes by the last follow-up visit based on the increased ≥ 1.00 D in K max readings, although no additional CXL treatment or corneal transplantation was performed in any patient, as none of these eyes lost Snellen lines in either UDVA or CDVA [19]. Mazzotta et al. also found a progression rate in 24% of the treated eyes, including 13 eyes (from 9 patients) with K max progression over 1.00 D and only two cases (4.2%) requiring CXL retreatment [20]. These data could indicate that the corneal collagen turnover might produce a short-term CXL effect with new KC instability or progression, especially among younger patients. Therefore, the CXL procedure is likely to be repeated in approximately 20–25% of the patients, if there is evidence of progression after follow-up and repeated examinations showing actual worsening; furthermore, parents should be advised before treatment [20].
In the pediatric population, few studies have evaluated the results for accelerated protocols with UV fluence of 30 mW/cm2 for 3 or 4 min, 18 mW/cm2 for 4 or 5 min, 10 mW/cm2 for 9 min, or 9 mW/cm2 for 10 min—some studies compared these results with SCXL and others with different ACXL protocols; all studies showed promising results, similar to those of the conventional method [20, 23, 27, 30, 32, 36]. Ulusoy et al., Badawi et al., and Eissa et al. found no evidence of KC progression after ACXL in all patients treated [71, 76, 87]. However, these studies had relatively short follow-up periods and used only a few patients. Agca et al. compared two different ACXL methods, 30 mW/cm2 for 4 min (7.2 J/cm2) and 18 mW/cm2 for 5 min (5.4 J/cm2), and found progression rates (K max progressed ≥ 1.00 D) of 23.3% and 16.8%, respectively, during a 5-year follow-up [72]. Nicula et al., in a retrospective analysis, compared ACXL (9 mW/cm2 for 10 min) and SCXL, which both led to similar keratometric and visual acuity results within a 4-year period. In two eyes from the ACXL group, progression was observed at the end of 4 years [77].
In the TCXL group (“epithelium-on”), no improvement in K readings was observed at the end of the follow-up period in the pediatric range [2]. Zhang et al. compared the results of conventional and transepithelial protocols in seven studies involving adult patients and found that, despite the lower occurrence of complications after the TCXL procedure, the corneal flattening effect was lower than that in SCXL [88]. This was also concluded by Li et al. and Wen et al. [89, 90]. Buzzonetti, in a study of the efficacy of TCXL in pediatric KC, with an average age group of 14.4 years (± 3.7 years), with an 18-month follow-up, observed worsening of K max, concluding that this method does not effectively reduce the progression of KC compared to SCXL [79]. In a comparative study, Henriquez et al. showed that although both procedures halted the progression of KC at 5 years of follow-up, SCXL was able to halt it at a significantly higher level and with a higher flattening effect than TCXL [84]. Compared with SCXL, I-CXL could stop KC progression as much as 12 months, as disease progression was found in 50% of cases at a 24-month follow-up [40]. These studies, as well as other reports involving the “epithelium-on” method for pediatric KC, have so far indicated insufficient evidence for the use of this protocol in pediatric patients with KC [2, 4].
In a recent meta-analysis, involving a total of 28 studies and 1300 eyes, the results demonstrated that CXL—in either SCXL or ACXL—is an effective modality to prevent pediatric KC progression, with significant improvement in uncorrected and corrected visual acuity at the end of the follow-up period [2]. In adult KC, the demarcation line depth after SCXL was deeper than that after ACXL, according to Kobashi et al.’s meta-analysis [91], which indicates that the biological effect of irradiation on a tissue differs when the total energy dose is maintained. Previous works have shown that the efficacy of CXL decreases when irradiance intensity > 10 mW/cm2 is used. This is due to the imbalance between conversion and replenishment of oxygen molecules [92,93,94]. When ACXL is used in the treatment of eyes with KC in pediatric patients, it is still not clear whether the highest irradiations with a short duration are sufficient to halt the progression of the disease for a long period. Thus, further studies and discussions are needed on this topic.
Among the articles reviewed (Table 1) [19, 20, 54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85], one used HPMC in SCXL and two in ACXL [65, 71, 73, 78, 85]; all have yielded good results in stabilizing the disease. Amer et al. compared SCXL and ACXL (9 mW/cm2, 10 min) using a HPMC-based riboflavin solution, and both protocols were efficient in pediatric KC management, with better outcomes in the SCXL [78]. The mean reduction in postoperative corneal pachymetry (at thinnest location) was significant in SCXL [19, 20, 54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85]. A study in the adult population with HPMC-based riboflavin in SCXL and ACXL has evidenced a marked keratocyte loss, as deep as in the preendothelial stroma, in a large number of eyes in particular after CXL with conventional UVA irradiation. This may be caused by a fast diffusion rate of HPMC-based riboflavin, leading to a deeper and more intense treatment [49]. Hammer et al. demonstrated, in various riboflavin formulations, that the concentration of riboflavin phosphate isomer compounds, mostly the riboflavin 5′-monophosphate, in the formulation is higher than the concentration of riboflavin; however, an inverse relationship occurs in the cornea, i.e., a higher concentration of riboflavin opposed to a lower concentration of riboflavin phosphate-isomer compounds [95]. Furthermore, the authors reported that HPMC-based riboflavin showed greater concentration of riboflavin and riboflavin 5′-monophosphate isomer than dextran-based riboflavin. Riboflavin is less electronegative and therefore shows better diffusion through the cornea; conversely, riboflavin 5′-monophosphate-isomer has a lower threshold for the photochemical reaction, i.e., it presents a more intense reaction for shorter irradiation times [95]. These findings could explain that HPMC-based riboflavin increases diffusion into the cornea and stronger photochemical reaction.
