Abstract
Introduction
The purpose of our study was to investigate the impact of ketorolac addition to the well-established combination of antibiotic-steroid agent in terms of vision-related quality of life.
Methods
Patients were randomized to: (1) fixed combination of tobramycin 0.3%–dexamethasone 0.1%, one drop qid (n = 68) and (2) fixed combination of tobramycin 0.3%–dexamethasone 0.1%, one drop qid, plus ketorolac tromethamine 0.5%, one drop tid (n = 70). All patients completed the VFQ-25 questionnaire to assess their functional vision before cataract surgery and postoperatively on days 7, 28 and 42. The statistical analysis comprised the point-wise comparison between the two groups at the four time points for all sub-scales of the VFQ-25 questionnaire, as well as the composite score.
Results
No significant differences were noted regarding the composite score, as well as all subscales in all examined time points.
Conclusions
The addition of ketorolac did not seem to offer any additional benefit in terms of vision-related quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cataracts are a main cause of blindness in the world [1, 2]. Ophthalmologists have become concerned about the visual problems caused by cataracts in everyday life and noted that visual impairment was not adequately measured by visual acuity [3, 4]. In fact, visual acuity as a sole index may not capture all meaningful aspects of cataract surgery-related outcomes, as it does not take into account postoperative functional improvement, changes in daily life activities and satisfaction with vision [4].
Several vision-specific health-related quality of life instruments have been used to assess vision-related functional impairment [3]. One such questionnaire is the National Eye Institute Visual Function Questionnaire-25 (VFQ-25), which was introduced by Mangione et al. to evaluate vision-related quality of life in patients with chronic eye diseases, including cataracts, age-related macular degeneration, glaucoma, diabetic retinopathy, keratoconus, CMV retinitis or branch retinal vein occlusion [5, 6]. Indeed, the VFQ-25 assesses not only visual function impairment, but also the impact of ocular diseases on patients’ daily life from a substantial number of viewpoints [7].
Our previous study has assessed the impact of cataracts in relation to various modifiers upon vision-related quality of life [8]; other studies examined the outcomes of cataract surgery, comparing VFQ scores pre- and postoperatively, as well as investigating the possible factors associated with the difference in vision-related quality of life before and after cataract surgery [3, 4, 7,8,9,10]. Nevertheless, no attempt has been made to evaluate whether postoperative pharmaceutical treatment may modify vision-related quality of life in cataract patients undergoing uneventful phacoemulsification. In one of our previous studies, we found that the addition of a non-steroidal agent i.e., ketorolac, did not seem to offer any additional benefit in terms of inflammation-related signs (corneal edema, redness, Tyndall reaction) [11]. In light of the above, our study aims to investigate the impact of ketorolac addition to the well-established combination of antibiotic-steroid agent in terms of vision-related quality of life. This effort envisages to extrapolate and further validate the results of our previous study [11].
Methods
All participants underwent a routine ophthalmological examination i.e., measurement of best-corrected visual acuity (BCVA) by means of Snellen charts, slit lamp examination, intraocular pressure measurement by Goldmann applanation tonometry and fundoscopy in addition to a complete medical history. Patient information included age, sex, educational and marital status, number of offspring and siblings, alcohol consumption, income, expenses, exercise, current smoking and presence of diabetes mellitus. Cataract was classified as: immature (partially opaque lens, disc view hazy), mature (completely opaque lens, no disc view), hypermature (liquefied cortical matter), Morganian. Exclusion criteria were the following: (1) history of intraocular surgery in the operated eye, (2) any previous episode of uveitis in the operated eye, (3) severe systemic disease (heart failure NYHA stage III of IV, end-stage renal failure, pulmonary failure, patients receiving chemotherapy), (4) regular, systemic use of steroid or non-steroidal anti-inflammatory drugs during the last three months, (5) uncontrolled glaucoma.
On the day of surgery, the pupil was dilated with tropicamide 0.5% (Tropixal, Demo) and phenylephrine hydrochloride 5% (Phenylephrine, Cooper) drops every 10 for 30 min before surgery. Phacoemulsification was performed using Alcon Series 20000® Legacy® by the same surgeon (LP).
Patients were randomized to one of the two postoperative treatment arms: (1) tobramycin 0.3%–dexamethasone 0.1% (TobraDex®, Alcon), one drop four times/day (TD group, n = 72) and (2) combination of tobramycin 0.3%–dexamethasone 0.1% (TobraDex®, Alcon), one drop four times/day, plus ketorolac tromethamine 0.5% (Acular®, Allergan), one drop three times/day (TD-K group, n = 73). The topical treatment was administered for 28 days after phacoemulsification. The study was masked to patients, i.e., patients received unmarked bottles so as to be unaware of which treatment they received. Patients who needed additional pupil devices for inadequate dilation or those whose underwent vitrectomy due to posterior capsule rupture (PCR) were excluded; as a result, 68 patients were ultimately included in the TD group and 70 patients in the TD-K group, as shown in the respective flowchart [11] (Fig. 1).
