Abstract
Introduction
In cases of inadequate capsular support for intraocular lens (IOL) implantation, iris-claw IOL is a practical option. Iris-claw IOL can be implanted anteriorly or retropupillary. In this study, we compare the outcome of implantation of iris-claw IOL between anterior and retropupillary locations.
Methods
We retrospectively examined the characteristics and outcomes of patients who underwent iris-claw “Artisan®” intraocular lens implantation (IOL) during the period of January 2014 to July 2020. The study population included all patients who underwent iris-claw IOL implantation, whether as a primary or secondary implantation, regardless of the causative indication. The study population was categorized by location of implantation and indication. The outcome was compared by visual acuity and postoperative complications.
Results
In this study, 171 eyes of 151 patients were included. Iris-claw IOL was implanted anteriorly in 110 (64.3%) eyes. The most common indication for iris-claw IOL was complicated cataract surgery, followed by ectopia lentis and by trauma. Patients with retropupillary position achieved better visual outcome whatever the causative indication. Anterior iris-claw IOL patients had more high intraocular pressure readings and macular edema.
Conclusions
This study revealed that retropupillary iris-claw IOL may achieve better visual outcome without significant postoperative complications. Further prospective studies and trials on larger sample sizes are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Iris-claw intraocular lens (IOL) can be implanted anteriorly or retropupillary. |
Most studies compare anterior versus retropupillary iris-claw IOL in aphakic eyes after complicated surgery only. |
They reveal no significant difference in the visual outcome between the two groups. |
What was learned from the study? |
Retropupillary iris-claw IOL achieved a better visual outcome. |
Also, the retropupillary location causes fewer postoperative complications such as high intraocular pressure. |
Proper preoperative preparation such as intraocular lens measurement and individualized case selection is a crucial step in managing those patients. |
Introduction
During cataract surgery, the best outcome can be achieved by implanting the intraocular lens (IOL) in the capsular bag as it resembles the natural location of the crystalline lens. However, for many reasons, the capsular support would be inadequate to implant the IOL in the bag of the sulcus. These situations included traumatic crystalline lens subluxation, complicated cataract surgery, dislocation of the IOL, zonular dehiscence, and after early congenital cataract extraction [1,2,3,4,5]. To overcome this problem, several techniques were utilized and included sutured scleral fixation, anterior chamber fixation, and iris fixation, either implanted into the anterior chamber or retropupillary [1,2,3,4,5].
Regarding anterior chamber IOL, surgical implantation is safe and fast. On the other hand, use of these IOLs is well known to cause secondary glaucoma, endothelial cell loss, bullous keratopathy, and uveitis [6]. Scleral-fixated IOLs have advantages over anterior chamber IOL in that they are far away from the angle, and they respect the anatomy of the eye despite the difficulty and long operation procedure with extensive intraocular manipulation. Many complications were reported after scleral-fixated IOL implantation and included vitreous incarceration, IOL decentration, chronic inflammation, retinal detachment, conjunctival erosions, scleromalacia, and pigment dispersion and have a lifelong risk of IOL drop [7, 8].
Regarding iris-claw IOL, the first model was fixated in 1972 to the midperipheral point of the iris, where the iris is less vascularized [9]. One of the latest versions of iris-claw IOL designed for aphakia is the Artisan Aphakia Model (convex/concave) (Ophtec BV, Groningen, The Netherlands). This Artisan IOL model was designated to be implanted in the anterior chamber and showed better visual outcome with fewer complications [10,11,12]. However, many surgeons have investigated their experience in implantation of the Artisan IOL retropupillary because of the possible risk of endothelial cell loss if the iris-claw IOL is implanted into the anterior chamber [2, 8]. This retrospective study aims to evaluate the overall practice of iris-claw IOL implantation and focuses on the differences between anterior and retropupillary locations.