The assessment of endothelial cell density (ECD) loss in standard and accelerated and transepithelial CXL protocols in the pediatric group has not produced a statistically significant decrease in ECD after the procedure [21, 23, 56, 58, 61, 69, 70, 76, 80, 82, 96, 97]; however, other studies reported that, depending on the protocol and carrier used, there is damage to endothelial cell density [68, 98,99,100]. In a retrospective SCXL study developed by Barbisan et al. in 2020, involving 105 eyes, a statistically significant reduction in ECD was observed, as well as corneal thinning after 1 year of follow-up, evidencing the potential impact of CXL on anatomy and corneal physiology [68]. It should be noted that in this study the authors did not mention whether HPMC or dextran was used, making it impossible to make inferences about the impact of the carrier on cell density [68]. In Hagem et al.’s study, at 1-year follow-up, the results did not show a reduction in ECD in SCXL and ACXL using HPMC-based riboflavin and a decrease in endothelial cell loss in eyes with the deepest keratocyte loss; however, the effects of these deep structural findings on the endothelium in the long term are still unknown [49]. Thus, safety and efficacy studies of diffusion depth with diverse riboflavin solutions in CXL are important because of the depth of riboflavin diffusion in the corneal stroma, which possibly influences the CXL depth; the strengthening effect of CXL, which is believed to occur in the anterior corneal stroma; and the UVA irradiation in the most posterior stroma, which could damage the endothelium [49].
Risks of SCXL include scarring, blepharitis, corneal haze, mild photophobia, and abrasion-related discomfort, in addition to severe pain, stromal haze, temporary vision loss, and infections, as “epithelium-off” corneal CXL involves the removal of the epithelium [3, 57, 88]. A transient reduction in finer pachymetry is also observed during the first 6 months after the procedure, which returns to baseline after 1 year [21, 23]; however, in some studies the cornea remained thinner when compared to baseline [63, 78]. Although microbial keratitis after CXL is uncommon, it has been observed after SCXL and ACXL protocols among children [23, 101]. Maharana et al. produced the largest series of microbial keratitis after CXL, describing 7 cases in 532 ACXL procedures, with cases occurring between the 1st and 7th days after the procedure [102]. Major complications have not been reported after different CXL protocols; therefore, it is a safe procedure with low risk of complications [23, 56].
For thin corneas, one of the proposed alternatives is the CACXL, which appears to show good efficacy and safety in adults, despite the small number of studies found and a short evaluation period. Also, special care should be taken with the choice of lens in view of the oxygen deprivation it may cause [103, 104]. The EI-CXL halted KC progression over a 1-year period; however, the cross-linking effect was lower in areas under the epi-on region [46]. In the Sub400 protocol, at a 12-month follow-up, a 90% success rate in slowing progression of KC was obtained in 39 eyes, being 8 eyes from patients between 13 and 20 years old [47]. Notably, there is still much to be clarified and monitored in these methods to assess their safety and efficacy. Thus, these new protocols are an alternative for thinner corneas and should be considered in younger patients—even if disease progression is not avoided for a long period of time—and still focusing on the great benefit of postponing a possible corneal transplant in this group of patients. Regarding the Sub400 protocol, special attention should be paid to the type of riboflavin used, which is hypoosmolar and without a carrier. Thus, the use of other riboflavin formulations is not recommended, given the changes in the corneal stroma, which can modify the safety parameters.
Pediatric KC is commonly associated with VKC, possibly related to the frequent rubbing of the eyes and chronic exposure of the cornea to inflammatory mediators and cytokines. Ocular allergy associated with eye rubbing causes thinning of keratocytes, and the degree of its effect depends on the strength and duration of rubbing of the eyes. It is well established that prevention against KC includes avoiding rubbing the eyes and treating dry eyes [105,106,107]. In this sense, the effect of CXL on pediatric VKC patients and eye rubbers needs to be evaluated. A study involving 89 eyes, divided into two groups (VKC and non-VKC), was developed to assess the safety and efficacy of SCXL in children and adolescents with KC and VKC [108]. In both groups, SCXL was associated with a similar rate of post-treatment progression, with similar adverse events, outcomes and progression of KC, demonstrating the safety and efficacy of SCXL in pediatric patients with and without VKC in a 2-year follow-up, with no significant differences between the baseline and last follow-up in mean UCVA, CDVA, manifest spherical equivalent, manifest astigmatism, keratometry values, and thinnest corneal area [108]. Further studies are also needed with longer follow-up periods to assess KC, its progression in VKC, and eye rubbers before and after the CXL procedures, given the short follow-up period of the study (2 years) and the lack of studies in literature addressing this subject.
