Abstract
Introduction
Interventional treatment options for the lumbar degenerative spine have undergone a significant amount of innovation over the last decade. As new technologies emerge, along with the surgical specialty expansion, there is no manuscript that utilizes a review of surgical treatments with evidence rankings from multiple specialties, namely, the interventional pain and spine communities. Through the Pacific Spine and Pain Society (PSPS), the purpose of this manuscript is to provide a balanced evidence review of available surgical treatments.
Methods
The PSPS Research Committee created a working group that performed a comprehensive literature search on available surgical technologies for the treatment of the degenerative spine, utilizing the ranking assessment based on USPSTF (United States Preventative Services Taskforce) and NASS (North American Spine Society) criteria.
Results
The surgical treatments were separated based on disease process, including treatments for degenerative disc disease, spondylolisthesis, and spinal stenosis.
Conclusions
There is emerging and significant evidence to support multiple approaches to treat the symptomatic lumbar degenerative spine. As new technologies become available, training, education, credentialing, and peer review are essential for optimizing patient safety and successful outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Low back pain due to degenerative spinal disease is one of the most common and costly forms of musculoskeletal pain, with an individual lifetime prevalence of approximately 49–90%. |
There are multiple surgical approaches to the treatment of the symptomatic lumbar degenerative spine but no balanced review article up to this point. |
The purpose of this article is to review and grade the current evidence for commercially available surgical treatments in the United States. |
Spinal surgery has been the staple in treatment of spinal pathology, now along with newly emerging minimally invasive techniques. |
Introduction
Degenerative disorders of the spine have many etiologies, including hypertrophied ligamentum flavum, facet joint hypertrophy, degenerative disc disease, and osteophyte formation. This cascade of degenerative changes is largely the result of aging, with a multitude of causes [1, 2]. Although symptoms may manifest in adolescence or early adulthood, the majority of patients present in the 6th, 7th, or 8th decades of life [3]. Acquired lumbar spinal stenosis (LSS), which is more prevalent, is a result of degenerative spondylosis, spondylolisthesis, synovial cysts, annular bulges, ligamentum flavum hypertrophy, facet hypertrophy, post-surgical fibrosis, other rheumatological and skeletal conditions, or a combination of these factors [4].
Spine surgery has undergone a significant evolution over the course of the last decade. The adoption of minimally invasive surgical techniques for treatment of lumbar spine pathology has resulted in lower complication rates, less blood loss, quicker recovery, and improved patient outcomes [3]. Although several surgical treatment options exist for those with degenerative spinal disease, discussion regarding patient selection characteristics and considerations has not been performed with a clear working group with equitable representation of surgical and interventional pain backgrounds.
Pacific Spine and Pain Society (PSPS) is a group of specialists that span multiple medical disciplines that diagnose, manage, and treat pain and spine disorders. By enhancing collaboration amongst surgical and interventional pain specialists, the society aims to enhance patient care and embrace innovation. This comprehensive treatment evidence review for lumbar degenerative disease is the first of its kind, assimilating information from both the spine and pain literature. As such, traditional evidence grading strategies were chosen for this pioneering effort, from the pain and spine literature, consistent with those commonly employed in each space.
Through the PSPS, the purpose of this manuscript is to provide a comprehensive evidence review of available surgical treatments for lumbar degenerative disease. This manuscript aims to utilize an unbiased and multidisciplinary approach in reviewing the evidence to foster collaboration between the spine and interventional pain communities. While it is not feasible to include every possible surgical treatment for lumbar degenerative disease, we hope to include the most commonly utilized surgical approaches.
Methods
The PSPS research committee members assigned, and oversaw the literature search methods, evidence table generation with validated assignment of evidence level based on the USPSTF (United States Preventative Services Taskforce) and NASS (North American Spine Society) evidence ranking criteria and edited and compiled the manuscript. Therefore, section authors performed a comprehensive literature search of PubMed, Ovid, and Google Scholar using key terms such as “lumbar spine surgery”, “lumbosacral spine surgery”, “lumbar degenerative disc disease”, “spondylolisthesis”, “lumbar spine stenosis”, “neurogenic claudication”, “posterior indirect spinal decompression”, “minimally invasive spinal decompression”, “interspinous fusion,” “interspinous spacer, and “discogenic pain treatment”.
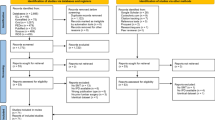
Articles were included if they were randomized controlled trials or systematic review and meta-analyses of randomized controlled trials with at least 1 year of follow-up. Retrospective comparative studies, case–control studies, case series, and articles with shorter than 1 year of follow-up were excluded. Prospective comparative studies are included if there were no randomized controlled trials available. For emerging technologies or treatments that lacked prospective randomized data with follow-up greater than a year, the available evidence is described and assigned the appropriate evidence grade. For reference, see Fig. 1.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Based on literature search, surgical treatments were separated by diagnoses of lumbar (1) degenerative disc disease, (2) spondylolisthesis, (3) spinal stenosis. The evidence ranking was performed by the individual working group authors and by two additional working group members for validation. Sections were defined by disease indication and evidence for each surgical treatment, with grades from USPSTF and NASS. USPSTF is commonly employed in the interventional pain literature as a methodology of criteria [5], where for the neurosurgical and orthopedic communities, the common evidence ranking nomenclature and methodology of the NASS were utilized also, therefore, each study reviewed received two evidence-level rankings.
The Level of Evidence, based on the current USPSTF criteria, adapted by NACC (Neuromodulation Appropriateness Consensus Committee) [6] and PACC (Polyanalgesic Consensus Conference) Guidance [7], as outlined in Tables 1 and 2.
Evidence on Surgical Treatments for Degenerative Disc Disease
Surgical treatment for lumbar degenerative disc disease (DDD) remains controversial. Delamination of the disc and posterior annular fissuring result in low back pain due to the mechanical loading to these areas, eventually resulting in sensitization of annular receptors [9]. During the degenerative cascade, neovascularization, neuronal penetration with unmyelinated nerve fibers and in growth of Schwann cells occurs and this neo‐innervation is a potential pain generator [1, 2, 6]. The most recent clinical guidelines from the NASS regarding diagnosis and treatment of low back pain determined that there were no studies to adequately address whether surgical vs. medical treatment alone decreased intensity of pain, decreased the duration of pain, increased the functional outcomes of treatment and improved return-to-work rate.
The two main surgical treatments for lumbar DDD are (1) lumbar fusion, traditional standard surgical treatment, and (2) lumbar disc arthroplasty (LDA), which is also known as total disc replacement (TDR). Lumbar fusion aims to relieve pain by fusing vertebrae together to eliminate movement and thus stabilizing the spinal segment. Although lumbar fusion is the most traditionally used treatment for DDD, drawbacks include reduced range of motion and segmental degeneration with time leading to adjacent segment disease. Various TDR devices aim to alleviate pain by replacing a degenerated intervertebral.
disc with a motion-preserving prosthesis. Lumbar TDR is intended to reproduce the biomechanics of an intervertebral disc, thereby avoiding issues with traditional fusion.