Methods
Patients and Data
After obtaining Institutional Review Board (IRB) approval, we retrospectively examined the clinico-demographic characteristics of 171 eyes of 151 patients who underwent iris-claw “Artisan®” IOL implantation during the period of January 2014 to July 2020. The study was conducted at King Abdullah University Hospital (KAUH), a tertiary care center located in North Jordan, which is affiliated with the Jordan University of Science and Technology (JUST). Using the hospital paper-based and electronic medical records, demographic data (age, sex), past medical history, and past ocular history were retrieved. Also, the indications and preoperative optical parameters were collected. Furthermore, the operative details [including the location of iris-claw IOL implantation (anterior or retropupillary)], visual outcome, and postoperative complications were studied.
This study received ethics approval from the Research and Ethics Committee, Jordan University of Science and Technology, with reference number 14/134/2020. We confirm that the privacy of the participants was maintained, and the data were anonymized and kept with confidentiality.
The study population included all patients who underwent iris-claw IOL implantation whether as a primary or secondary implantation regardless of the causative indication. The exclusion criteria comprised patients with insufficient pre- or postoperative data. Most of these cases were referral cases with complicated procedures from secondary care centers. The study population was categorized by location of implantation and by indication. The patients were divided by location into two main groups: anterior iris-claw IOL and retropupillary iris-claw IOL. By indication, the patients were divided into several groups. First was patients who suffered from trauma which resulted in subluxation or total dislocation of the crystalline lens or ruptured cataractous lens without adequate capsular support. This group also included patients with iris trauma or iris tissue loss who required iridoplasty and patients with posterior segment involvement including patients with traumatic retinal detachment. The second group comprised cataract surgery complications which included posterior capsular rupture (PCR) with or without dropped crystalline lens material with inadequate capsular support, dislocated IOL, and dropped IOL. The iris-claw IOL was implanted either as a primary IOL intraoperatively during complicated cataract surgery or as a secondary IOL in cases of dropped IOL, dropped nucleus material, and dislocated IOL and some cases of PCR where the patients were left aphakic. The third group implicated pediatric patients with congenital cataract extraction with or without anterior vitrectomy. The fourth group comprised patients with non-traumatic ectopia lentis (subluxated crystalline lens), which could be due to inherited disease such Marfan’s syndrome or zonular dialysis in patients with exfoliation syndrome. In this group, the iris-claw IOL was implanted uniformly as a primary IOL implantation during the planned procedure. The fifth group included patients who underwent anterior chamber (AC) IOL and then suffered from ocular complications such as secondary glaucoma and bullous keratopathy. The last group included the implantation of iris-claw IOL for refractive indications such as high myopia.
The optical parameters included iris-claw IOL power (using an A-constant of 115 for anterior iris-claw and 117 for retropupillary iris-claw IOL), keratometry readings, and axial length. All parameters were retrieved from visits at 1 week, 1 month, 3 months, 1 year, and the last follow-up visits postoperatively.
The outcome was compared between the two main groups using different measures. First, the mean change in visual acuity was compared and studied pre- and postoperatively during all follow-up visits. Visual acuity was measured in decimal visual acuity and converted to LogMAR visual acuity. For patients with visual acuity of counting fingers, hand motion, light perception, or “no light perception,” they were converted according to the study of Schulze-Bonsel et al. [13].
Furthermore, postoperative complications were compared between the two groups and included: irregular iris shape (new postoperative irregularity or aggravated preoperative irregularity), iris tissue loss, iris-claw IOL decentration or tilt, spontaneous disenclavation, clinical signs of endothelial cell loss (including long-term corneal edema and the development of bullous keratopathy), pigment dispersion, postoperative high intraocular pressure (IOP) that affected the vision and required using antiglaucoma agents or glaucoma surgery, macular edema requiring treatment, retinal detachment, endophthalmitis, and epiretinal membrane proliferation.