Regarding CXL’s cost-effectiveness compared to traditional KC management, early CXL’s superiority over standard management with penetrating keratoplasty is observed [17, 109, 110]. Following the hypothetical Markov model, a 10-year effect after early CXL treatment would produce an increase in quality-adjusted life-years (QALYs) by 50–51 years with early CXL, as well as higher cost-effectiveness rates compared with the previously standard management [21, 23, 109, 110]. However, there is no consensus among specialists on some aspects related to the indication of the CXL surgery in all cases of KC in children and adolescents at the time of diagnosis. Ambrósio et al. [9] draw attention to the fact that early indication of CXL may increase risk of complications; on the other hand, a late indication will produce a significant risk factor for treatment failure [4, 9, 111]. So, an individualized approach should be carried out according to each patient’s clinical condition and the therapeutic options and availability of each center. Thus, in some situations, in which SCXL or ACXL cannot be performed, other approaches of corneal cross-linking should be considered, such as riboflavin in oral supplementation, although we do not have studies (only congress case reports) which prove the effectiveness, or even with crosslinking without removing the epithelium [9, 112].
The criteria for assessing the progression of KC after CXL in the pediatric group and the need for a re-approach are not standardized, making it difficult to assess CXL results. Although several systematic reviews have been published on the improvement of clinical outcomes after CXL in pediatric KC, they have not specifically investigated the failure rate of CXL [113]. Achiron et al. assessed KC progression rates after CXL in the pediatric group in a literature review study of 37 studies (2078 eyes). The most common methods for reporting progression were increased K max, K mean, or steep K by 1.0 diopter or more. According to these criteria, the mean pooled progression rate after “epithelium-off” CXL amounted to 9.9% with high heterogeneity between studies and p value < 0.0001 [113]. Defining progression, therefore, is rather challenging in pediatric KC, as typical changes in progression often do not happen concomitantlyin a patient, with variability within and between observers and between corneal topography and CT scanners.
In addition, the need for retreatment of CXL (Re-CXL) and the analysis of its amount and effectiveness remain unclear in the literature [113]. Most of the articles evaluated do not mention the need for re-CXL or performance of this procedure. Few studies, involving mostly adult populations, show progressive KC and Re-CXL, in which they were performed with similar methods to primary CXL. They yielded stable results, but with short follow-up periods, which are insufficient to draw long-term conclusions [114,115,116]. An example for this discussion is the study performed by Antoun et al., which reported that 7 in a total of 221 eyes (3.17%) after SCXL needed Re-CXL, repeating the standard CXL protocol, with no complications and effective results at 1-year follow-up. The authors suggested that allergic conjunctivitis and eye rubbing were risk factors for the need for Re-CXL [114]. In an in vitro study performed on human corneas, comparison was made between corneas that were submitted to CXL once to three times within a maximum 24-h interval between procedures [117]. In this article, differences between groups were not significant using scanning acoustic microscopy to evaluate results, indicating that no further cross-links are induced when Re-CXL is performed [117].
Studies with clear KC progression criteria are necessary; they should evaluate the effectiveness of CXL in the pediatric population and the need for retreatment and new CXL approaches in this age group using long-term follow-up studies. In this scenario, the use of integrated, multimodal propaedeutics in corneal imaging, biomechanics, molecular biology, and genetics associated with artificial intelligence (AI) can be of great value in the future. In this sense, it can be the gold standard for the diagnosis of the progression and worsening of KC, especially in children, who usually pose a real challenge for doctors during examinations, as well as to ensure reliability of the results, promoting a more efficient and safe treatment for the patient.
Conclusion
Pediatric KC is likely to be diagnosed at an advanced stage, with rapid progression and associated ocular comorbidities. Optimal decision-making in the management of KC involves deep knowledge of the clinical challenges that may occur during treatment, and the procedure should be individualized for each patient.
Standard CXL and ACXL can be considered effective and safe in the management of pediatric KC. On the other hand, TCXL—although safe—has not proved to be as efficient as the other existing techniques to this date. However, it can be considered in some situations. Studies have confirmed the benefit of HPMC-based riboflavin in maintaining corneal thickness throughout the treatment and reducing the soaking time with a good result in ACXL.
Special attention should be paid to the type of CXL protocol used (irradiation time and energy), the soaking time, and the type of riboflavin used, as they greatly impact the safety and final result of CXL. Therefore, in terms of long-term outcomes and safety of corneal CXL in children, we consider using the standard CXL protocol with dextran-riboflavin or the use of the accelerated CXL protocol with 9 mW/cm2 for 10 min with HPMC-based riboflavin and soaking time of 10–15 min.
For thinner corneas, where 400 mm thickness without epithelium is not achieved with hypoosmolar riboflavin, the CACXL, EI-CXL, and Sub400 protocols should be considered in patients who have a chance of visual rehabilitation, although further studies are still needed to prove safety and efficacy.