Lumbar fusion includes various approaches including but not limited to anterior lumbar interbody fusion (ALIF), oblique lumbar interbody fusion (OLIF), lateral lumbar interbody fusion (LLIF), posterior lumbar interbody fusion (PLIF), posterolateral lumbar fusion (PLF), transforaminal lumbar interbody fusion (TLIF), and the combined anterior and posterior lumbar fusion (APLF) [10]. More recently, minimally invasive techniques have been widely adopted. Posterior lumbar fusion is typically performed with pedicle screws with intent of stabilization while preserving the disc space. For that reason, posterior instrumentation is not addressed in the DDD section. There is limited comparative evidence for fusion approaches in the context of discogenic low back pain. Comparative evidence mostly investigates surgical fusion versus nonoperative management for discogenic low back pain with still no conclusive recommendations as to which is the most effective [11, 12]. For discogenic pain, precedence has been the ALIF technique, although PLIF and TLIF are also commonly utilized surgical approaches.
A review of major studies comparing lumbar fusion with non-operative management in the treatment of lumbar degenerative disc disease can be found in Table 3.
Summary: There is level I and II evidence indicating that there is no significant difference in pain scores and ODI when comparing lumbar fusions to conservative management in patients with degenerative disc disease. There is level I and II evidence indicating there is a significantly higher complication rate in the lumbar fusion group compared to conservative management.
Anterior Lumbar Interbody Fusion (ALIF)
With ALIF, the disc space is fused by approaching the spine via an anterior approach. A lower abdominal incision is made to access the peritoneum. The retroperitoneal approach is the most common means of accessing the spine. This involves cutting through the external oblique muscles and retracting the peritoneum and vasculature.
ALIF can restore lumbar lordosis, reduce spondylolisthesis with distraction, and achieve coronal and sagittal balance. Caution must be taken due to larger vascular structures that need to be retracted in order to access the anterior disc space. As a result, vascular surgery is often included in the surgical planning. Independent use of ALIF may be utilized, but it is often combined with anterior or posterior fusion or fixation.
The ALIF approach is advantageous in that, unlike the PLIF and PLF approaches, both the paraspinal muscles and their innervation remain undisturbed. Additional advantage over the posterior approach is that nerve root retraction and entrance into the spinal canal is unnecessary and thus avoids the possibility of epidural scarring and perineural fibrosis.
The ideal candidate for ALIF has chronic, disabling back pain of discogenic origin for 1 or 2 lumbar levels with loss of disc height, stability and mobility of the diseased segment or neurological deficit [13]. Burkus et al. completed one of the largest prospective studies on DDD, with 279 patients investigated post-ALIF treatment. Clinical outcomes were based on comparing preoperative and postoperative Oswestry Disability Index (ODI) scores, neurological function, back and leg pain. Authors reported an overall 81% clinical success, and the study had a complication rate of 9% [14]. Several prospective, non-controlled studies validate the use of various types of ALIF devices [15, 16]. Of note, ALIF has been utilized in the presence of spondylolisthesis (discussed in a separate section) and degenerative lumbar scoliosis (not addressed directly in this manuscript). As previously noted, ALIF has also been used in combination with a PLF [17].
Posterior Lumbar Interbody Fusion (PLIF)
One of the original approaches for interbody fusion is PLIF. The posterior approach may be more suitable for degenerative indications requiring a fusion procedure. Patients with segmental instability, recurrent disc herniation, spinal stenosis and pseudoarthrosis may also benefit from a PLIF procedure. A posterior exposure allows for visualization of nerve roots without compromising blood supply. PLIF allows for adequate interbody height restoration and neural decompression while maintaining posterior support structures [18]. Disadvantages and/or complications of this technique include (1) prolonged retraction may lead to paraspinal muscle denervation, (2) delayed recovery and mobilization due to trauma, (3) potential for neurological sequelae secondary to nerve root injury and/or perineural fibrosis, (4) cerebrospinal fluid leak.
A prospective multicenter clinical study was conducted on eight-seven patients with chronic low back pain due to DDD treated by posterior lumbar interbody fusion (PLIF). Visual Analog Scale and Oswestry Disability Index decreased by 60% and 58%, respectively [19].
In a prospective, nonrandomized clinical series, 89 patients underwent PLIF with an allograft spacer and posterior pedicle fixation. All patients had experienced at least 6 months of low back pain that had been unresponsive to nonsurgical treatment. Follow-up visits were at intervals of 6 weeks, 6 months, 12 months, and 24 months. At each interval, radiographs and patient outcome measures were recorded, including SF-36 Bodily Pain Score, visual analog scale (VAS) pain rating and Oswestry Disability Index (ODI). The authors concluded that PLIF is a safe and effective surgical treatment for low back pain caused by degenerative disc disease when performed with machined allograft spacers and posterior pedicle fixation [20].
Transforaminal Lumbar Interbody Fusion (TLIF)
TLIF is a modification of the PLIF technique where the intervertebral disc is exposed unilaterally through a transforaminal approach with subtotal facetectomy in conjunction with pedicle screw instrumentation. Although risks are similar between these two techniques, a potential advantage of TLIF is less nerve root retraction to place the interbody spacer. Similar to PLIF, contralateral posterolateral fusion can be added to improve fusion rates. Takahashi et al. reported significant improvement with TLIF for the treatment of intractable chronic lumbar discogenic pain [21].
Lateral Lumbar Interbody Fusion (LLIF)
LLIF was developed as a trans-psoas approach to the anterior disc space allowing for complete discectomy, distraction, and interbody fusion without the need for an approach surgeon [22]. The lateral aspect of the disc is approached through the psoas muscles with serial dilators and a retractor. Complete diskectomy is performed and a large interbody is placed. There is no need to retract the great vessels or the sympathetic chain. LLIF techniques access the lateral aspect of the disc space via a retroperitoneal, trans-psoas approach and serve as an alternative to ALIF. There is no need to mobilize the great vessels or sympathetic chain and thus serves as a less-invasive alternative to ALIF. Similar to ALIF, advantages LLIF compared to PLF include: preservation of paraspinal muscles, interbody placement without retraction of nerve roots, and essentially no risk of dural injury. The LLIF approach is limited by the iliac crest and thus cannot be performed below L4-5 and is contra-indicated in cases where the lumbar plexus and psoas muscles are positioned past the anterior half of the disc space. Additional anatomical challenges include > grade 2 spondylolisthesis, significant rotatory scoliosis, retroperitoneal scarring, anomalous lateral position of the great vessels and prior fusion of L5/S1. Multiple studies have demonstrated high fusion rates for LLIF including Berjano et al. (2015) showing a fusion rate of 98% in 77 patients using a combination of autologous bone, calcium triphosphate and Attrax (Nuvasive) and Rodgers et al. (2010) with a fusion rate of 93.2% with at 17.3 months utilizing autograft and demineralized bone matrix with bone marrow aspirated from the iliac crest [23, 24]. In a retrospective, single surgeon cohort study of patients undergoing single level ALIF or LLIF for degenerative disc disease or spondylolisthesis, Malham et al. reported equivalent results for relief of back pain (64 vs. 56%), leg pain (65 vs. 57%), ODI (60 vs. 52%), and fusion on CT (100 vs. 95%) at 24-month follow-up [25].