Perioperative Setting
Visual acuity was assessed by Snellen decimal projectors. IOP was measured by Goldmann tonometry; anterior and posterior segment examination was performed through slit-lamp biomicroscopy with the required non-contact hand-held lenses. The ophthalmic examination was done by well-trained residents and confirmed by the attending consultant ophthalmologist. The IOL power was measured by either ultrasonic biometry (Digital A/B scan 5500; Sonomed Inc., Lake Success, NY, USA) or IOL Master when needed.
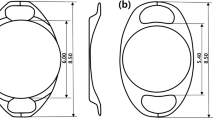
Seven consultant surgeons performed the operations and selected to implant the IOL either anteriorly or retropupillary depending on their individual experience and the characteristics of the specific surgical condition. The same standardized surgical technique was applied in both groups. The lens used in this study was the Artisan® aphakia IOL (Ophtec BV, Groningen, The Netherlands), which is a polymethyl methacrylate IOL with 8.5-mm length, 1.04-mm maximum height, and 5.4-mm optical zone width. The desired IOL power was calculated using the SRK/T formula in most cases (other formulas were utilized such as Haigis formula in patients with high myopia, Shammas and Haigis formulas for those who had a previous refractive surgery, and Holladay II and Hoffer Q for patients with short axial length) aiming to achieve slight myopia (0.0 D to − 0.5 D) except in certain cases such as pediatric patients. The manufacturer’s recommendation for A constant is 115.0 for implantation above the iris but we used an A constant of 117.0 when the IOL was implanted retropupillary. All operations were performed under either general or local anesthesia. Two corneal side ports were performed at the 3 and 9 o’clock positions. After performing the primary associated procedure, acetylcholine 1% (Miochol®-E) was injected intracamerally through the paracentesis for miosis, followed by injection of a cohesive viscoelastic material. A 5.5-mm corneal incision was made at 12 o’clock. For retropupillary implantation, the iris-claw IOL was inserted upside down (with its convex surface facing posteriorly), rotated by an Artisan lens forceps to a horizontal position, and centered over the pupil. The optic of the reversed iris-claw IOL was held securely using a special forceps. Next, the two haptics were gently slid behind the iris, and the optic was lifted slightly forward toward the posterior surface of the iris so that the claw configuration of both haptics could be recognized from above on the iris anterior surface. With the other hand, a long micro-spatula was used through the side ports to tuck iris tissue into the claw. The second haptic was fixated in the same way, using the same spatula. For anterior implantation, the convex surface was placed anteriorly, and the iris was enclavated at midperiphery between the claw haptics. The corneal incision was closed and secured with three simple buried interrupted 10–0 nylon sutures.
A peripheral iridotomy (PI) was performed based on the surgeon’s experience, and it was done mostly for anterior chamber location. In most cases, the Artisan implantation was combined with other operative procedures. Anterior vitrectomy was done in many cases. IOL exchange was performed for patients with anterior chamber IOL or for dislocated IOL. A pars plana vitrectomy was performed for dropped IOL and dropped nucleus material. Also, keratoplasty was performed for patients with bullous keratopathy. Silicon oil removal was done also in combination with iris-claw IOL implantation. Postoperative therapy included antibiotic, steroid and nonsteroidal anti-inflammatory eye drops for 1 month.
Statistical Analysis
Extracted data were entered into a spreadsheet. Statistical analysis was performed using the IBM SPSS statistical package for Windows v.22 (Armonk, New York, USA). Data were expressed as frequency (percentage) for nominal data, mean ± standard deviation of the mean (SD). Statistical significance between the study groups regarding the previously mentioned parameters was determined using chi-square test for categorical variables, and Student’s t-test and ANOVA test for continuous variables. P ≤ 0.05 was considered statistically significant. Simple linear regression test was applied to study the relation between two continuous variables. Multiple logistic regression analyses were performed to study the multiple effects of different variables. The sample size was confirmed retrospectively at alpha level of 0.05 and power of analysis at 90%.