Given the concern of regression of keratoconus after CXL in children, a close follow-up after CXL in the pediatric group must monitor for early signs of returned progression, and Re-CXL should be considered as an attempt to delay a future corneal transplant.
Thus, further reports should be carried out, especially long-term prospective studies, to assess the progression of KC after CXL in the pediatric group, as well as the need for retreatment to allow for a better definition of the optimal method of CXL. In the future, the use of multimodal propaedeutics and AI could offer better therapeutic treatment for this group.
References
Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319.
Fard AM, Reynolds AL, Lillvis JH, Nader ND. Corneal collagen cross-linking in pediatric keratoconus with three protocols: a systematic review and meta-analysis. J AAPOS. 2020;24(6):331–6.
Mukhtar S, Ambati BK. Pediatric keratoconus: a review of the literature. Int Ophthalmol. 2018;38(5):2257–66.
Anitha V, Vanathi M, Raghavan A, Rajaraman R, Ravindran M, Tandon R. Pediatric keratoconus—current perspectives and clinical challenges. Indian J Ophthalmol. 2021;69:214–25.
El Rami H, Chelala E, Dirani A, et al. An update on the safety and efficacy of corneal collagen cross-linking in pediatric keratoconus. Biomed Res Int. 2015;2015:257927.
Moshirfar M, Heiland MB, Rosen DB, Ronquillo YC, Hoopes PC. Keratoconus screening in elementary school children. Ophthalmol Ther. 2019;8:367–71.
Ambrósio R Jr, Lopes B, Amaral J, Correia FF, Canedo ALC, Marcella Salomão M, Silva RS, Sena N Jr. Keratoconus: breaking paradigms and contradictions of a new subspecialty. Ver Bras Oftalmol. 2019;78(2):81–5.
Salomão M, Hoffling-Lima AL, Lopes B, Belim MW, Sena N, Dawson DG, et al. Recent developments in keratoconus diagnosis. Expert Rev Ophthalmol. 2018;13(6):329–41.
Ambrósio R Jr, Faria-Correia F, Silva-Lopes I, Azevedo-Wagner A, Tanos FW, Lopes B, et al. Paradigms, paradoxes, and controversies on keratoconus and corneal ectatic diseases. Int J Kerat Ectatic Corneal Dis. 2018;7(1):35–49.
Gokhale NS. Epidemiology of keratoconus. Indian J Ophthalmol. 2013;61(8):382–3.
Torres Netto EA, Al-Otaibi WM, Hafezi NL, Kling S, Al-Farhan HM, Randleman JB, Hafezi F. Prevalence of keratoconus in paediatric patients in Riyadh, Saudi Arabia. Br J Ophthalmol. 2018;102:1436–41.
Buzzonetti L, Bohringer D, Liskova P, Lang S, Valente P. Keratoconus in children: a literature review. Cornea. 2020;39(12):1592–8.
Cheung IM, McGee CN, Sherwin T. A new perspective on the pathobiology of keratoconus: interplay of stromal wound healing and reactive species-associated processes. Clin Exp Ophthalmol. 2013;96:188–96.
Jun AS, Cope L, Speck C, et al. Subnormal cytokine profile in the tear fluid of keratoconus patients. PLoS One. 2011;6:e16437.
Wojcik KA, Blasiak J, Szaflik J, et al. Role of biochemical factors in the pathogenesis of keratoconus. Acta Biochem Pol. 2014;61:55–62.
Tuft SJ, Moodaley LC, Gregory WM, et al. Prognostic factors for the progression of keratoconus. Ophthalmology. 1994;101:439–47.
Chang H-YP, Chodosh J. The genetics of keratoconus. Semin Ophthalmol. 2013;28:275–80.
Wollensak G, Spoerl E, Steiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–7.
Godefrooij DA, Soeters N, Imhof SM, Wisse RPL. Corneal cross-linking for pediatric keratoconus: long-term results. Cornea. 2016;35(7):954–8.
Mazzotta C, Traversi C, Baiocchi S, Bagaglia S, Caporossi O, Villano A, Caporossi A. Corneal collagen cross-linking with riboflavin and ultraviolet a light for pediatric keratoconus: ten-year results. Cornea. 2018;37(5):560–6.
McAnena L, Doyle F, O’Keefe M. Cross-linking in children with keratoconus: a systematic review and meta-analysis. Acta Ophthalmol. 2017;95(3):229–39.
Vanathi M, Panda A, Vengayil S, et al. Pediatric keratoplasty. Surv Ophthalmol. 2009;54:245–71.
Perez-Straziota C, Gaster RN, Rabinowitz YS. Corneal cross-linking for pediatric keratcoconus review. Cornea. 2018;37:802–9.
Leoni-Mesplie S, Mortemousque B, Touboul D, et al. Scalability and severity of keratoconus in children. Am J Ophthalmol. 2012;154:56-62.e1.
Aasuri MK, Garg P, Gokhle N, Gupta S. Penetrating keratoplasty in children. Cornea. 2000;19:140–4.
