Abstract
Introduction
Painful idiopathic distal sensory polyneuropathy (IDSP) and fibromyalgia syndrome (FMS) are cryptogenic chronic pain syndromes. The contribution of small fibre pathology (SFP) in FMS remains controversial. This study aims to quantify small nerve pathology in participants with IDSP and FMS and identify relationships of SFP with sensory phenotypes.
Methods
In this study, 73 individuals (FMS: 25, IDSP: 23, healthy volunteers: 25) underwent comprehensive assessment, including neurological exams, questionnaires, sensory tests, and corneal confocal microscopy.
Results
IDSP participants displayed lower wind-up ratio (WUR) relative to FMS (p < 0.001), loss of function to thermal and mechanical stimuli and elevated neuropathy disability scores compared to FMS and healthy volunteers (all p < 0.001). FMS participants demonstrated gain of function to heat and blunt pressure pain responses relative to IDSP, and healthy volunteers (heat: p = 0.002 and p = 0.003; pressure: both p < 0.001) and WUR (both p < 0.001). FMS participants exhibited reduced corneal nerve fibre density (p = 0.02), while IDSP participants had lower global corneal nerve measures (density, branch density, and length) relative to healthy volunteers (all p < 0.001). Utilising corneal nerve fibre length, SFP was demonstrated in 66.6% of participants (FMS: 13/25; IDSP: 22/23).
Conclusion
Participants with SFP, in both FMS and IDSP, reported symptoms indicative of small nerve fibre disease. Although distinctions in pain distributions are evident between individuals with FMS and IDSP, over 50% of participants between the two conditions displayed both a loss and gain of thermal and mechanical function suggestive of shared mechanisms. However, sensory phenotypes were associated with the presence of SFP in IDSP but not in FMS.
Plain Language Summary
In people with painful idiopathic neuropathy (pain related to nerve damage where the cause of nerve damage is unknown), fibromyalgia syndrome (a long-term condition causing widespread pain), and healthy volunteers, the small nerve fibres of the peripheral nervous system, which may be involved in generating pain were assessed. These nerve fibres can be measured at the front of the eye (cornea) which can provide details on whether they are damaged in the body. The response to temperature, light touch, pressure and pinprick stimuli can also be used to determine if there is a loss or gain of sensation, which may contribute to pain. The aim of this study was to identify the degree of damage to these nerve fibres and to determine whether this damage is associated with a loss (cannot feel or requires more intense stimulus to feel) or gain (stimulus is felt earlier or is painful earlier at lower intensity) of sensory function. The pattern of loss or gain in sensory function is known as a sensory phenotype. It was found that people with painful idiopathic neuropathy had more severe nerve damage, loss of function to temperature and touch, and fewer small nerve fibres in the cornea compared to those with fibromyalgia syndrome and healthy volunteers. People with fibromyalgia syndrome were more sensitive to heat and pressure and had fewer corneal nerve fibres relative to healthy volunteers. The presence of corneal nerve fibre damage was associated with sensory phenotypes (types of sensation felt) in painful idiopathic neuropathy but not in fibromyalgia syndrome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Why carry out this study? |
Painful idiopathic distal sensory polyneuropathy (IDSP) and fibromyalgia syndrome (FMS) are chronic pain conditions with unclear pathophysiology and substantial patient burden. |
Corneal nerve fibre deficits may provide objective evidence of a lesion of the somatosensory system. |
The contribution of small fibre pathology (SFP) in FMS is controversial, necessitating a detailed investigation to understand sensory phenotypes and their relationship with SFP. |
What did the study ask? |
This study aimed to quantify small nerve pathology in participants with IDSP and FMS and to identify the relationships between SFP and sensory phenotypes. |
What was learned from the study? |
IDSP participants were distinguished by pronounced sensory loss and higher neuropathy scores relative to FMS and healthy volunteers, with FMS participants characterised by a gain of function to heat and pressure pain. |
SFP was demonstrated in two-thirds of participants using corneal nerve fibre length, with IDSP showing greater corneal nerve fibre loss and FMS exhibiting reduced corneal nerve fibre density relative to healthy volunteers. |
Despite distinct pain distributions, half of participants from each condition had both a loss and gain of sensory function, suggesting shared mechanisms. |
Sensory phenotypes are associated with the presence of SFP in IDSP but not in FMS. |
Introduction
Fibromyalgia syndrome (FMS) and painful idiopathic distal sensory polyneuropathy (IDSP) are chronic pain conditions of unclear pathophysiology. The recently updated diagnostic criteria for idiopathic neuropathies published by Freeman and colleagues use IDSP as an umbrella term incorporating idiopathic small fibre, mixed fibre and large fibre neuropathy [1]. The global prevalence of FMS is ~ 2–7% with a predilection for women [2]. FMS is characterised by diffuse regional or widespread pain, while IDSP is typically a length-dependent process, presenting in a stocking and glove distribution, often advancing proximally with time [3]. Pain generation in FMS has traditionally been attributed to abnormal central mechanisms [4]. However, preclinical, and experimental human studies indicate a role for peripheral afferents in spontaneous diffuse pain and tenderness in FMS [5]. Studies have identified small fibre pathology (SFP) in FMS, including reduced intra-epidermal and corneal nerve fibres [6,7,8]. Similarly, reductions in corneal confocal microscopy (CCM) measures and intra-epidermal nerves have been demonstrated in IDSP [9, 10]. Our meta-analysis demonstrates that SFP occurs in approximately 50% of individuals with FMS [11]. This suggests a possible co-existing peripheral mechanism of pain generation and/or maintenance in a sub-group of participants with FMS. While there is evidence to support a sensory overlap between FMS and idiopathic neuropathy, there is a paucity of data which compare phenotypes and pathophysiology. The aim of this study was to compare sensory phenotypes and small nerve fibre pathology using CCM in people with IDSP and FMS. We hypothesised that there would be significant group differences in corneal nerve fibre metrics and quantitative sensory testing (QST) parameters between individuals with IDSP, FMS, and healthy volunteers. In addition, we hypothesised that the presence of SFP is associated with the resultant sensory phenotypes in these patient groups.