Results
General Characteristics
In this study, 171 eyes of 151 patients were included. Of those 151 patients, 89 (58.9%) were of males. The mean age of the patients was 47.9 years. Of the 171 eyes, the left eye was involved in 89 (52%) procedures. Iris-claw IOL was implanted anteriorly in 110 (64.3%) eyes.
The most common indication for iris-claw IOL was complicated cataract surgery, which was performed in 96 (56.1%) eyes followed by ectopia lentis in 23 (13.5%) and by trauma in 22 (12.9%) eyes. Congenital cataract and AC IOL complications were involved in 14 patients for each. The refractive implant was done only for two eyes for the same patient.
Diabetic retinopathy was found in 12.3% of the patients. Also, 9.9% of the eyes had an associated previous retinal detachment. The iris-claw IOL implantation was done along with PI in 61.4% of the eyes. Irregular iris shape and high IOP were the two most common complications after iris-claw IOL implantation. Table 1 summarizes the overall characteristics of the study sample.
Retropupillary Versus Anterior Artisan
There was no difference between retropupillary and anterior iris-claw IOL in terms of sex, age, laterality, indications, previous ocular history, and comorbidities. Regarding the associated surgical procedure, PI was performed more when the implantation was anterior (79.6% for anterior location versus 31.1% for retropupillary). In addition, IOL exchange was achieved more in the retropupillary location.
The visual outcome was statistically different between the two groups, and Table 2 shows the difference in the visual outcome. For the uncorrected visual acuity (UCVA) change at the 1-year postoperative period, the mean change in visual acuity in the retropupillary group was − 0.845 LogMAR, which corresponds to an improvement in visual acuity of about 42 letters. On the other hand, the mean change of visual acuity was − 0.315 LogMAR in the anterior group, which corresponds to about 16 letters of improvement. Similarly, for the best corrected visual acuity (BCVA) at 1-year postoperative period, the mean change in visual acuity for retropupillary group was − 0.728 LogMAR (an improvement of about 36 letters) and the mean change for the anterior group was − 0.121 LogMAR (an improvement of about 6 letters). The differences between anterior and retropupillary location in each indication group (such as the comparison of BCVA between retropupillary and anterior groups in patients with complicated cataract surgery) were studied and revealed a similar pattern of results (in that retropupillary achieved better visual outcome).
Multiple linear regression analysis was done to justify the factors that affected visual acuity independently (including location, age, gender, previous ocular diseases, and surgery, and indications). It was revealed that the location of the iris-claw IOL and the presence of previous retinal detachment were the only two independent factors affecting the visual acuity outcome (being a retropupillary implant and the absence of retinal detachment were associated with improved outcome).
The postoperative complications varied between the two groups as most complications developed in the anterior location. Iris-claw IOL decentration occurred more in the anterior location (20.6% for the anterior location versus 6.6% for the retropupillary). Furthermore, high IOP readings and/or the prolonged use of postoperative antiglaucoma agents was associated much more in the anterior location (28% for the anterior location and 13.1% for the retropupillary). Regarding high IOP, it is important to notice that in the anterior group, patients without iridotomy had more high IOP readings (52.4% patient without iridotomy in anterior group had high IOP versus 22.6% for patients with iridotomy; P = 0.009). This was not applicable for retropupillary position where performing iridotomy did not decrease the high IOP readings.
Moreover, macular edema was developed significantly more in the anterior group (12.1% for the anterior group and 3.3% for the retropupillary). Table 2 summarizes the differences between anterior and retropupillary iris-claw IOL.
Differences Between Indication Groups
There was no difference between indication groups in gender, location of iris-claw IOL, and laterality. The “complicated cataract surgery” group was older than any other group (P < 0.001). Diabetes and hypertension were found significantly more in the “complicated cataract surgery” group, which tended to be older in age (P value < 0.001 for both). Marfan’s syndrome was found exclusively in the “ectopia lentis” group (P value < 0.0001). Preoperative retinal detachment was associated more with the trauma group (P = 0.011). Also, preoperative diabetic retinopathy was associated with the “complicated cataract surgery group (P = 0.012). In addition, preoperative glaucoma was correlated with the “AC IOL complications” group (P = 0.004).