Wollensak G, Iomdina E. Long-term biomechanical properties of rabbit cornea after photodynamic collagen crosslinking. Acta Ophthalmol. 2009;879(1):48–51.
Panos GD, Kozeis N, Balidis M, et al. Collagen cross-linking for paediatric keratoconus. Open Ophthalmol J. 2017;11:211–6.
Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg. 2015;41:41–6.
Soeters N, Van der Lelij A, Van der Valk R, Tahzib NG. Corneal crosslinking for progressive keratoconus in four children. J Pediatr Ophthalmol Strabismus. 2011;21:48.
Gatzioufas Z, Richoz O, Brugnoli E, et al. Safety profile of high-fluence corneal collagen cross-linking for progressive keratoconus: preliminary results from a prospective cohort study. J Refract Surg. 2013;29:846–8.
Schumacher S, Oeftiger L, Mrochen M. Equivalence of biomechanical changes induced by rapid and standard corneal cross-linking, using riboflavin and ultraviolet radiation. Investig Ophthalmol Vis Sci. 2011;52:9048–52.
Bunsen RW, Roscoe HE. Photochemical researches—part V. On the measurement of the chemical action of direct and diffuse sunlight. Proc R Soc Lond. 1862;12:306–12.
Nicula C, Nicula D, Pop RN. Results at 7 years after cross-linking procedure in keratoconic patients. J Fr Ophtalmol. 2017;40:535–41.
Koppen C, Wouters K, Mathysen D, et al. Refractive and topographic results of benzalkonium chloride-assisted transepithelial crosslinking. J Cataract Refract Surg. 2012;38:1000–5.
Filippello M, Stagni E, O’Brart D. Transepithelial corneal crosslinking: bilateral study. J Cataract Refract Surg. 2012;38:283–91.
Leccisotti A, Islam T. Transepithelial corneal collagen cross-linking in keratoconus. J Refract Surg. 2010;26:942–8.
Caporossi A, Mazzotta C, Baiocchi S, et al. Transepithelial corneal crosslinking for keratoconus: qualitative investigation by in vivo HRT II confocal analysis. Eur J Ophthalmol. 2012;22(Suppl 7):S81-88.
Alhamad TA, O’Brart DP, O’Brart NA, et al. Evaluation of transepithelial stromal riboflavin absorption with enhanced riboflavin solution using spectrophotometry. J Cataract Refract Surg. 2012;38:884–9.
Mazzotta C, Bagaglia SA, Vinciguerra R, Ferrise M, Vinciguerra P. Enhanced-fluence pulsed-light iontophoresis corneal cross-linking: 1-year morphological and clinical results. J Refract Surg. 2018;34(7):438–44.
Buzzonetti L, Petrocelli G, Valente P, Iarossi G, Ardia R, Petroni S, Parrilla R. Iontophoretic transepithelial collagen cross-linking versus epithelium-off collagen cross-linking in pediatric patients: 3-year follow-up. Cornea. 2019;38:859–63.
Freeman RD. Oxygen consumption by the component layers of the cornea. J Physiol. 1972;225:15–32.
Spoerl E, Seiler T. Techniques for stiffening the cornea. J Refract Surg. 1999;15:711–3.
Kolozsvári L, Nógrádi A, Hopp B, Bor Z. UV absorbance of the human cornea in the 240- to 400-nm range. Investig Ophthalmol Vis Sci. 2002;43:2165–8.
Hafezi F, Mrochen M, Iseli HP, Seiler T. Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. J Cataract Refract Surg. 2009;35:621–4.
Jacob S, Kumar DA, Agarwal A, Basu S, Sinha P, Agarwal A. Contact lens-assisted collagen cross-linking (CACXL): a new technique for cross-linking thin corneas. J Refract Surg. 2014;30:366–72.
Mazzotta C, Ramovecchi V. Customized epithelial debridement for thin ectatic corneas undergoing corneal crosslinking: epithelial island cross-linking technique. Clin Ophthalmol. 2014;8:1337–43.
Hafezi F, Kling S, Gilardoni F, Hafezi N, Hillen M, Abrishamchi R, Gomes JA, Mazzotta C, Randleman JB, Torres-Netto AA. Individualized corneal cross-linking with riboflavin and UV-A in ultrathin corneas: the Sub400 protocol. Am J Ophthalmol. 2021;224:133–42.
Mark T, Ngounou F, Tamon J, Gross SM, Preussner PR. Modulatory effect of different riboflavin compositions on the central corneal thickness of African keratoconus corneas during collagen crosslinking. MEAJO Middle East Afr J Ophthalmol. 2014;21(1):66–71.
Hagem A, Thorsrud A, Sandvik GF, Råen M, Drolsum L. Collagen crosslinking with conventional and accelerated ultraviolet-A irradiation using riboflavin with hydroxypropyl methylcellulose. J Cataract Refract Surg. 2017;43:511–7.
Kymionis GD, Kounis GA, Portaliou DM, Grentzelos MA, Karavitaki AE, Coskunseven E, Jankov MR, Pallikaris IG. Intraoperative pachymetric measurements during corneal collagen cross-linking with riboflavin and ultraviolet A irradiation. Ophthalmology. 2009;116:2336–9.