Research Design and Methods
Participants with IDSP were recruited sequentially from pain clinics at The Walton Centre. Participants with FMS were recruited from fibromyalgia services, musculoskeletal clinics and pain clinics at The Walton Centre and Aintree University Hospital sites. Ethical approval was obtained a priori (North West—Preston Research Ethics Committee – REC reference: 19/NW/0078; South West—Frenchay Research Ethics Committee REC reference: 20/SW/0138) and written informed consent was obtained from each participant and the study conduct adhered to good clinical practice guidelines and the tenets of the Declaration of Helsinki. Data were collected from two separate studies with similar protocols during a similar period of time.
Study Population
Between 2019 and 2022, 108 participants were screened and 73 adult participants were subsequently recruited (55 women, 18 men; mean age 49 ± 13 years). A total of 35 participants either could not participate or were excluded from the study. Eighteen participants reported being too unwell to participate or encountered logistical problems during the COVID-19 lockdowns in the United Kingdom. Fifteen participants did not meet eligibility criteria such as a diagnosis of diabetes mellitus, systemic disease, or corneal pathology. Participants with painful IDSP (n = 23) and FMS (n = 25) were recruited sequentially. Participants with a clinical diagnosis by exclusion of idiopathic SFN were included in this study. Participants were excluded if they reported or had a formal diagnosis of diabetes mellitus, systemic disease, corneal pathology, or co-morbid neurological conditions. All participants underwent laboratory tests as part of the study protocol to exclude other causes of SFP. Participants without a history of chronic pain and all above exclusions were recruited as healthy volunteers (n = 25).
Polyneuropathy Subtyping
The ACTTION criteria for IDSP were applied to further classify these participants according to signs and symptoms, QST, point-of-care nerve conduction studies, and CCM [12]. The classification of painful IDSP was accomplished through the utilisation of established and validated pain questionnaires. The diagnosis of primary FMS was based on current or past guideline criteria [13].
Sample Size Calculation
A sample size analysis determined the minimum sample size required to test the primary study hypothesis regarding participants with IDSP relative to healthy volunteers. The required sample size to achieve 80% power at a significance threshold of α = 0.05 was 17 patients in each group to detect a meaningful group difference in corneal nerve fibre length (CNFL). A post hoc power calculation was performed to account for the inclusion of participants with FMS. A power calculation using the group means and standard deviation of CNFL indicated a 94.6% power at a significance threshold of α = 0.05 with our included cohort.
Neurological Examination
All participants underwent neurological examination and a detailed assessment of neuropathy using the modified composite system neuropathy disability score (NDS). Vibration perception threshold of the hallux was measured using a neurothesiometer (Horwell Scientific, UK), with readings in millivolts (mV) and an abnormality defined as ≥ 15 mV.
Questionnaires
Neuropathic symptoms, location and pain characteristics were assessed using the Neuropathy Symptom Profile (NSP), McGill Short Form Questionnaire (MPQ), PainDetect (PDQ) and Small Fibre Neuropathy Screening List (SFNSL). The composite scores of the SFNSL and PDQ were used to indicate the probability of small fibre neuropathy (SFN) and a neuropathic pain component, respectively.
Point-of-Care Nerve Conduction
Sural nerve conduction velocity (SNCV) and amplitude (SNAP) were assessed in the right lower limb using the NC-Stat DPN Check (Neurometrix, Waltham, MA, USA) point-of-care device. Nerve conduction abnormality was determined by ≥ 1 measure below threshold (SNCV: ≤ 44 m/s; SNAP: < 6 µV) [14]. Current perception threshold testing with the Neurometer (MDE Diagnostics, Budapest, Hungary) was undertaken in a subgroup of participants with IDSP and healthy volunteers on the right index finger (C7) at the following frequencies: 2000, 250 and 5 Hz.