Oblique Lumbar Interbody Fusion (OLIF)
OLIF was developed as an alternative to LLIF and ALIF and approaches the lumbar spine via a retroperitoneal, anterior to psoas muscle trajectory. A retractor is placed between the great vessels and psoas muscle at the anterolateral aspect of the disc space. Diskectomy is performed and the interbody is placed across the intervertebral space in an oblique anterior to posterior trajectory. OLIF may be performed from L1-S1 and because the approach is anterior to the iliac crest may be an alternative to ALIF at L5/S1 or cases where LLIF may not be performed due to a high-riding iliac crest or anterior lumbar plexus/psoas. Anatomic limitations of OLIF include > grade 1 spondylolisthesis where the overlap of the endplates may limit the space for an oblique interbody and cases of high-grade stenosis where there may be concern that the posteromedial approach could push disc fragments into the central canal or contralateral foramen [10].
OLIF has shown favorable outcomes in VAS and ODI for patients with back pain and radiculopathy caused by spondylosis [26,27,28]. Fusion rates have been reported to comparable to those for ALIF and LLIF. In 29 patients with OLIF and posterior pedicle screw fixation, Kim et al. reported a 12-month fusion rate of 92.9% [29]. Lin et al. reported a fusion rate of 81.9% at 12 months in 52 patients treated with stand-alone OLIF [30].
Compared to LLIF, OLIF has been shown to have less risk of postoperative neurological deficits but significantly higher rates of abdominal complications, system failure, and vascular injury [31]. A vascular injury during OLIF, compared to ALIF, is potentially more threatening because with OLIF the access does not permit direct repair and a vascular surgeon is typically not utilized the procedure.
Lumbar Disc Arthroplasty (LDA)
There is no conclusive evidence of LDA superiority over fusion in long-term level I studies. However, studies of LDA have reported satisfactory clinical results and implant survival along with comparable complication profiles to fusion. [32, 33]. The impetus for development of this technique has been motion preservation. Zigler et al. reported adjacent segment degeneration was significantly lower at 5 years for LDA (6.7%) versus APLF (23.8%). In a randomized controlled trial (RCT) Radcliff et al. compared 5-year outcomes for 2-level LDA to 2-level APLF in 229 subjects. The authors found equivalent success rates and a significantly lower rate of re-operation for the LDA group (5.6 vs. 19.1%). In another study, David et al. concluded that the rate of reoperation secondary to adjacent segment disease was ten times lower than the rates for fusion [34]. Other advantages for LDA include the absence of grafting or hardware, which present their own complications [35].
Earlier, some of the initial LDA designs led to inconsistent outcomes. Reasons for implant failures reported included failure of osseointegration, elastomeric tears, and osteolysis [36]. While reportedly lower than fusion, complications rates remain significantly lower than fusion (29.1% for arthroplasty and 50.2% for fusion at 2-year follow-up). Complications include those related to the anterior surgical approach (e.g., vascular injury, nerve root injury, retrograde ejaculation), prosthesis/fusion failure (e.g., subsidence, osteolysis, migration, implant fracture, endplate fracture, pseudoarthrosis), heterotopic ossification (up to 76% at 3 years) both hyper- and hypomobility of the implant, as well as donor site complications [36]. Device failures necessitating repeat operations have been reported at 5.4–6.3%.
Evidence on Surgical Treatments for Spondylolisthesis
The need to fuse patients who have lumbar spinal stenosis (LSS) in the setting of degenerative spondylolisthesis (DS) is one of the most debated topics in the surgical spine community. The concern with standalone decompression for LSS in the setting of DS is inadequate relief of leg pain or persistent back pain secondary to abnormal motion or instability at the decompressed segment. For decades, the surgical treatment of DS was based on a landmark 1991 study by Herkowitz et al. of 50 patients who were randomized to either a decompressive laminectomy or laminectomy plus un-instrumented posterolateral fusion [37]. The study included patients who had a “single level of DS seen on plain radiographs”. The degree of listhesis or kyphosis was not used as a criterion. The authors reported that patients who had a fusion accompanied by a decompression had “more excellent and good” outcomes compared to the decompression alone group. Over the last 15 years, our understanding of DS has evolved to include discerning stable vs. unstable DS, determining predictors of post decompressive instability, and introducing minimally invasive ligament preserving decompressive techniques.
Degenerative spondylolisthesis has traditionally been described as the slipping forward of one lumbar vertebra on another with an intact neural arch, with Meyerding classifying the slippage as grades 1–4 [38]. It is important for clinicians to understand the differences between stable and unstable DS, even though there is no universally accepted standard. Unstable DS is traditionally described as > 10 angulation or 4-mm translation between flexion–extension X-rays. The treatment of stable vs. unstable DS is clouded by the lack of standardization in large-scale clinical studies as some include stable and unstable DS [37,38,39] while others include only patients with stable DS [40]. The Spine Patient Outcomes Research Trial (SPORT) examined the effectiveness of surgical vs. non-surgical treatment of DS. This study included any patient who had DS shown on lateral radiographs in a standing position [38]. An analysis of patient demographics in this trial revealed that only 47/601 patients (8%) had unstable DS. Despite only 8% of patients presenting with unstable DS, 95% of patients with any DS underwent a fusion.