All patients completed the VFQ-25 in order to assess their functional vision before cataract surgery and postoperatively on day 7, 28 and 42. The VFQ-25 measures the following vision-dependent sub-scales: general health (GH), general vision (GV), ocular pain (OP), near activities (NA), distance activities (DA), social functioning (VSSF), mental health (VSMH), role difficulties (VSRD), dependency (VSD), driving (D), colour vision (CV) and peripheral vision (PV). The subscales score ranges from 0 to 100. A score of 0 indicates the worst possible score and a score of 100 the best possible score. We have used the official VFQ-25 Greek translation by the Laboratory of Experimental Ophthalmology of Aristotle University, Thessaloniki, Greece, 2000 [12].
This study is in accordance with the Declaration of Helsinki and has been approved by the local Institutional Review Board. Written informed consent was obtained from all patients. The authors declare no conflict of interest.
Statistical Analysis
The statistical analysis comprised the point-wise comparison between the two groups (TD vs. TD-K group) at the four time points (baseline, day 7, day 28 and day 42) for all sub-scales of the VFQ-25 questionnaire, as well as the composite score. Student’s t test was appropriately implemented when subscales were normally distributed (GH, GV, OP). On the other hand, in case of significant deviation from normality (NA, DA, VSSF, VSMH, VSRD, VSD, D, PV, as well as the composite score) Mann–Whitney–Wilcoxon test for independent samples (MWW for brevity) was performed. The deviation from normality was assessed by means of the Shapiro–Wilk test. Given that four comparisons took place for each outcome-subscale (baseline, day 7, day 28 and day 42), the Bonferroni correction for multiple comparisons was adopted; as a result, the threshold of statistical significance for p was set at 0.05/4 = 0.0125.
For the post hoc additional, secondary comparison aiming to examine whether the overall condition of patients improved after the completion of the follow-up period, Wilcoxon matched-pairs signed-rank test was performed on the VFQ-25 composite score, separately for each group. Statistical analysis was performed with STATA 11.1 statistical software (StataCorp, College Station, TX, USA).
Results
The study design, as well as the randomization of patients to the two groups, is depicted in the respective flow chart (Fig. 1), which has been presented in our previous study [11]. Patients’ data are summarized in Table 1 [11].
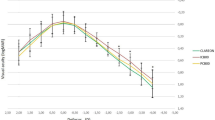
The VFQ-25 sub-scales scores of the participants are shown in Table 2, by study group for all time points. No significant differences were noted regarding the composite score in all examined time points (90.8 ± 2.1 vs. 90.8 ± 1.7, p = 0.833 on day 7; 91.0 ± 2.2 vs. 90.8 ± 1.7, p = 0.360 on day 28; 90.8 ± 2.3 vs. 90.7 ± 1.8, p = 0.467 on day 42). Accordingly, the lack of significant differences between the TD and TD-K group pertained to all subscales. A borderline trend that may merit highlighting pertains to the relatively higher values of NA subscale in TD-K group compared to TD group, which were consistently observed in all three time points (p = 0.014 on day 7, p = 0.025 on day 28, p = 0.024 on day 42) (Fig. 2).
Τhe post hoc additional, secondary comparison aiming to examine whether the overall condition of patients improved after the completion of the follow-up period pointed to significant improvement in VFQ-25 composite score in both groups (p < 0.0001, Wilcoxon matched-pairs signed-rank test for each group).
In addition, the BCVA (logMAR) did not differ between the two groups at any time point (for TD and TD-K respectively: 0.22 ± 0.11 vs. 0.22 ± 0.10 on day 7, p = 0.955, MWW; 0.08 ± 0.09 vs. 0.06 ± 0.07 on day 14, p = 0.425, MWW; 0.04 ± 0.07 vs. 0.04 ± 0.06 on day 21, p = 0.887, MWW; 0.03 ± 0.06 vs. 0.03 ± 0.05 on day 28, p = 0.373, and 0.03 ± 002 vs. 0.03 ± 0.01 on day 42, p = 0.198). Worthy of note, there was a significant correlation between BCVA and VFQ-25 at all time-points in both groups (Spearman’s rho = −0.085 on day 42, p < 0.001).
Discussion
The principal message of this study is that the addition of a non-steroidal anti-inflammatory agent (ketorolac) after uneventful phacoemulsification surgery did not seem to offer any additional benefit in terms of quality of life when compared to the antibiotic/steroid group.
The rationale for the addition of ketorolac after phacoemulsification cataract surgery lies to the prevention of cystoid macular edema (CME) [11]. Advances in cataract surgery have taken place, including the minimally invasive cataract surgery, as well as the femtosecond laser assisted cataract surgery (FLACS), which led to reduction of postoperative inflammation [13]. Therefore, one could hypothesize that there is no need for the addition of ketorolac after uneventful phacoemulsification.
Our study shows that phacoemulsification with IOL implantation is an effective and safe method for improving vision-related quality of life, as both groups presented improvement in composite score postoperatively in comparison to preoperative scores. This is in line with previous studies, which also found a significant difference in vision-related quality of life between pre- and post-operative measurements [3, 4, 7,8,9,10]. Previous studies also suggest that visual acuity is not a good predictor of patients’ satisfaction after cataract surgery and that vision-related quality of life questionnaires are more accurate in measuring patients’ postoperative function [3, 4, 7,8,9,10].