Quantitative Sensory Testing (QST)
All participants underwent QST of the right forearm according to the German Research Network on Neuropathic Pain (DNFS) protocol [15]. Participants with IDSP also underwent QST of the dorsum of the foot. For analysis, raw QST data were logarithmically transformed, followed by a Z-transformation to standardise all values (except paradoxical heat sensations and dynamic mechanical allodynia). This was performed by subtracting the published age and sex reference population mean for each participant’s raw scores for every parameter and dividing this value by the reference population standard deviation [16]. A positive Z-score value denotes a gain in function, and a negative Z-score denotes a loss of function in each parameter. This is a standard research methodology allowing comparisons between participants differing in age and sex.
Sensory Phenotypes
The frequency of sensory abnormalities and their combinations was characterised using the “L0G0” coding system as described by Maier and colleagues [17]. A loss of function was defined as two standard deviations (≤ 1.96) below 0 of the normalised published reference data from the DNFS [18]. Loss of function to thermal stimuli [cold detection threshold (CDT); warm detection threshold (WDT)] was coded as L1; loss of function to mechanical detection threshold (MDT) or vibration detection threshold (VDT) was coded as L2. Gain of function was defined as two standard deviations (≥ 1.96) above 0 of the published reference data from the DNFS. Gain of function to cold (CPT) or heat (HPT) pain threshold was coded as G1; gain of function to the mechanical pain threshold (MPT), mechanical pain sensitivity (MPS), dynamic mechanical allodynia (DMA) or pressure pain threshold (PPT) was coded as G2. When both thermal and mechanical abnormalities were present, this was coded as L3 or G3, respectively. Participants without a detected loss or gain were coded as L0G0. These codes were subsequently categorised into one of four patterns: no abnormality detected, loss of function, gain of function, and both loss and gain of function.
Corneal Confocal Microscopy (CCM)
CCM was performed using a standard internationally accepted protocol in both eyes using a laser scanning corneal confocal microscopy device (Heidelberg Retina Tomograph 3 Rostock Cornea Module; Heidelberg Engineering, Heidelberg, Germany) to acquire images of the corneal sub-basal plexus. On average, 6–8 images (3–4 from each eye) were selected in a masked fashion (from group allocation) according to image quality and apical positioning of the central sub-basal nerve plexus. Automated image analysis was undertaken using ACCMetrics in a masked and randomised manner. The corneal metrics quantified include corneal nerve fibre density (CNFD), corneal nerve fibre branch density (CNBD), and corneal nerve fibre length (CNFL). Published automated corneal nerve fibre thresholds were used to indicate abnormal corneal nerve counts and thus the threshold for SFP [19]. These were: CNFL < 15.3 mm/mm2; CNBD < 18.7 branches/mm2; CNFD < 16.7 fibres/mm2. Participants were considered to have SFP if they were below the CNFL threshold.
Statistical Analysis
The statistical package R (v.4.0.2) was used for statistical analysis. Data were assessed for normality using quantile–quantile plots and Shapiro–Wilk test. Summary statistics were reported as mean ± standard deviation or median ± interquartile range as appropriate. Groups (IDSP, FMS and healthy volunteers) were compared using a one-way ANOVA followed by a Tukey’s HSD test or Kruskal–Wallis test followed by a Dunn’s test for multiple comparisons. For two group comparisons, parametric data and non-parametric variables were evaluated using an independent t est or Mann–Whitney U test, respectively. For all tests, a p value of < 0.05 after corrections for multiple comparisons was considered statistically significant. Categorical data were reported as frequencies and percentages by group. The mode of each item was used to broadly characterise groups as an indication of intensity, severity, or frequency of each descriptor on a Likert or ordinal scale. Fisher’s exact test was used to test the association between the presence of SFP and sensory phenotype.
Results
Clinical and Laboratory Patient Characteristics
Data from 73 participants were included in this analysis. Participants with IDSP were older relative to healthy volunteers (Table 1). There was a comparable duration in symptoms prior to a formal diagnosis and disease duration after diagnosis in participants with IDSP and FMS. Overall, blood tests and anthropometrics were unremarkable between the three groups. Participants with FMS or IDSP were prescribed 1 (n = 6, 12.5%), or ≥ 2 (n = 27, 56.3%) analgesic medicines. In this study, participants were on a diverse range of medications, making it challenging to conduct a systematic assessment of how these drugs influenced our findings.
Neurological Examination and Questionnaires
Participants with IDSP scored higher on the neuropathy disability score relative to participants with FMS and healthy volunteers (Table 2). Participants with FMS and IDSP had higher scores in the SFNSL, MPQ, PDQ and NSP questionnaires relative to healthy volunteers (Table 2). The total scores of NSP and SFNSL were higher in participants with FMS compared to IDSP participants. Pain scores were comparable between the two groups.
Pain Characteristics
The total cohort frequently reported deep aching pain, burning discomfort, sensitivity to touch and symptoms consistent with paraesthesia, with over half evaluating their experience as punishing or cruel (Fig. 1A–C). Participants with FMS characterised their pain as throbbing and tender, often triggered by slight pressure and overall evaluating the experience as exhausting (Fig. 1D). In contrast, IDSP participants described their pain as stabbing or sharp. Sudden pain attacks and radiating pains were reported by most participants (Fig. 1B, C).