Wollensak G, Spörl E, Reber F, Pillunat L, Funk R. Corneal endothelial cytotoxicity of riboflavin/UVA treatment in vitro. Ophthalmic Res. 2003;35:324–8.
Jain V, Gazali Z, Bidayi R. Isotonic riboflavin and HPMC with accelerated crosslinking protocol. Cornea. 2014;33:910–3.
Oltulu RS, Satirtav G, Donbaloglu M, Kerimoglu H, Ozkagnici A, Karaibrahimoglu A. Intraoperative corneal thickness monitoring during corneal collagen cross-linking with isotonic riboflavin solution with and without dextran. Cornea. 2014;33:1164–7.
Caporossi A, Mazzotta C, Baiocchi S, et al. Riboflavin-UVA-induced corneal collagen cross-linking in pediatric patients. Cornea. 2012;31:227–31.
Arora R, Gupta D, Goyal JL, Jain P. Results of corneal collagen cross-linking in pediatric patients. J Refract Surg. 2012;28:759–62.
Vinciguerra P, Albé E, Frueh BE, Trazza S, Epstein D. Two-year corneal cross-linking results in patients younger than 18 years with documented progressive keratoconus. Am J Ophthalmol. 2012;154:520–6.
Kodavoor KS, Ariswala AZ, Ramamurthy D. One year clinical study of efficacy of corneal cross-linking in Indian children with progressive keratoconus. Cornea. 2014;33(9):919–22.
Soeters N, Van der Valk R, Tahzib NG. Corneal cross-linking for treatment of progressive keratoconus in various age groups. J Refract Surg. 2014;30:454–60.
Viswanathan D, Kumar NL, Males JJ. Outcome of corneal crosslinking for progressive keratoconus in paediatric patients. Biomed Res Int. 2014;2014:140461.
Sarac O, Caglayan M, Cakmak HB, Cagil N. Factors influencing progression of keratoconus 2 years after corneal collagen cross-linking in pediatric patients. Cornea. 2016;35:1503–7.
Ucakhan OO, Bayraktutar BN, Saglik A. Pediatric corneal collagen cross-linking: long-term follow-up of visual, refractive, and topographic outcomes. Cornea. 2016;35:162–8.
Wise S, Diaz C, Termote K, et al. Corneal cross-linking in pediatric patients with progressive keratoconus. Cornea. 2016;35:1441–3.
Padmanabhan P, Rachapalle Reddi S, Rajagopal R, et al. Corneal collagen cross-linking for keratoconus in pediatric patients-long-term results. Cornea. 2017;36:138–43.
Zotta PG, Diakonis VF, Kymionis GD, Grentzelos M, Moschou KA. Long-term outcomes of corneal cross-linking for keratoconus in pediatric patients. J AAPOS. 2017;21:397–401.
Henriquez MA, Villegas S, Rincon M, et al. Long-term efficacy and safety after corneal crosslinking in pediatric patients: three-year follow-up. Eur J Ophthalmol. 2018;28:415–8.
Knutsson KA, Paganoni G, Matuska S, et al. Corneal collagen cross-linking in paediatric patients affected by keratoconus. Br J Ophthalmol. 2018;102:248–52.
Or L, Rozenberg A, Abulafia A, et al. Corneal cross-linking in pediatric patients: evaluating treated and untreated eyes-5-year follow-up results. Cornea. 2018;37:1013–7.
Barbisan PR, Pinto RD, Gusmão CC, Castro RS, Arieta CE. Corneal collagen cross-linking in young patients for progressive keratoconus. Cornea. 2020;39:186–91.
Ozgurhan EB, Kara N, Cankaya KI, et al. Accelerated corneal cross-linking in pediatric patients with keratoconus: 24-month outcomes. J Refract Surg. 2014;30:843–9.
Shetty R, Nagaraja H, Jayadev C, et al. Accelerated corneal collagen cross-linking in pediatric patients: two-year follow-up results. Biomed Res Int. 2014;2014:894095.
Badawi AE. Accelerated corneal collagen cross-linking in pediatric keratoconus: one year study. Saudi J Ophthalmol. 2017;31:11–8.
Agca A, Tulu B, Yasa D, Yildiz BK, Sucu ME, Genç S, Fazil K, Yildirim Y. Accelerated corneal crosslinking in children with keratoconus: 5-year results and comparison of 2 protocols. J Cataract Refract Surg. 2020;46:517–23.
McAnena L, O’Keefe M. Corneal crosslinking in children with keratoconus. J AAPOS. 2015;19:228–32.
Henriquez MA, Rodriguez AM, Izquierdo L Jr. Accelerated epi-on versus standard epi-off corneal collagen cross-linking for progressive keratoconus in pediatric patients. Cornea. 2017;36:1503–8.
Sarac O, Caglayan M, Uysal BS, et al. Accelerated versus standard corneal collagen cross-linking in pediatric keratoconus patients: 24 months follow-up results. Cont Lens Anterior Eye. 2018;41:442–7.