Regarding the visual outcome, Fig. 1 illustrates the BCVA at different postoperative periods for all indication groups. The statistical tests (parametric and nonparametric) showed no statistical difference between indication groups at all postoperative periods. This could be attributed to the small sample size for some groups. However, the “AC IOL complications” group had the least improvement in visual acuity compared to other groups. This is explained by the statistical fact that keratoplasty was done along with Artisan implantation mostly in the “AC IOL complications” and the trauma groups (P < 0.001).
The postoperative complications are shown in Fig. 2. Spontaneous disenclavation occurred mostly in the “congenital cataract” group (P = 0.002). Similarly, pigment dispersion developed significantly in the “congenital cataract” group (P = 0.025). Retinal detachment developed mostly in the trauma group (P = 0.021). Other postoperative complications were not statistically different.
Discussion
Although some previous studies described using iris-claw IOL implantations, very few compared the anterior and retropupillary positions [2, 8, 14,15,16]. Toro et al. conducted a retrospective study on 180 aphakic patients with secondary iris-claw IOL implantation with a follow-up of 5-year duration [8]. Their study revealed that both the anterior and retropupillary Iris IOL implantation is effective in the treatment of aphakia without sufficient capsule support by improving visual acuity significantly without serious intra- and postoperative complications [8]. Similarly, Helvacı et al. found in their study on 40 aphakic eyes that there were no significant differences between anterior and retropupillary locations [2]. In addition, the Mora et al. study showed that both retropupillary and anterior locations were equally effective and safe for managing cases of aphakia with inadequate capsular support [14]. In our study, we included all cases of iris-claw implantation for all possible indications and investigated the effect of all associated procedures, ocular diseases, and clinical status. Relatively, the study included a good sample size. It was revealed that the retropupillary implantation achieved better visual outcome, fewer cases of high IOP, and fewer cases of macular edema.
All cataract surgeries may cause endothelial cell loss [2]. Güell et al. reported that anterior iris-claw IOL implantation caused approximately 10.9% endothelial cell loss in the 3-year follow-up [12]. Anbari and Lake reported a mean drop of 267 cells/mm2 (about 11.7%) of endothelial cell density at 2 years after retropupillary iris-claw IOL implant [17]. Gicquel et al. reported that anterior iris-claw IOL results in more endothelial cell loss than the retropupillary implantation [18]. Yueqin et al. proposed that the corneal endothelial cell loss may be due to a mechanical irritation between the endothelium and the instruments or the IOL haptics [19]. By using a sufficient amount of viscoelastic material during the surgery, endothelial cells loss can be minimized. In our study, anterior location resulted in more cases of clinical signs of endothelial cell loss although this was not statistically different.
In most studies, there was no significant difference in the risk of IOP elevation events between the two groups [2, 14]. Some studies showed that IOPs are elevated more in the anterior group [8, 15]. Subsequently, retropupillary iris-claw IOL may be better for eyes with ocular hypertension or borderline IOP before surgery. Performing a peripheral iridotomy has been recommended when the iris-claw is placed on the anterior surface of the iris to prevent pupillary block and subsequent IOP elevation problems [8]. Also, this may be explained by the directed contact of IOL haptics with the angle of the anterior chamber in the anterior group. In our study, high postoperative IOP that required the use of antiglaucoma was significantly higher in the anterior group. Also, patients without iridotomy in the anterior group had many more high IOP readings than patients with iridotomy.