Summary of self-reported outcomes of participants with Idiopathic Distal Sensory Polyneuropathy (IDSP) and fibromyalgia syndrome (FMS). A Participants with IDSP presented with an acral pain distribution which more frequently involved both the feet and hands. B Participants with FMS more frequently reported symptoms across three or more regions including distal and proximal regions of the body. C Participants with FMS and IDSP reported radiating pains at a similar rate but elected different dynamic descriptors of their pain. The most frequently elected pain dynamic in participants with IDSP was ‘pain attacks with pain between them’ while participants with FMS commonly elected 'persistent pain with pain attacks' to represent their pain. D Diverging bar chart showing the McGill Short-Form Pain Questionnaire (MPQ) descriptors of participants with FMS (left) and IDSP (right). E Diverging bar chart showing the Pain Detect Questionnaire (PDQ) responses of participants with FMS (left) and IDSP (right). F Diverging bar chart showing the frequency of SFNSL symptoms reported. G Diverging bar chart showing the seriousness of SFNSL symptoms reported
Pain Location
In participants with FMS, pain was reported more frequently in the shoulders and neck (70.8%), back and upper chest (71%), legs (67%), and lower back and buttocks (63%). In IDSP, all participants reported pain in acral distributions, over a third reported pain in their feet only (39%), both hands and feet (52%), hands only (9%) and over half (57%) also had pain in their legs. Pain in FMS participants was frequently widespread (63%) or regional (38%), encompassing both proximal and distal distributions or two or more proximal sites (Table S1).
Autonomic Symptoms
Participants with FMS frequently reported muscle weakness, painful arms, muscle cramps, and autonomic symptoms including bowel problems, orthostatic hypotensive and sicca symptoms, and rated hypersensitivity of their legs, hot flushes, and tingling sensations as their most distressing symptoms (Fig. 1G).
Quantitative Sensory Testing (QST)
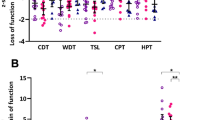
Participants with IDSP demonstrated a loss of function to thermal (WDT, CDT and TSL; all p < 0.001), tactile (MDT; p < 0.001) and wind-up ratio (WUR) (WUR; p < 0.001) stimuli relative to FMS and control groups. Participants with FMS had reduced tactile detection thresholds (MDT; p = 0.01) and increased responsiveness to mechanical stimulation (MPS; p = 0.01) and allodynia (DMA; p = 0.008) at the group level, relative to healthy volunteers (Fig. 2; Table 2). Participants with FMS exhibited gain of function to heat pain (HPT; IDSP: p = 0.002; healthy volunteers: p = 0.003) blunt pressure (PPT; both p < 0.001) and WUR (WUR; both p < 0.001) stimuli relative to IDSP and healthy volunteers.
Quantitative sensory testing (QST) and corneal confocal microscopy measures (CCM) in participants with idiopathic distal sensory polyneuropathy (IDSP) and fibromyalgia syndrome (FMS). Top The Z-scores of individual participants by group of thermal (A) and mechanical (B) QST measures represented as a dot plot. The error bars indicate the 95% confidence interval. Idiopathic SFN idiopathic small fibre neuropathy (orange); FMS fibromyalgia syndrome (green); HV healthy volunteers (dark blue). Bottom Representative CCM images (C left) from a healthy volunteer, a participant with fibromyalgia syndrome (FMS) and idiopathic distal sensory polyneuropathy (IDSP). CCM measurements from individual participants by group represented as a raincloud plot. The raincloud plot is a box plot with individual data points with the group distribution represented as a ‘raincloud’. As can be seen, there appears to be a binomial distribution in corneal nerve branch density in the fibromyalgia syndrome group. CDT cold detection threshold, CNBD corneal nerve branch density, CNFD corneal nerve fibre density, CNFL corneal nerve fibre length, CPT cold pain threshold, HPT heat pain threshold, MDT mechanical detection threshold, MPS mechanical pain sensitivity, MPT mechanical pain threshold, PPT pressure pain threshold, TSL thermal sensory limen, VDT vibration detection threshold, WDT warm detection threshold, WUR wind-up ratio
Corneal Confocal Microscopy
Corneal sub-basal nerve plexus measures CNFL, CNFD and CNBD were reduced in participants with IDSP relative to healthy volunteers (all p < 0.001). In participants with FMS, CNBD was reduced relative to healthy volunteers (p = 0.02) (Table 2; Fig. 2C).
Sensory Phenotypes
Half of the participants (FMS: 11/25; IDSP: 16/23) exhibited both gain and loss of function to thermal and/or mechanical stimuli (Table 3). Sensory loss was more frequent in IDSP (IDSP: 6/23; FMS: 1/25), while FMS participants frequently had gain of function only (FMS: 12/25; IDSP: 1/23). According to the SFNSL, over 83% of participants (FMS: 24/25; IDSP: 16/23) had small fibre symptoms (Table 3). Additionally, more than two-thirds (FMS: 21; IDSP: 16; Total: 37/48) were indicated to have a > 90% probability of a neuropathic pain component according to the PDQ.