In 2016, the New England Journal of Medicine (NEJM) published consecutive articles on the surgical treatment of LSS [29, 30]. Försth et al. examined 247 patients as part of the Swedish Spinal Stenosis Study that consisted of 135 patients with both stable and unstable DS [39]. The average pre-operative listhesis measured on radiographs 7.4 mm. Sixty-eight patients underwent decompression and 67 underwent decompression and fusion. Patient-reported outcome (PRO) measures included the VAS, EQ-5D, ZCQ, ODI, and 6-min walk test. At 2 and 5 years, fusion did not result in better PRO than decompression alone. The overall re-operation rate at 6.5 years was 22% in the fusion group and 21% in the decompression group. Most revisions in the fusion group were for adjacent segment stenosis while most of the revisions in the decompression group were for recurrent stenosis or foraminal stenosis. The second NEJM article by Ghogawala et al., reported on a randomized control trial of laminectomy vs. laminectomy and fusion performed across multiple sites in the United States. Contrary to the Forsyth study, only patients who had a grade 1 stable DS were included; patients with > 3 mm of motion on flexion–extension radiographs were specifically excluded. The average slippage was 6 mm with 1.5 mm of translation. Sixty-six patients were included in the study with 35 treated by laminectomy, and 31 treated by laminectomy and fusion. A 1-, 2-, and 4-year follow-up was performed with PRO including SF-36 and ODI. This study demonstrated that fusion was better than decompression for the physical component score (PCS) of the SF-36 at all endpoints, although there were no significant differences in ODI. The authors also reported a re-operation rate of 34% in the decompression group for subsequent instability despite already excluding patients with an unstable DS. It should be noted that decompression in the Ghogawala et al. study was an open laminectomy while 20% of the patients in the Forsyth et al. study received a minimally invasive interspinous ligament sparing decompression [40]. These were the first two large-scale clinical trials that challenged the traditional dogma of the need to fuse all patients with a DS.
Several authors have reported on the predictors of post-operative instability following decompression in patients with a DS [41, 42]. Blumenthal et al. demonstrated in patients with a stable DS that risk factors for progressive instability and re-operation included patients who had greater than > 1.25 mm of motion, disc height > 6.5 mm, or facet angle > 50 degrees (more vertically oriented facet joint). Patients with all three criteria had a re-operation rate of 75%. Inui et al. reported on patients with both stable and unstable DS and found that even in the setting of a minimally invasive decompression, pre-operative translation (on flexion–extension radiographs) of more than 5 mm led to an 80% risk of postoperative instability. Patients who had an unstable DS, tall disc, fluid filled facets, and vertically oriented facets may be better candidates for a fusion than a decompression and require less re-operation rates at the index level.
A traditional laminectomy removes the midline structures including the supraspinous and interspinous ligaments. The advent of minimally invasive decompressions performed through a mini-open incision or tubular retractor has given surgeons the ability to decompress the spine without disrupting stabilizing ligaments. A unilateral laminotomy bilateral decompression (ULBD) is performed through a traditional mini-open unilateral approach followed by decompression of the contralateral side. Theoretically, a ULBD leads to a decreased risk of post decompression instability in patients who have a DS. Kuo et al. reported on a retrospective cohort of 164 patients who underwent a ULBD vs. 437 matched fusion controls in patients with a DS [43]. Although there was no indication of DS grade or stability, the authors reported that at 5-year follow-up the reoperation rate was 10% in ULBD vs. 17% in the Fusion cohort. A systematic review of 37 studies w/1156 patients comparing traditional midline laminectomy vs. ULBD in the setting of DS demonstrated secondary fusion rates of 12.8% and 3.3% in the laminectomy and ULBD groups, respectively [44]. Seventy-two percent of the patients who had an open laminectomy had slippage progress while 0% of patients in the ULBD group progressed. Patients who have a stable DS, collapsed disc, and more horizontally oriented facets may be good candidates for a ULBD rather than a fusion. There still may be a requirement to fuse the index level albeit less than in the setting of an unstable DS.
Some clinicians prefer to fuse patients with a DS when back pain is also a presenting symptom presuming that degenerative disc disease and facet joint arthrosis which often accompanies DS contributes to back pain [45]. Sigmundsson et al. analyzed 1624 patients with stable and unstable DS from the Swedish Spine Registry treated by decompression vs. decompression and fusion [46]. Patients who received a fusion had a greater decrease in low back pain but a similar reduction in leg pain. Similarly, Austevoll reported from a cohort of 294 patients with a fusion vs. 260 with a decompression and found that the fusion group was superior in reducing low back pain at 1 year. There were no statistical differences in leg pain or ODI [47]. The degree of relief from pre-surgical diagnostic injections such as lumbar epidural steroid injections, facet joint injections, and functional anesthetic discogram can be used to help delineate what structural pathology is contributing to back pain in DS. It is important to remember that even though the primary purpose of LSS surgery is to reduce leg pain, decompression alone in the absence of fusion may also decrease low back pain [48].
While there are studies that show that instrumentation increases fusion rates, and that increased fusion rates lead to better PRO, it is still unproven if instrumentation directly correlates to improved PRO. Further study and research are required. Further, there are no studies directly comparing direct vs. indirect decompression.
A review of major studies comparing surgical vs. non-surgical management, as well as surgical management for lumbar degenerative spondylolisthesis can be found in Tables 4 and 5.
Summary: There is level I and II evidence showing significantly greater improvement in pain and functional scores in patients treated surgically for lumbar DS.
Evidence on Surgical Treatment for Lumbar Spinal Stenosis
The spectrum of surgical and non-surgical procedures in the treatment of LSS has been previously explored by many different groups [49,50,51]. The North American Spine Society (NASS) has more recently published evidence-based guidelines for the “Diagnosis and Treatment of Lumbar Spinal Stenosis” which elaborates on epidural injections and surgical treatment [49]. Failure of conservative management or worsening of neurological deficits, as in most other types of lumbar pathology, warrants consideration for interventional and surgical treatment options. A recent guideline suggests the use of minimally invasive treatments as an alternative to more traditional and open surgical procedures [52], particularly addressing the use of percutaneous indirect and direct decompression.
Surgical Decompression with and without Fusion
Surgical decompression remains the “gold standard” treatment for LSS. The main aim of decompression for LSS is to relieve impingement of neural structures and compression of vascular elements by freeing up space within the spinal canal [53,54,55,56,57]. This is accomplished by removing posterior spinal elements such as laminae, facets, ligaments, synovial cysts, and osteophytes. There are a multitude of open and minimally invasive procedures by which this can be accomplished, including traditional laminectomy, bilateral laminotomy, bilateral decompression with unilateral laminotomy, laminoplasty, and indirect decompression with lateral lumbar interbody fusion [56, 58]. Among these, there is no particular approach which is convincingly superior for LSS, and the ultimate choice of procedure often comes down to individual surgeon preference and case presentation [53, 59, 60]
Although decompressive surgery has been performed worldwide for well over a century, there have been very few high-quality studies demonstrating its efficacy. Unlike many of the novel therapies described above, there is no industry to sponsor randomized controlled studies for decompression. Malimivarra et al. published the first randomized trial that compared the effectiveness of traditional surgery in comparison with nonoperative and conservative treatment for spinal stenosis [61]. In this multicenter trial, 94 patients with LSS were randomized to undergo surgery (laminectomy n = 40, laminectomy with instrumented fusion n = 10) or nonoperative treatment (n = 44). Although both treatment groups showed improvement, patients in the surgery group had a more favorable outcome at 2 years.