Macular edema has been reported to occur after iris-claw implantation [8, 14]. The exact cause is not well known but may be as a result of chronic irritation of the iris, or might even be a result of the primary cause of aphakia [20]. The risk of macular edema seemed to be higher in the anterior group in other studies [8]. In this study, macular edema developed more in the anterior group. This might be explained by two points: first, the different degree of inflammation or pigment dispersion between the anterior and posterior iris surfaces; second, the larger degree of iris-claw IOL movement with subsequent iris irritation in the anterior iris-claw IOL group due to the insufficient iris tissue and the possible insufficient capturing of the claw compared to the retropupillary location where the IOL might be able to enclave more iris tissue [21].
The limitation of iris claw IOL, regardless of the location of implantation, includes the 5.5-mm large incision and the consequent corneal astigmatism. Baykara et al. preferred a scleral tunnel incision with a surgical procedure that normally does not require sutures [22]. Another difficulty of retropupillary implantation is the probability of IOL dislocation into the vitreous due to enclavation failure. However, these cases can often be treated by re-enclavation [8].
Another complication of iris-claw IOL is the possible damage to the iris, which occurred in 16.4% of the cases in this study, and pigment dispersion, which developed in 7% of the cases. Toro et al. suggested the use of subconjunctival corticosteroid injections to reduce the incidence of pigment dispersion and iris atrophy at the end of implantation [8].
Our study is not without limitations. First, our inability to measure endothelial cell count limits the ability to investigate the effect of the location of implantation on corneal endothelium. Second, the retrospective nature of the study along with the unequal size of both groups is a major pitfall. Third, the broad range of indications is another point due to the complicated nature of the analysis.
Conclusions
Iris-claw IOL is an important solution for patients without adequate capsular support to achieve significant visual outcome. This study revealed that retropupillary iris-claw IOL may achieve better visual outcome if implanted properly without significant postoperative complications. Further prospective studies and trials on larger sample sizes are needed to investigate the best model in patients with inadequate capsular support.
References
Güell JL, Verdaguer P, Elies D, Gris O, Manero F, Mateu-Figueras G, et al. Secondary iris-claw anterior chamber lens implantation in patients with aphakia without capsular support. Br J Ophthalmol. 2014;98:658–63.
Helvaci S, Demirduzen S, Oksuz H. Iris-claw intraocular lens implantation: anterior chamber versus retropupillary implantation. Indian J Ophthalmol. 2016;64:45–9.
Saleh M, Heitz A, Bourcier T, Speeg C, Delbosc B, Montard M, et al. Sutureless intrascleral intraocular lens implantation after ocular trauma. J Cataract Refract Surg. 2013;39:81–6.
Ohta T, Toshida H, Murakami A. Simplified and safe method of sutureless intrascleral posterior chamber intraocular lens fixation: Y-fixation technique. J Cataract Refract Surg. 2014;40:2–7.
Agarwal A, Jacob S, Kumar DA, Agarwal A, Narasimhan S, Agarwal A. Handshake technique for glued intrascleral haptic fixation of a posterior chamber intraocular lens. J Cataract Refract Surg. 2013;39:317–22.
Moschos MM, Nitoda E. The correction of aphakiausing anterior chamber intraocular lens. Vivo. 2016;30:733–8.
McCluskey P, Harrisberg B. Long-term results using scleral fixated posterior chamber intraocular lenses. J Cataract Refract Surg. 1994;20:34–9.
Toro MD, Longo A, Avitabile T, Nowomiejska K, Gagliano C, Tripodi S, et al. Five-year follow-up of secondary iris-claw intraocular lens implantation for the treatment of aphakia: anterior chamber versus retropupillary implantation. PLoS ONE. 2019;14:e0214140.
Worst JG. Iris claw lens. J Am Intrakcul Implant Soc. 1980;6:166–7.
Schallenberg M, Dekowski D, Hahn A, Laube T, Steuhl KP, Meller D. Aphakia correction with retropupillary fixated iris-claw lens (Artisan)—long-term results. Clin Ophthalmol. 2014;8:137–41.