Small Fibre Pathology
Signs and symptoms suggestive of small fibre neuropathy, along with reduced corneal nerve fibre length, were found in 66% of participants (IDSP: 19/23; FMS: 13/25; Total: 34/48). The presence of SFP presence was significantly associated with sensory phenotypes in IDSP (p = 0.017) but not in FMS (p ≥ 0.9).
Discussion
The primary aim of this study was to identify small nerve fibre abnormalities in participants with IDSP and FMS and to relate those to neuropathic pain phenotypes. Using physical examination, pain questionnaires and QST, we have demonstrated signs, symptoms, and objective evidence of SFP in over two-thirds of our included participants. Our data demonstrate that sensory loss is more frequently associated with participants with IDSP, although a sub-group of participants with FMS and IDSP exhibit sensory loss concurrent with a gain of function to thermal and/or mechanical stimuli. Despite this, participants with FMS more frequently exhibit increased sensitivity to heat and pressure pain, despite a mild loss of thermal and/or mechanical detection function.
Approximately 50% of participants with FMS showed signs of SFP in either CNFL or CNBD. Our CCM findings broadly agree with findings presented by Klitsch and colleagues of moderate reductions in CNFL and CNFD seen in participants with FMS, and greater decrements in all corneal nerve metrics in participants with SFN [20]. We found participants with IDSP had loss of function in mechanical, thermal and vibration senses. Similar findings in mixed-fibre polyneuropathies and SFN have been reported [21]. Interestingly, participants with SFN exhibited thermal hyperalgesia and mechanical hyperalgesia, while mixed-fibre polyneuropathies were more frequently associated with sensory loss [21]. Unfortunately, there were too few participants with pure isolated SFN to meaningfully test this.
Small fibre damage has been associated with abnormalities in thermal and mechanical parameters [6, 21,22,23]. A recently published retrospective cohort study identified pathological alterations in corneal nerve fibre metrics, with FMS participants primarily exhibiting differences in branch density [24]. We also identified prevalent thermal and mechanical loss of function in IDSP and found that participants with FMS showed a gain of function to pressure pain. Both IDSP and FMS exhibit a comparable prevalence of mechanical and thermal hyperalgesia. However, mechanical hyperalgesia and sensory loss rarely occur in isolation in IDSP and FMS, respectively. Moreover, we found that SFP was associated with neuropathic pain phenotype in IDSP, but not in FMS. This is similar to recently published studies which indicates that SFP may not be associated with sensory phenotype in FMS [23].
Our data demonstrate that participants with FMS report burning pain and paraesthesia, with 84% of participants likely to have a neuropathic pain component. These findings agree with previous studies [7, 8]. However, the certainty of the evidence for using the PDQ for the likelihood of a neuropathic pain component is deemed to be low by the Special Interest Group of the International Association for the Study of Pain [25]. This might also clarify why a neuropathic pain component was considered likely in only 70% of participants with painful IDSP. Indeed, symptoms alone are unreliable for a diagnosis of small fibre neuropathy and require an objective test such as abnormal intra-epidermal nerve fibre density (IENFD) at the distal leg [26]. The Besta criteria propose that such clinical signs and symptoms be used in conjunction with QST and IENFD for a reliable diagnosis. The proposed IDSP criteria by ACTTION provide a more comprehensive framework for sub-grouping using symptomatology, neurological examination, exclusion of other conditions, together with QST and IENFD to delineate small from mixed-fibre polyneuropathy [12].
Notably, both FMS and idiopathic IDSP groups showed a trend towards reduced tactile detection and increased mechanical pain sensitivity, a characteristic noted in previous studies [27, 28]. Participants with IDSP exhibited reduced pain summation while participants with FMS retained sensitivity to pressure pain suggestive of differing pathophysiology. Overall, participants with IDSP and FMS with SFP demonstrated similarities.
Both sensory loss and hyperalgesia can co-occur after neuronal injury [29], leading to both ascending and descending alterations along the neuroaxis [30]. Sensory characteristics associated with neuropathic pain have been successfully replicated in studies involving healthy volunteers through experimental neuropathic pain models [31]. These characteristics seem to align with proposed models explaining the generation and persistence of pain [32]. Importantly, the use of neuropathic pain phenotypes enables the categorization of individuals into different response groups regarding analgesic medications [33]. Indeed, surrogate models of central sensitisation, including those induced by capsaicin, heat, and electrical stimulation, have demonstrated pharmacological profiles that exhibited greater responsiveness to antihyperalgesic medicines [34].