Patients in the surgical group had better disability (11.3, 95% confidence interval [CI] 4.3–18.4), leg pain (1.7, 95% CI, 0.4–3.0), and back pain (2.3, 95% CI 1.1–3.6) scores at the 1-year follow-up. The surgical group advantage was slightly less in all three variables at the 2-year follow-up: disability (7.8, 95% CI 0.8–14.9), leg pain (1.5, 95% CI 0.3–2.8), and back pain (2.1, 95% CI 1.0–3.3). There was no significant difference between the walking ability of both groups.
The Maine Lumbar Spine Study prospectively studied a cohort of 148 patients with symptomatic lumbar stenosis [62]. In this non-randomized cohort study, 81 patients were treated surgically and 67 treated nonsurgically. The surgical cohort tended to present with more severe symptoms, imaging findings, and worse functional status. At 1 year, 55% of the patient’s that underwent surgical treatment showed definite improvement in their predominant symptoms compared to only 28% in the non-surgical group (p = 0.003). Patient’s in the surgical group had the maximum benefit from surgery at the 3-month follow-up. There was minimal improvement in both symptoms and functional status in the non-surgical group. At the 8–10-year follow-up, patients in the surgical arm of the study showed greater leg pain relief and back-related functional status. However, there was no statistically significant difference for low back pain relief, predominant symptom improvement and satisfaction between the two groups.
The Spine Patient Outcomes Research Trial (SPORT) reported on the 2-year outcomes of patients with spinal stenosis without degenerative spondylolisthesis to compare the efficacy of surgical versus nonsurgical treatment [63]. In this multicenter RCT, 289 patients were enrolled in the randomized cohort, and 365 patients were enrolled in the observational cohort. At the 2-year mark, the intention-to-treat analysis showed no significant difference in change on physical function or on the Oswestry Disability Index. However, the randomized cohort did show a significant improvement favoring surgical treatment with better results on the SF-36 scale for bodily pain (7.8, 95% CI 1.5–14.). The study had a high crossover rate with 43% of the patients initially randomized to receive nonsurgical care undergoing surgical treatment and only 67% of patients randomly assigned to receive surgical treatment having undergone surgery. The combined cohorts, when adjusted for potential confounders, in the as-treated analysis did show a significant advantage in all primary outcomes (change in bodily pain, physical function, and ODI) for the surgical treatment group at the 3-month mark. These changes continued to remain significant at the 2- and 4-year follow-up.
Fusion, in addition to decompression, is often considered when mechanical back pain is the predominant presenting symptom. Further, pre-existing instability, spondylolisthesis, scoliosis, foraminal stenosis necessitating resection of greater than 50% of the facet joint leading to iatrogenic instability may support the need for fusion.
Försth et al. randomized 247 with LSS at one or two adjacent vertebral levels to either decompression plus fusion surgery (fusion group) or decompression surgery alone (decompression-alone group) [29]. At the 2-year follow-up, there was no significant difference in the ODI (27 vs. 24, p = 0.24) and 6-min walk test (397 vs. 405 m, p = 0.72) scores between the fusion and decompression-alone groups. The presence or absence of spondylolisthesis did not have an effect on the results. There was no significant difference in the clinical outcomes or the need for further surgery between the groups at the 5-year follow-up. However, the addition of fusion surgery along with decompression was associated with longer hospitalization, longer operative times, increased bleeding, and higher surgical costs.
Fusion is often considered an option for patients with LSS and spondylolisthesis with or without evidence of pre-existing radiographic instability. The Spinal Laminectomy versus Instrumented Pedicle Screw Fusion (SLIP) Trial randomized 66 patients who had stable degenerative grades 1–2 spondylolisthesis and symptomatic lumbar spinal stenosis to undergo either decompressive laminectomy alone or laminectomy with posterolateral instrumented fusion [40]. At the 2-year follow-up, the group that underwent instrumented pedicle screw fusion had a significantly greater increase in SF-36 physical component summary (15.2) vs. the decompression-alone group (9.5, 95% confidence interval, 0.1–11.3; p = 0.046). This increase in the SF-36 physical component remained at the 3- and 4-year follow-up visits (p = 0.02 for both years). However, there was no difference between the two groups with respect to reduction in disability related to back pain. The changes in the Oswestry Disability Index scores did not differ significantly between the groups at the 2-year follow-up (− 17.9 in the decompression-alone group and – 26.3 in the fusion group, p = 0.06). The study did note that there was increased blood loss and longer hospital stay in the fusion group when compared to the decompression-alone group (p < 0.001 for both comparisons). The rate of reoperation was also significantly higher (p = 0.05) in the decompression alone group (34%) when compared to the fusion group (14%).
Several systematic reviews have compared decompression alone versus decompression with fusion [64, 65]. Current literature suggests that the addition of fusion in the management of LSS yields no clinical improvements over decompression alone. The addition of fusion did result in longer duration of operation, increased blood loss, and a higher incidence of complications.
However, a meta-analysis that included four RCTs comparing the two treatments in the setting of degenerative spondylolisthesis found that fusion had advantages of improvement of clinical satisfaction, as well as reduction of postoperative leg pain, with similar complication rate to decompression alone [66]. Taken together, the systematic reviews are limited by a paucity of included studies, inconsistency in the type of fusion or instrumentation placed, heterogeneity of the included patients, and the lack of a clear definition for “stability” vs. “instability” in LSS [17].
Ultimately, whether a surgeon opts for decompression alone or in combination with fusion for LSS comes down to a combination of mechanical factors, symptomatology, radiographic findings, and personal preference. The latter is a significant factor involved in the choice of procedure because there is not definitive evidence that laminectomy alone is superior to fusion for LSS, or vice versa.
A review of major studies comparing different types of surgical management for lumbar spinal stenosis can be found in Table 5.
Summary: There are mixed results showing whether or not decompression plus fusion versus decompression alone improved pain and function scores in patients with lumbar DS.
Percutaneously Implanted Interspinous Spacers
Interspinous spacers (ISS) were originally developed as an alternative to posterior decompression and fusion. In the evolution of the ISS, there have been various types of sizes, shapes and materials used. Presently, ISS can be characterized as static or dynamic, with both providing distraction of the interspinous space, leading to flexion or anti-extension at the targeted spinal level. Biomechanically, this increases the cross-sectional area in both the central canal and neural foramen, and results in tightening of the ligamentum flavum. This prevents buckling of the ligamentum flavum along the posterior canal, which further leads to opening of the central canal [67].
Static devices are made of non-compressive materials [68], while dynamic implant devices have a degree of compression. Examples of static ISS devices include X-Stop (Medtronic), Wallis (Zimmer), and Superion (Boston Scientific); whereas the DIAM (Medtronic) implant is considered to be a dynamic ISS [69].