According to one cross-sectional study conducted by our team on 220 patients who were eligible for phacoemulsification cataract surgery, baseline vision-related quality of life is affected by inherent sociodemographic and lifestyle parameters, such as gender, educational level, marital status, current working status and exercise [8]. In the present study, as the two groups were randomized, the various modifiers did not differ significantly. As a result, although potential risk factors may confer meaningful discrepancies in vision-related quality of life in cataract patients, in our current study, they seemed not to have any effect depending on the postoperative treatment regimen.
Another interesting finding of the present study was the borderline trend for relatively higher values of NA subscale in TD-K group compared to TD group, which were consistently observed in all three time points. Future studies may focus on the examination of the possible pathophysiological mechanism for this trend, which remains elusive.
Nevertheless, some limitations of the study should be declared. Firstly, it should be noted that sample size calculation was not performed. Furthermore, the randomization procedure was based on “random numbers” and did not include the cataract stage or the visual needs of patients, although the two groups seemed to have similar characteristics. Moreover, other treatment regimens would be desirable to be evaluated, as the results of the study may be influenced by regimens including other treatment combinations.
Conclusions
In conclusion, this is the first study evaluating the impact of ketorolac in quality of life after uneventful phacoemulsification, suggesting that the addition of a non-steroidal anti-inflammatory agent (ketorolac) after uneventful phacoemulsification surgery did not seem to offer any additional benefit in terms of quality of life when compared to the antibiotic/steroid group, in line with our previous study, according to which the addition of ketorolac was not associated with additional benefit regarding clinical signs and recovery in BCVA.
References
Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85.
Greenberg PB, Tseng VL, Wu WC, Liu J, Jiang L, Chen CK, Scott IU. Friedmann. Prevalence and predictors of ocular complications associated with cataract surgery in United States veterans. Ophthalmology. 2011;118:507–14.
Bilbao A, Quintana JM, Escobar A, García S, Andradas E, Baré M. Elizalde B; IRYSS-Cataract Group. Responsiveness and clinically important differences for the VF-14 index, SF-36, and visual acuity in patients undergoing cataract surgery. Ophthalmology. 2009;116:418–24.
Rosen PN, Kaplan RM, David K. Measuring outcomes of cataract surgery using the quality of Well-Being Scale and VF-14 Visual Function Index. J Cataract Refract Surg. 2005;31:369–78.
Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Hays RD, Berry S. National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute visual function questionnaire. Arch Ophthalmol. 2001;119:1050–8.
Awdeh RM, Elsing SH, Deramo VA, Stinnett S, Lee PP, Fekrat S. Vision-related quality of life in persons with unilateral branch retinal vein occlusion using the 25-item National Eye Institute Visual Function Questionnaire. Br J Ophthalmol. 2010;94:319–23.
Yamada M, Mizuno Y, Miyake Y. A multicenter study on the health-related quality of life of cataract patients: baseline data. Jpn J Ophthalmol. 2009;53:470–6.
Chatziralli IP, Sergentanis TN, Peponis VG, Papazisis LE, Moschos MM. Risk factors for poor vision-related quality of life among cataract patients. Evaluation of baseline data. Graefes Arch Clin Exp Ophthalmol. 2013;251:783–9.
Steinberg EP, Tielsch JM, Schein OD, Javitt JC, Sharkey P, Cassard SD, Legro MW, Diener-West M, Bass EB, Damiano AM. The VF-14. An index of functional impairment in patients with cataract. Arch Ophthalmol 1994;112 630–8.
Alonso J, Espallargues M, Andersen TF, Cassard SD, Dunn E, Bernth-Petersen P, Norregaard JC, Black C, Steinberg EP, Anderson GF. International applicability of the VF-14. An index of visual function in patients with cataracts. Ophthalmology. 1997;104:799–807.
Chatziralli IP, Papazisis L, Sergentanis TN. Ketorolac plus tobramycin/dexamethasone versus tobramycin/dexamethasone after uneventful phacoemulsification surgery: a randomized controlled trial. Ophthalmologica. 2011;225:89–94.
Laboratory of Experimental Ophthalmology of Aristotle University, Thessaloniki, Greece 2000. http://www.rand.org/health/surveys_tools/vfq.html.
Moshirfar M, Churgin DS, Hsu M. Femtosecond laser-assisted cataract surgery: a current review. Middle East Afr J Ophthalmol. 2011;18:285–91.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Irini P. Chatziralli, Theodoros N. Sergentanis, Efstratios A. Parikakis, Leonidas E. Papazisis, Panagiotis Mitropoulos and Marilita M. Moschos have nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Data Availability
The dataset analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/8527F0602EBCC959.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chatziralli, I.P., Sergentanis, T.N., Parikakis, E.A. et al. The Impact of Non-Steroidal Anti-Inflammatory Agents after Phacoemulsification on Quality of Life: A Randomized Study. Ophthalmol Ther 6, 133–140 (2017). https://doi.org/10.1007/s40123-016-0073-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-016-0073-3