Eissa SA, Yassin A. Prospective, randomized contralateral eye study of accelerated and conventional corneal cross-linking in pediatric keratoconus. Int Ophthalmol. 2019;39:971–9.
Nicula CA, Rednik AM, Bulboaca AE, Nicula D. Comparative results between “epi-off” conventional and accelerated corneal crosslinking for progressive keratoconus in pediatric patients. Ther Clin Risk Manag. 2019;15:1483–90.
Amer I, Elaskary A, Mostafa A, Omar A, Abdou A. Long-term visual, refractive and topographic outcomes of “epi-off” corneal collagen cross-linking in pediatric keratoconus: standard versus accelerated protocol. Clin Ophthalmol. 2020;14:3747–54.
Buzzonetti L, Petrocelli G. Transepithelial corneal cross-linking in pediatric patients: early results. J Refract Surg. 2012;28:763–7.
Salman AG. Transepithelial corneal crosslinking for progressive keratoconus in a pediatric age group. J Cataract Refract Surg. 2013;39:1164–70.
Tian M, Jian W, Sun L, Shen Y, Zhang X, Zhou X. One-year follow-up of accelerated transepithelial corneal collagen cross-linking for progressive pediatric keratoconus. BMC Ophthalmol. 2018;18:75.
Magli A, Forte R, Tortori A, et al. Epithelium-off corneal collagen cross-linking versus transepithelial cross-linking for pediatric keratoconus. Cornea. 2013;32:597–601.
Eraslan M, Toker E, Cerman E, Ozarslan D. Efficacy of epithelium-off and epithelium-on corneal collagen cross-linking in pediatric keratoconus. Eye Contact Lens. 2017;43:155–61.
Henriquez MA, Hernandez-Sahagun G, Camargo J, Izquierdo L Jr. Accelerated epi-on versus standard epi-off corneal collagen cross-linking for progressive keratoconus in pediatric patients: five years of follow-up. Cornea. 2020;39:1493–8.
Iqbal M, Elmassry A, Saad H, Am Gad A, Ibrahim O, Hamed N, Saeed A, SKhalil A, Tawfik M, Said A, et al. Standard cross-linking protocol versus accelerated and transepithelial cross-linking protocols for treatment of paediatric keratoconus: a 2-year comparative study. Acta Ophthalmol. 2020;98:e352–62.
Chatzis N, Hafezi F. Progression of keratoconus and efficacy of pediatric [corrected] corneal collagen cross-linking in children and adolescents. J Refract Surg. 2012;28:753–8.
Ulusoy DM, Goktas E, Duru N, Ozkose A, Atas M, Yuvaci I, Arifoglu HB, Zararsiz G. Accelerated corneal crosslinking for treatment of progressive keratoconus in pediatric patients. Eur J Ophthalmol. 2017;27(3):319–25.
Zhang X, Zhao J, Li M, et al. Conventional and transepithelial corneal cross-linking for patients with keratoconus. PLoS One. 2018;13:e0195105.
Li W, Wang B. Efficacy and safety of transepithelial corneal crosslinking surgery versus standard corneal crosslinking surgery for keratoconus: a meta-analysis of randomized controlled trials. BMC Ophthalmol. 2017;17:262.
Wen D, Song B, Li Q, et al. Comparison of epithelium-off versus transepithelial corneal collagen cross-linking for keratoconus: a systematic review and meta-analysis. Cornea. 2018;37:1018–24.
Kobashi H, Tsubota K. Accelerated versus standard corneal cross-linking for progressive keratoconus: a meta-analysis of randomized controlled trials. Cornea. 2020;39:172–80.
Wernli J, Schumacher S, Spoerl E, Mrochen M. The efficacy of corneal cross-linking shows a sudden decrease with very high intensity UV light and short treatment time. Invest Ophthalmol Vis Sci. 2013;54:1176–80.
Hammer A, Richoz O, Arba Mosquera S, Tabibian D, Hoogewoud F, Hafezi F. Corneal biomechanical properties at different corneal cross-linking (CXL) irradiances. Investig Ophthalmol Vis Sci. 2014;55:2881–4.
Bao F, Zheng Y, Liu C, Zheng X, Zhao Y, Wang Y, Li L, Wang Q, Chen S, Elsheikh A. Changes in corneal biomechanical properties with different corneal cross-linking irradiances. J Refract Surg. 2018;34(1):51–8.
Hammer A, Rudaz S, Guinchard S, Kling S, Richoz O, Hafezi F. Analysis of riboflavin compounds in the rabbit cornea in vivo. Curr Eye Res. 2016;41(9):1166–72.
Cantemir A, Alexa AI, Galan BG, Anton N, Ciuntu RE, Danielescu C, Chiselita D, Costin D. Outcomes of iontophoretic corneal collagen crosslinking in keratoconic eyes with very thin córneas. Medicine. 2017;96(47):e8758.
Goukon H, Kamiya K, Takahashi M, Shoji N. Effect of corneal cross-linking on endothelial cell density and morphology in the peripheral córnea. BMC Ophthalmol. 2020;20:139.