The X-stop device was historically one of the most popular ISS devices in the United States. Though X-stop is no longer on the market, it bears mentioning here to understand the evolution of ISS. Initial data showed promising results on short-term follow-up, but this did not translate to long-term symptomatic relief. Furthermore, complications rates were found to be high, including issues of spinous process fracture, heterotopic ossification with possible intrusion of bone within the central and foraminal spaces, dislocation, spinous process erosion, infection, and neurological sequelae [70].
Since the discontinuation of X-stop, there has been a resurgence of ISS brought to market both static and dynamic, with modifications to help prevent the aforementioned list of complications. With the advent of improved technology and less invasive methods of placement, there presently is a need for a new classification of ISS devices by the method in which they are implanted; percutaneous ISS and open ISS.
The Superion implant is a percutaneously implanted interspinous spacer that is FDA approved for treatment of neurogenic claudication in the presence of moderate degenerative LSS (L1-L5). The use of Superion has been compared to the X-stop device and showed similar improvement outcomes at the 2-year follow-up [71, 72]. A study comparing the two devices showed more patients in the Superion group (63 of 120 patients, 52.5%) achieved statistically significant (p = 0.023) Composite Clinical Success (CCS) when compared to the X-Stop cohort (40 of 120, 38%) at the 36-month follow-up. CCS was defined as a clinically significant improvement in two of the three domains including the Zurich Claudication Questionnaire (ZCQ); no reoperations, revision, removals, or supplemental fixation at the treated level(s); no major implant or procedure-related complications; and no significant confounding treatments such as epidural injections, nerve blocks or rhizotomies. At the 5-year follow-up, 84% of patients (74 of 88) Leg and back pain success rates (50% or greater relief) were seen in 80% (68 of 85) and 65% (55 of 85), in Superion and X-stop groups, respectively.
Review of present evidence supports the use of percutaneously implanted interspinous spacer devices in patients with radiological evidence of mild-to-moderate degenerative LSS and no worse than a grade I spondylolisthesis, with flexion-based relief of neurogenic claudication symptoms. For patients who do not fit these criteria, or exhibit any of the aforementioned contraindications, other interventional or surgical treatment options should be highly considered.
A review of major studies comparing different types of interspinous spacers can be found in Table 6. A review of major studies comparing interspinous spacers to lumbar decompression can be found in Table 7.
Summary: There is level I and II evidence suggesting improved pain and function scores over 2 and 5 years with the treatment of ISS for LSS with stable LDS.
Percutaneous Image-Guided Lumbar Decompression (PILD)
Percutaneous image-guided lumbar decompression (PILD) is a decompressive strategy to treat neurogenic claudication and spinal stenosis that focuses on removal of the ligamentum flavum. The ligamentum flavum PILD is not intended to debulk lateral foramen or primary bony abnormalities [73, 74]. Ligamentum flavum is very commonly a contributor to the radiographic evidence of spinal stenosis, with estimates as high as 85% [75]. In order to be a candidate for removal (or debulking) the ligamentum, the degree of hypertrophy needs to be 2.5 mm or greater, in the presence of neurogenic claudication.
To date, there have been multiple studies demonstrating the efficacy of this approach [75,76,77]. There have been three randomized prospective studies investigating percutaneous lumbar decompression of the ligamentum flavum to treat symptomatic spinal stenosis, compared to conservative management or epidural steroid injection.
In addition to reporting superiority to lumbar epidural steroid injections at 1 year, the results had continued improvements at 2 years [75, 77]. In the 2-year MiDAS ENCORE study, Staats et al. followed 143 Medicare patients with central LSS having undergone the MILD procedure. Study patients were required to be 65 years or older and Medicare beneficiaries. Significant improvements were seen in the study endpoints.
A review of major studies on posterior image-guided lumbar decompression (PILD) can be found in Table 8.
Summary: There is level I and II evidence suggesting improved pain and function scores in patients with LSS with neurogenic claudication with the treatment of PILD.
Lumbar Posterior Interspinous Fixation (PISF)
The use of interspinous fixation systems have been proposed as an alternative to pedicle screw fixation in patients undergoing decompression for the treatment of LSS in the presence of spondylolisthesis with or without dynamic instability [35, 36, 78]. Further, interspinous fusion devices have been around for several years as a method of supplemental fixation following lumbar laminectomy or interbody fusion [78]. While pedicle screw fixation constructs are still the most widely used and biomechanically sound method of stabilization, the use of interspinous fusion systems has been proposed to allow for stabilization results with reduced risk. These devices are used at a single level in the thoracic and lumbar spine (T1-S1) for adjunctive fixation in interbody fusion. These devices have been used for degenerative disc disease (defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies), spondylolisthesis with or without dynamic instability, trauma (i.e., fracture or dislocation), and/or tumor.
The use of interspinous fixation devices has been more recently proposed as a means to distract the spinous processes for patients with LSS. Implantation is accomplished by a percutaneous posterior or posterolateral approach, allowing for bony fusion along the spinous processes spanning the index level, along with the barrel of the device. Currently available interspinous fixation devices are intended to be used with bone graft material to provide immobilization and stabilization of the spinal segments and are not intended for stand-alone use. The safety and efficacy of the use of interspinous fixation devices are currently being studied.
A review of major studies on interspinous fixation devices can be found in Table 9.
Summary: There is level II and III evidence suggesting improvement in pain and function scores in patients with DDD plus LSS or LDS treated with PISF.
Decompression with Surgical Interlaminar Devices
The Coflex device (Paradigm Spine, New York) is an interlaminar device approved in the lumbar spine from L1 through L5 [79, 80]. Patient selection includes those who have moderate limitations or impairment from spinal stenosis that is improved in flexion. The procedure includes decompression of the stenosis before stabilization with the Coflex device. It can be placed at 1–2 contiguous levels. It is generally accepted for use in patients with up to a grade I spondylolisthesis. It is also avoided with those patients who have had previous decompression, dynamic instability, or severe osteoporosis.
Placement of the device is done via an open approach with a midline incision with minimal removal of spinous process. A laminotomy is performed to decompress the neural structures while maintaining the presence of the spinous process above and below, as well as the central lamina.
The Coflex device was compared to lumbar laminectomy for moderate LSS in a 2-year, multicenter, randomized controlled trial [79]. Patients with moderate to severe spinal stenosis were included and were followed for 2 years. Primary and secondary endpoints were compared within the two groups. Primary endpoints included a composite of four measures including Oswestry Disability Index, secondary surgery or injections, neurological status, and adverse events related to the procedure or device. Secondary endpoints included visual analog scale (VAS) scores, Zürich Claudication Questionnaire (ZCQ) scores, narcotic usage, walking tolerance, and radiographs. There was no significant difference in patient-reported outcomes (ODI, VAS, ZCQ) between the two groups. However, the Coflex cohort did show superiority in the primary endpoints and led to two times the improvement in walking distance as compared to decompression alone. Furthermore, patients in the decompression alone group were more likely to undergo a secondary intervention or injections and were associated with a higher rate of opioid use.