Widespread FMS pain is accompanied by tenderness and deep aching pain of the muscles. Interestingly, lidocaine injections increased local pain-thresholds and decreased secondary thermal hyperalgesia in participants with FMS [5, 35]. These data suggest pain generation and maintenance through peripheral afferents and descending pain inhibition. Indeed, abnormal spontaneous activity of peripheral c-nociceptors have been demonstrated in FMS and SFN [36]. After-sensations and anhedonia in response to c-afferent stimuli have been reported in participants with FMS, suggesting peripheral c-fibre pathology may contribute to hyperalgesia and FMS pain [6, 8, 37, 38]. Furthermore, both ISDP and FMS are characterised by chronic nociceptive input which worsens at night and disrupted sleep which are implicated in central sensitisation and pain amplification [39, 40]. An immune mediated mechanism has been suggested to be a cause in some individuals with FMS, since passive transfer of IgG from participants with FMS was demonstrated, resulting in peripheral sensitisation of nociceptive afferents in mice [41]. Indeed, a potential incipient pro-inflammatory insult may instigate both peripheral and central sensitivity in participants with FMS and IDSP, representing a possible shared mechanism [30, 42].
A potential limitation of the present study is the sample size, which was further reduced in subsequent exploratory sub-group analyses. Further, the selection of the right forearm for QST may not be reflective of small fibre function of other dermatomes. This is important considering the widespread and regional nature of sensory disturbances and pain reported by participants with FMS and the contrasting stocking and glove distribution seen in participants with IDSP. Additionally, contrasts between participants with FMS and IDSP may be more pronounced at the dorsum of the foot. The lack of formal nerve conduction studies of the peroneal, tibial, radial, median, and ulnar motor and sensory nerves would facilitate a more precise delineation between small and mixed-fibre neuropathies. Furthermore, a pure sample of participants with small fibre neuropathy would require a formalised screening protocol performed close to the date of testing within one dedicated laboratory to limit sample testing variability. Reductions of cutaneous innervation are evidenced in SFN and are recommended for the diagnosis of neuropathic pain [25]. While CCM has shown utility in the diagnosis of peripheral neuropathy, structural deficits of intra-epidermal nerves in IDSP and FMS would have strengthened our findings. However, evidencing a lesion or disease of the somatosensory system using CCM in patients with an irregular distribution of pain could prove useful considering the lack of established normative thresholds for intra-epidermal nerve fibre density in proximal or regional distributions.
Conclusion
We have demonstrated SFP in most participants with IDSP and half of participants with FMS. Despite clinical heterogeneity, the presence of SFP is broadly associated with sensory loss in IDSP but not in FMS. While there is overlap in the symptomatology of IDSP and FMS, the presence of SFP does not appear to be associated with the sensory phenotype in FMS. These findings highlight the importance of refining diagnostic criteria. Future studies should include longitudinal multi-modal imaging of the neuroaxis and peripheral nervous system to delineate the precise pathophysiology of these conditions, enabling more targeted treatments and improved clinical outcomes.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request but may require data transfer agreement and associated costs.
References
Freeman R, Gewandter JS, Faber CG, Gibbons C, Haroutounian S, Lauria G, Levine T, Malik RA, Singleton JR, Smith AG, Bell J, Dworkin RH, Feldman E, Herrmann DN, Hoke A, Kolb N, Mansikka H, Oaklander AL, Peltier A, Polydefkis M, Ritt E, Russell JW, Sainati S, Steiner D, Treister R, Üçeyler N. Idiopathic distal sensory polyneuropathy. Neurology. 2020;95(22):1005.
Marques AP, Santo ASE, Berssaneti AA, Matsutani LA, Yuan SLK. Prevalence of fibromyalgia: literature review update. Revista Brasileira de Reumatologia (English Edition). 2017;57(4):356–63.
Häuser W, Brähler E, Ablin J, Wolfe F. Modified 2016 American College of Rheumatology Fibromyalgia Criteria, the Analgesic, Anesthetic, and Addiction Clinical Trial Translations Innovations Opportunities and Networks-American Pain Society Pain Taxonomy, and the prevalence of fibromyalgia. Arthritis Care Res (Hoboken). 2021;73(5):617–25.
Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2-s15.
Affaitati G, Costantini R, Fabrizio A, Lapenna D, Tafuri E, Giamberardino MA. Effects of treatment of peripheral pain generators in fibromyalgia patients. Eur J Pain. 2011;15(1):61–9.
Evdokimov D, Frank J, Klitsch A, Unterecker S, Warrings B, Serra J, Papagianni A, Saffer N, Meyer Zu Altenschildesche C, Kampik D, Malik RA, Sommer C, Üçeyler N. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann Neurol. 2019;86(4):504–16.
Giannoccaro MP, Donadio V, Incensi A, Avoni P, Liguori R. Small nerve fiber involvement in patients referred for fibromyalgia. Muscle Nerve. 2014;49(5):757–9.
Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, Casanova-Molla J, Reiners K, Sommer C. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136(Pt 6):1857–67.
Tavakoli M, Marshall A, Pitceathly R, Fadavi H, Gow D, Roberts ME, Efron N, Boulton AJ, Malik RA. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol. 2010;223(1):245–50.
Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, Broglio L, Granieri E, Lauria G. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131(Pt 7):1912–25.
Grayston R, Czanner G, Elhadd K, Goebel A, Frank B, Üçeyler N, Malik RA, Alam U. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: implications for a new paradigm in fibromyalgia etiopathogenesis. Semin Arthritis Rheum. 2019;48(5):933–40.