Gokhale NS. Corneal endothelial damage after collagen cross-linking treatment. Cornea. 2011;30:1495–8.
Bagga B, Pahuja S, Murthy S, Sangwan VS. Endothelial failure after collagen cross-linking with riboflavin and UV-A: case report with literature review. Cornea. 2012;31:1197–200.
Bhandari V, Lohia M, Reddy JK, Haritha. Effect of accelerated corneal collagen cross linking (CXL) on corneal endothelium. Adv Ophthalmol Vis Syst. 2015;3(1):228–31.
Rana M, Lau A, Aralikatti A, et al. Severe microbial keratitis and associated perforation after corneal crosslinking for keratoconus. Contact Lens Anterior Eye. 2015;38:134–7.
Maharana PK, Sahay P, Sujeeth M, et al. Microbial keratitis after accelerated corneal collagen cross-linking in keratoconus. Cornea. 2018;37:162–7.
Knyazer B, Kormas RM, Chorny A, Lifshitz T, Achiron A, Mimouni M. Corneal cross-linking in thin corneas: 1-year results of accelerated contact lens-assisted treatment of keratoconus. J Refract Surg. 2019;35(10):642–8.
Srivatsa S, Jacob S, Agarwal A. Contact lens assisted corneal cross linking in thin ectatic corneas—a review. Indian J Ophthalmol. 2020;68:2773–8.
Sharma N, Rao K, Maharana PK, Vajpayee RB. Ocular allergy and keratoconus. Indian J Ophthalmol. 2013;61:407–9.
Shetty R, Sureka S, Kusumgar P, Sethu S, Sainani K. Allergen-specific exposure associated with high immunoglobulin E and eye rubbing predisposes to progression of keratoconus. Indian J Ophthalmol. 2017;65:399–402.
Najmi H, Mobarki Y, Mania K, Altowairqi B, Basehi M, Mahfouz MS, Elmahdy M. The correlation between keratoconus and eye rubbing: a review. Int J Ophthalmol. 2019;12(11):1775–81.
Alrobaian M, Elsayed M, Alotaibi AK, et al. Safety and efficacy of corneal cross-linking in pediatric patients with keratoconus and vernal keratoconjunctivitis. Middle East Afr J Ophthalmol. 2019;26:95–100.
Salmon HA, Chalk D, Stein K, et al. Cost effectiveness of collagen crosslinking for progressive keratoconus in the UK NHS. Eye (Lond). 2015;29:1504–11.
Leung VC, Pechlivanoglou P, Chew HF, et al. Corneal collagen cross-linking in the management of keratoconus in Canada: a cost-effectiveness analysis. Ophthalmology. 2017;124:1108–19.
Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35(8):1358–62.
Stulting RD, Trattler WB, Woolfson JM, Rubinfeld RS. Corneal crosslinking without epithelial removal. J Cataract Refract Surg. 2018;44(11):1363–70.
Achiron A, El-Hadad O, Leadbetter D, Hecht I, Hamiel U, Avadhanam V, Tole D, Darcy K. Progression of pediatric keratoconus after corneal cross-linking: a systematic review and pooled analysis. Cornea. 2021;00:1–5.
Antoun J, Slim E, Hachem R, Chelala E, Jabbour E, Cherfan G, Jarade EF. Rate of corneal collagen crosslinking redo in private practice: risk factors and safety. J Ophthalmol. 2015;2015:1–8 (ID 690961).
Hafezi F, Tabibian D, Richoz O. Additive effect of repeated corneal collagen cross-linking in keratoconus. J Refract Surg. 2014;30(10):716–8.
Wu H, Li L, Luo S, Fang X, Shang X, Xie Z, Xiao X, He H, Lin Z, Liu Z. Safety and efficacy of repeated crosslinking assisted by transepithelial double-cycle iontophoresis in keratoconus progression after primary corneal crosslinking. Eye. 2021;35:3020–7.
Beshtawi IM, Akhtar R, Hillarby MC, O’Donnell C, Zhao X, Brahma A, Carley F, Derby B, Radhakrishnan H. Biomechanical changes after repeated collagen cross-linking on human corneas assessed in vitro using scanning acoustic microscopy. Investig Ophthalmol Vis Sci. 2014;55(3):1549–54.
Acknowledgements
Funding
No funding or sponsorship was received for this study or for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
The first draft of the manuscript was written by Julia Polido and João Gabriel Alexander. All authors commented on previous versions of the manuscript. All authors contributed significantly and in agreement with the content of the article. All authors read and approved the final manuscript.
Disclosures
Dr. Ambrósio is a consultant for Alcon, Allergan, Oculus, Mediphacos, and Zeiss. The other authors have no conflicts of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The datasets analyzed during the current study are available in the PUBMED repository [https://pubmed.ncbi.nlm.nih.gov].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Polido, J., dos Xavier Santos Araújo, M.E., Alexander, J.G. et al. Pediatric Crosslinking: Current Protocols and Approach. Ophthalmol Ther 11, 983–999 (2022). https://doi.org/10.1007/s40123-022-00508-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00508-9