The Coflex device with decompression was also studied in a multicenter, randomized controlled trial in which it was compared to decompression with pedicle screw fusion [80]. Patients with moderate to severe lumbar stenosis at 1 to 2 contiguous levels were evaluated and followed for 5 years. The study looked at four main endpoints including ODI, repeat surgery, further lumbar injections, or adverse events. At the 5-year follow-up, 50.3% of patients in the Coflex group and 44% of patients in the pedicle screw fusion group met all 4 endpoints. ZCQ scores were significantly better in the Coflex cohort. However, the two groups were similar when it came to re-operate rates, improvements in ODI, VAS, and SF-12.
A review of major studies comparing interlaminar stabilization vs. surgery alone can be found in Table 10.
Summary: There is level I and II evidence indicating improved pain and function scores in patients with LSS with grade I LDS treated with the Coflex device.
Conclusions
Degeneration within the spine is largely the result of time and aging with a cascade of events that include hypertrophied ligamentum flavum, facet joint hypertrophy, degenerative disc disease, and osteophyte formation. Perhaps more prevalent, and a contributing factor, is lumbar spinal stenosis that includes degenerative spondylosis, spondylolisthesis, synovial cysts, annular bulges, ligamentum flavum hypertrophy, facet hypertrophy, post-surgical fibrosis, or a combination of these factors. Spinal surgery has been a staple in the treatment of spinal pathology, but it has also undergone changes in its utility and approach over time.
Minimally invasive techniques have grown tremendously, as highlighted in this paper. Some have level one evidence, while others have none. There is level I and II evidence indicating there is no significant difference in pain scores and ODI when comparing lumbar fusions to conservative management in patients with degenerative disc disease. There is level I and II evidence showing significantly greater improvement in pain and functional scores in patients treated surgically for lumbar DS. There are mixed results showing whether decompression plus fusion versus decompression alone improved pain and function scores in patients with lumbar DS. There is level I and II evidence suggesting improved pain and function scores over 2 and 5 years with the treatment of ISS for LSS with stable LD. There is level I and II evidence suggesting improved pain and function scores in patients with LSS with neurogenic claudication with the treatment of PILD.
All too often, we ask or have been asked the question “where is the evidence?” It is up to the physician stewardship of our spine and pain space to demand the generation of data and to practice evidence-based guidelines. With this also comes an important consideration for training, appreciation for mechanics of the spine, and to develop a team to help manage potential complications with surgical care. We need to embrace this emerging field and approach it responsibly and civilly.
Through PSPS this evidence review may help outline the evidence for transitional and emerging surgical treatment options for the degenerative spine. It is by no means exhaustive, but purposeful to qualify the strength of evidence available for treatment, and to begin to develop a common language for both spine and pain communities.
Although the review was extensive, this paper did have some limitations. The manuscript is confined to lumbar spine pathology, focusing solely on degenerative disc disease, spondylolisthesis and spinal stenosis. In an effort to publish a concise manuscript featuring the most commonly used approaches, certain surgical techniques, including endoscopic techniques were omitted. Due to the scope of the review, the PSPS committee also did not include any recommendations in this manuscript. Future publications can be considered to include these omitted techniques and recommendations.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Clarençon F, Law-Ye B, Bienvenot P, Cormier É, Chiras J. The degenerative spine. Magn Reson Imaging Clin N Am. 2016;24(3):495–513.
Gallucci M, Limbucci N, Paonessa A, Splendiani A. Degenerative disease of the spine. Neuroimaging Clin N Am. 2007;17(1):87–103.
Parenteau CS, Lau EC, Campbell IC, et al. Prevalence of spine degeneration diagnosis by type, age, gender, and obesity using Medicare data. Sci Rep. 2021;11:5389.
Binder DK, Schmidt MH, Weinstein PR. Lumbar spinal stenosis. Semin Neurol. 2002;22(2):157–66.
Harris RP, Helfand M, Woolf SH, for the Methods Work Group, Third U.S. Preventive Services Task Force, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20:21–35.
Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17(6):515–50. https://doi.org/10.1111/ner.12208.
Deer TR, Pope JE, Hayek SM, et al. The polyanalgesic consensus conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation. 2017;20(2):96–132. https://doi.org/10.1111/ner.12538. (Epub 2017 Jan 2).
Watters WC, Bono CM, Gilbert TJ, et al. An evidence based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J. 2009;9(7):6009–14.
Mobbs RJ, Loganathan A, Yeung V, Rao PJ. Indications for anterior lumbar interbody fusion. Orthop Surg. 2013;5(3):153–63.
Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1(1):2–18.
Bydon M, De la Garza-Ramos R, Macki M, Baker A, Gokaslan AK, Bydon A. Lumbar fusion versus nonoperative management for treatment of discogenic low back pain: a systematic review and meta-analysis of randomized controlled trials. J Spinal Disord Tech. 2014;27(5):297–304.
Teng I, Han J, Phan K, Mobbs R. A meta-analysis comparing ALIF, PLIF, TLIF and LLIF. J Clin Neurosci. 2017;44:11–7. https://doi.org/10.1016/j.jocn.2017.06.013. (Epub 2017 Jul 1).
Mummaneni PV, Lin FJ, Haid RW Jr, Rodts GE Jr, Subach BR, Miller JS. Current indications and techniques for anterior approaches to the lumbar spine. Contemp Spine Surg. 2002;3:57–64.
Burkus JK, Gornet MF, Dickman CA, Zdeblick TA. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337–49.
Kuslich SD, Danielson G, Dowdle JD, Sherman J, Fredrickson B, Yuan H, Griffith SL. Four-year follow-up results of lumbar spine arthrodesis using the Bagby and Kuslich lumbar fusion cage. Spine (Phila Pa 1976). 2000;25(20):2656–62.
Rao PJ, Loganathan A, Yeung V, Mobbs RJ. Outcomes of anterior lumbar interbody fusion surgery based on indication: a prospective study. Neurosurgery. 2015;76(1):7–23 (discussion 23-4).
Strube P, Hoff E, Hartwig T, Perka CF, Gross C, Putzier M. Stand-alone anterior versus anteroposterior lumbar interbody single-level fusion after a mean follow-up of 41 months. J Spinal Disord Tech. 2012;25(7):362–9.
Lestini WF, Fulghum JS, Whitehurst LA. Lumbar spinal fusion: advantages of posterior lumbar interbody fusion. Surg Technol Int. 1994;3:577–90.
Folman Y, Lee SH, Silvera JR, Gepstein R. Posterior lumbar interbody fusion for degenerative disc disease using a minimally invasive B-twin expandable spinal spacer: a multicenter study. J Spinal Disord Tech. 2003;16(5):455–60.