Freeman R, Gewandter JS, Faber CG, Gibbons C, Haroutounian S, Lauria G, Levine T, Malik RA, Singleton JR, Smith AG, Bell J, Dworkin RH, Feldman E, Herrmann DN, Hoke A, Kolb N, Mansikka H, Oaklander AL, Peltier A, Polydefkis M, Ritt E, Russell JW, Sainati S, Steiner D, Treister R, Üçeyler N. Idiopathic distal sensory polyneuropathy: ACTTION diagnostic criteria. Neurology. 2020;95(22):1005–14.
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–29.
Scarr D, Lovblom LE, Cardinez N, Orszag A, Farooqi MA, Boulet G, Weisman A, Lovshin JA, Ngo M, Paul N, Keenan HA, Brent MH, Cherney DZ, Bril V, Perkins BA. Validity of a point-of-care nerve conduction device for polyneuropathy identification in older adults with diabetes: results from the Canadian Study of Longevity in Type 1 Diabetes. PLoS One. 2018;13(4): e0196647.
Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10(1):77–88.
Magerl W, Krumova EK, Baron R, Tölle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151(3):598–605.
Maier C, Baron R, Tölle TR, Binder A, Birbaumer N, Birklein F, Gierthmühlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihöfner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uçeyler N, Valet M, Wasner G, Treede RD. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150(3):439–50.
Magerl W, Krumova EK, Baron R, Tolle T, Treede R-D, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;3:598.
Perkins BA, Lovblom LE, Bril V, Scarr D, Ostrovski I, Orszag A, Edwards K, Pritchard N, Russell A, Dehghani C, Pacaud D, Romanchuk K, Mah JK, Jeziorska M, Marshall A, Shtein RM, Pop-Busui R, Lentz SI, Boulton AJM, Tavakoli M, Efron N, Malik RA. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia. 2018;61(8):1856–61.
Klitsch A, Evdokimov D, Frank J, Thomas D, Saffer N, Meyer Zu Altenschildesche C, Sisignano M, Kampik D, Malik RA, Sommer C, Üçeyler N. Reduced association between dendritic cells and corneal sub-basal nerve fibers in patients with fibromyalgia syndrome. J Peripher Nerv Syst. 2020;25(1):9–18.
Üçeyler N, Vollert J, Broll B, Riediger N, Langjahr M, Saffer N, Schubert AL, Siedler G, Sommer C. Sensory profiles and skin innervation of patients with painful and painless neuropathies. Pain. 2018;159(9):1867–76.
Doppler K, Rittner HL, Deckart M, Sommer C. Reduced dermal nerve fiber diameter in skin biopsies of patients with fibromyalgia. Pain. 2015;156(11):2319–25.
Leone C, Galosi E, Esposito N, Falco P, Fasolino A, Di Pietro G, Di Stefano G, Camerota F, Vollert J, Truini A. Small-fibre damage is associated with distinct sensory phenotypes in patients with fibromyalgia and small-fibre neuropathy. Eur J Pain. 2023;27(1):163–73.
Jänsch S, Evdokimov D, Egenolf N, Meyer zu Altenschildesche C, Kreß L, Üçeyler N. Distinguishing fibromyalgia syndrome from small fiber neuropathy: a clinical guide. PAIN Reports 2024;9(1).
Truini A, Aleksovska K, Anderson CC, Attal N, Baron R, Bennett DL, Bouhassira D, Cruccu G, Eisenberg E, Enax-Krumova E, Davis KD, Di Stefano G, Finnerup NB, Garcia-Larrea L, Hanafi I, Haroutounian S, Karlsson P, Rakusa M, Rice ASC, Sachau J, Smith BH, Sommer C, Tölle T, Valls-Solé J, Veluchamy A. Joint European Academy of Neurology-European Pain Federation-Neuropathic Pain Special Interest Group of the International Association for the Study of Pain guidelines on neuropathic pain assessment. Eur J Neurol. 2023;30(8):2177–96.
Devigili G, Rinaldo S, Lombardi R, Cazzato D, Marchi M, Salvi E, Eleopra R, Lauria G. Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain. 2019;142(12):3728–36.
Egenolf N, Zu Altenschildesche CM, Kreß L, Eggermann K, Namer B, Gross F, Klitsch A, Malzacher T, Kampik D, Malik RA, Kurth I, Sommer C, Üçeyler N. Diagnosing small fiber neuropathy in clinical practice: a deep phenotyping study. Ther Adv Neurol Disord. 2021;14:17562864211004318.
Fasolino A, Di Stefano G, Leone C, Galosi E, Gioia C, Lucchino B, Terracciano A, Di Franco M, Cruccu G, Truini A. Small-fibre pathology has no impact on somatosensory system function in patients with fibromyalgia. Pain. 2020;161(10):2385–93.
Schmelz M. Lessons learned—moving on from QST sensory profiles. Scand J Pain. 2022;22(4):670–2.
Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52(1):77–92.