Hsing YE, Lee GA. Retropupillary iris claw intraocular lens for aphakia. Clin Exp Ophthalmol. 2012;40:849–54.
Güell JL, Velasco F, Malecaze F, Va’zquez M, Gris O, Manero F. Secondary Artisan-Verysise aphakic lens implantation. J Cataract Refract Surg. 2005;31:2266–71.
Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion“ and “counting fingers“ can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47(3):1236–40.
Mora P, Calzetti G, Favilla S, Forlini M, Tedesco S, Date P, et al. Comparative analysis of the safety and functional outcomes of anterior versus retropupillary iris-claw IOL fixation. J Ophthalmol. 2018. https://doi.org/10.1155/2018/8463569.
Hazar L, Kara N, Bozkurt E, Ozgurhan EB, Demirok A. Intraocular lens implantation procedures in aphakic eyes with insufficient capsular support associated with previous cataract surgery. J Refract Surg. 2013;29:685–91.
Tourino Peralba R, Lamas-Francis D, Sarandeses-Diez T, Martinez-Perez L, Rodriguez-Ares T. Iris-claw intraocular lens for aphakia: can location influence the final outcomes? J Cataract Refract Surg. 2018;44:818–26.
Anbari A, Lake DB. Posteriorly enclavated iris claw intraocular lens for aphakia: long-term corneal endothelial safety study. Eur J Ophthalmol. 2015;25:208–13.
Gicquel JJ, Guigou S, Bejjani RA, Briat B, Ellies P, Dighiero P. Ultrasound biomicroscopy study of the Verisyse aphakic intraocular lens combined with penetrating keratoplasty in pseudophakic bullous keratopathy. J Cataract Refract Surg. 2007;33:455–64.
Yueqin C, Qinghuai L, Chunyan X, Zhenping H, Yin C. Three-year follow-up of secondary anterior iris fixation of an aphakic intraocular lens to correct aphakia. Cataract Refract Surg. 2012;38:1595–601.
Faria M, Pinto Ferreira N, Medeiros Pinto J, Cordeiro-Sousa D, Cardoso-Leal I, Neto E, et al. Retropupillary iris claw intraocular lens implantation in aphakia for dislocated intraocular lens. Int Med Case Rep J. 2016;9:261–5.
Massa HF, Gobej I, Jacquier P, Jonescu-Cuypers C, Le Quoy O. Cystoid macular oedema and iris-fixated intraocular lens treated with intraocular lens exchange: a case series and review. J Int Med Res. 2019;47:188–95.
Baykara M, Ozcetin H, Yilmaz S, Timucin OB. Posterior iris fixation of the iris-claw intraocular lens implantation through a scleral tunnel incision. Am J Ophthalmol. 2007;144:586–91.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed significantly and in agreement with the content of the article. All authors were involved in project design, data collection, analysis, statistical analysis, data interpretation and writing the manuscript. All authors presented substantial contributions to the article and participated of correction and final approval of the version to be submitted.
Disclosures
Rami Al-Dwairi, Omar Saleh, Abdelwahab Aleshawi, Zeinab Alladkanie, Osama Al Deyabat, Acil Alasheh, Sharaf Adi and Mohammed Al-Howthi confirm that they have no financial ties or conflicts of interest to disclose.
Compliance with Ethics Guidelines
This study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendment. This research has obtained ethical approval from Research and Ethics Committee, at Jordan University of Science and Technology with a reference number of 14/134/2020. We confirm that the privacy of the participants was saved, and the data was anonymized and maintained with confidentiality. The need for consent was waived by our institutional review board due to the retrospective nature of the study.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Al-Dwairi, R., Saleh, O., Aleshawi, A. et al. Anterior Versus Retropupillary Iris-Claw Intraocular Lens: Indications, Visual Outcome and Postoperative Complications. Ophthalmol Ther 11, 771–784 (2022). https://doi.org/10.1007/s40123-022-00474-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00474-2