Vollert J, Magerl W, Baron R, Binder A, Enax-Krumova EK, Geisslinger G, Gierthmühlen J, Henrich F, Hüllemann P, Klein T, Lötsch J, Maier C, Oertel B, Schuh-Hofer S, Tölle TR, Treede RD. Pathophysiological mechanisms of neuropathic pain: comparison of sensory phenotypes in patients and human surrogate pain models. Pain. 2018;159(6):1090–102.
Vollert J. Sensory testing might not be perfect– but it is the best biomarker for pain phenotypes we have right now. Scandinavian J Pain. 2022;22(4):673–5.
Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, Jensen TS, Sindrup SH. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014;155(11):2263–73.
Quesada C, Kostenko A, Ho I, Leone C, Nochi Z, Stouffs A, Wittayer M, Caspani O, Brix Finnerup N, Mouraux A, Pickering G, Tracey I, Truini A, Treede RD, Garcia-Larrea L. Human surrogate models of central sensitization: a critical review and practical guide. Eur J Pain. 2021;25(7):1389–428.
Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain. 2009;145(1–2):96–104.
Serra J, Collado A, Solà R, Antonelli F, Torres X, Salgueiro M, Quiles C, Bostock H. Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol. 2014;75(2):196–208.
Berwick RJ, Andersson DA, Goebel A, Marshall A. Aftersensations and lingering pain after examination in patients with fibromyalgia syndrome. Pain Med. 2022;23(12):1928–38.
Morrison I, Löken LS, Minde J, Wessberg J, Perini I, Nennesmo I, Olausson H. Reduced C-afferent fibre density affects perceived pleasantness and empathy for touch. Brain. 2011;134(Pt 4):1116–26.
Choy EH. The role of sleep in pain and fibromyalgia. Nat Rev Rheumatol. 2015;11(9):513–20.
Vierck CJ Jr. Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia). Pain. 2006;124(3):242–63.
Goebel A, Krock E, Gentry C, Israel MR, Jurczak A, Urbina CM, Sandor K, Vastani N, Maurer M, Cuhadar U, Sensi S, Nomura Y, Menezes J, Baharpoor A, Brieskorn L, Sandström A, Tour J, Kadetoff D, Haglund L, Kosek E, Bevan S, Svensson CI, Andersson DA. Passive transfer of fibromyalgia symptoms from patients to mice. J Clin Invest. 2021. https://doi.org/10.1172/JCI144201.
Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32(12):611–8.
Acknowledgements
The author team would like to acknowledge the University of Liverpool, Institute of Life Course and Medical Sciences and the Shared Research Facility Histology laboratory. We acknowledge MDE diagnostics for providing Neurometer equipment for current perception threshold testing. MDE diagnostics did not contribute to the funding or design of this study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
J.B. received a PhD studentship from the Pain Relief Foundation. Participants with fibromyalgia syndrome were included from DEFINE-FMS funded by Versus Arthritis. Grant number 22471. The funding for this article was waived.
Author information
Authors and Affiliations
Contributions
All persons who meet authorship criteria are listed as authors. All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Conceptualization: Uazman Alam, Bernhard Frank, Andrew Marshall, Stephen Kaye, and Jamie Burgess. Writing—original draft preparation: Jamie Burgess, Anne Marshall, Leandros Rapteas, and Uazman Alam. Writing—review and editing: Jamie Burgess, Anne Marshall, Kohei Matsumoto, David Riley, Leandros Rapteas, Cheng Boon, Alia Alchawaf, Maryam Ferdousi, Rayaz A. Malik, David Gosal, Andrew Marshall, Bernhard Frank, Stephen Kaye, and Uazman Alam. Visualization: Jamie Burgess. Supervision: Uazman Alam, Andrew Marshall, Bernhard Frank, Stephen Kaye.
Corresponding author
Ethics declarations
Conflict of Interest
All authors; Jamie Burgess, Anne Marshall, Leandros Rapteas, David Riley, Kohei Matsumoto, Cheng Boon, Alia Alchawaf, Maryam Ferdousi, Rayaz A. Malik, Andrew Marshall, Stephen Kaye, David Gosal and Bernhard Frank declare that they have no competing interests relevant to the current publication. Uazman Alam has no direct conflict of interest but discloses the following: honoraria received from Procter & Gamble, Viatris, Eli Lilly, Grunenthal, and Sanofi for educational meetings; investigator-led funding from Procter & Gamble; and sponsorship for travel to an international conference from Daiichi Sankyo.
Ethical Approval
Ethical approval was obtained a priori (North West – Preston Research Ethics Committee—REC reference: 19/NW/0078; South West—Frenchay Research Ethics Committee REC reference: 20/SW/0138) and written informed consent was obtained from each participant and the study conduct adhered to good clinical practice guidelines and the tenets of the Declaration of Helsinki. Data was collected from two separate studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Burgess, J., Marshall, A., Rapteas, L. et al. Idiopathic Distal Sensory Polyneuropathy and Fibromyalgia Syndrome: A Comparative Phenotyping Study. Pain Ther (2024). https://doi.org/10.1007/s40122-024-00646-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40122-024-00646-x