Arnold PM, Robbins S, Paullus W, Faust S, Holt R, McGuire R. Clinical outcomes of lumbar degenerative disc disease treated with posterior lumbar interbody fusion allograft spacer: a prospective, multicenter trial with 2-year follow-up. Am J Orthop (Belle Mead NJ). 2009;38(7):E115–22.
Takahashi T, Hanakita J, Minami M, et al. Surgical outcome and postoperative work status of lumbar discogenic pain following transforaminal interbody fusion. Neurol Med Chir (Tokyo). 2011;51:101–7.
Ozgur BM, Aryna HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435–43.
Berjano P, Langella F, Damilano M, et al. Fusion rate following extreme lateral interbody fusion. Eur Spine J. 2015;24:369–71.
Rogers WB, Gerber EJ, Patterson JR. Fusion after minimally disruptive anterior lumbar interbody fusion: analysis of extreme lateral interbody fusion by computed tomography. SAS J. 2010;4:63–6.
Malham GW, Parker RM, Bleacher CM, Seex KA. Choice of approach does not affect clinical and radiographic outcomes: a comparative cohort of patients having anterior lumbar interbody fusion and patient having lateral interbody fusion at 24 months. Glob Spine. 2016;6(5):472–81.
Silvester C, Mac-Thiong JM, Hilmi R, et al. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusions: oblique lumbar interbody fusion in 179 patients. Asian Spine J. 2012;6:89–97.
Ohroti S, Ortita S, Yamauchi K, et al. Mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for lumbar spinal degenerative disease. Yonsei Med J. 2015;56:1051–9.
Zairi F, Sunna TP, Westwick HJ, et al. Mini-open oblique lumbar interbody fusion (OLIF) approach for multi-level discectomy and fusion involving L5–S1: preliminary experience. Orthop Traumatol Surg Res. 2017;103:295–9.
Kim JS, Choi WS, Sung JH. 314 minimally invasive oblique lateral interbody fusion for L4–5: clinical outcomes and perioperative complications. Neurosurgery. 2016;63:190–1.
Lin JF, Lundusi R, Tarantino U, et al. Intrarvertebral plate and cage system via lateral trajectory for lumbar interbody fusion—a novel fixation device. Spine J. 2010;10:S86.
Ricciardi L, Piazza A, Capobianco M, Della Pepa GM, Miscusi M, Raco A, Scerrati A, Somma T, Lofrese G, Sturiale CL. Lumbar interbody fusion using oblique (OLIF) and lateral (LLIF) approaches for degenerative spine disorders: a meta-analysis of the comparative studies. Eur J Orthop Surg Traumatol. 2023;33(1):1–7.
Lu SB, Hai Y, Kong C, et al. An 11-year minimum follow-up of the Charite III lumbar disc replacement for the treatment of symptomatic degenerative disc disease. Eur Spine J. 2015;24:2056–64.
Siepe CJ, Mayer HM, Heinz-Leisenheimer M, Korge A. Total lumbar disc replacement: different results for different levels. Spine (Phila Pa 1976). 2007;32:782–90.
David T. Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the CHARITE artificial disc in 106 patients. Spine (Phila Pa 1976). 2007;32:661–6.
Meir AR, Freeman BJ, Fraser RD, Fowler SM. Ten-year survival and clinical outcome of the AcroFlex lumbar disc replacement for the treatment of symptomatic disc degeneration. Spine J. 2013;13:13–21.
Guyer RD, McAfee PC, Banco RJ, Bitan FD, Cappuccino A, Geisler FH, Hochschuler SH, Holt RT, Jenis LG, Majd ME, Regan JJ, Tromanhauser SG, Wong DC, Blumenthal SL. Prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: five-year follow-up. Spine J. 2009;9(5):374–86.
Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Jt Surg. 1991;73(6):802–8.
Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson ANA, Blood EA, Birkmeyer NJO, Hilibrand AS, Herkowitz H, Cammisa FP, Albert TJ, Emery SE, Lenke LG, Abdu WA, Longley M, Errico TJ, Hu SS. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356(22):2257–70.
Försth P, Ólafsson G, Carlsson T, Frost A, Borgström F, Fritzell P, Öhagen P, Michaëlsson K, Sandén B. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med. 2016;374(15):1413–23.
Ghogawala Z, Dziura J, Butler WE, Dai F, Terrin N, Magge SN, Coumans J-VCE, Harrington JF, Amin-Hanjani S, Schwartz JS, Sonntag VKH, Barker FG 2nd, Benzel EC. Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med. 2016;374(15):1424–34.
Blumenthal C, Curran J, Benzel EC, Potter R, Magge SN, Harrington JFJ, Coumans JV, Ghogawala Z. Radiographic predictors of delayed instability following decompression without fusion for degenerative grade I lumbar spondylolisthesis. J Neurosurg Spine. 2013;18(4):340–6.
Inui T, Murakami M, Nagao N, Miyazaki K, Matsuda K, Tominaga Y, Kitano M, Hasegawa H, Tominaga S. Lumbar degenerative spondylolisthesis: changes in surgical indications and comparison of instrumented fusion with two surgical decompression procedures. Spine. 2017;42(1):E15–24.
Kuo CC, Merchant M, Kardile MP, Yacob A, Majid K, Bains RS. In degenerative spondylolisthesis, unilateral laminotomy for bilateral decompression leads to less reoperations at 5 years when compared to posterior decompression with instrumented fusion: a propensity-matched retrospective analysis. Spine. 2019;44(21):1530–7.
Schöller K, Alimi M, Cong G-T, Christos P, Härtl R. Lumbar spinal stenosis associated with degenerative lumbar spondylolisthesis: a systematic review and meta-analysis of secondary fusion rates following open vs. minimally invasive decompression. Neurosurgery. 2017;80(3):355–67.
Schroeder GD, Kepler CK, Kurd MF, Vaccaro AR, Hsu WK, Patel AA, Savage JW. Rationale for the surgical treatment of lumbar degenerative spondylolisthesis. Spine. 2015;40(21):E1161–6.
Sigmundsson FG, Jönsson B, Strömqvist B. Outcome of decompression with and without fusion in spinal stenosis with degenerative spondylolisthesis in relation to preoperative pain pattern: a register study of 1,624 patients. Spine J. 2015;15(4):638–46.
Austevoll IM, Gjestad R, Brox JI, Solberg TK, Storheim K, Rekeland F, Hermansen E, Indrekvam K, Hellum C. The effectiveness of decompression alone compared with additional fusion for lumbar spinal stenosis with degenerative spondylolisthesis: a pragmatic comparative non-inferiority observational study from the Norwegian Registry for Spine Surgery. Eur Spine J. 2017;26(2):404–13.
Weinstein JN, Tosteson TD, Lurie JD, Tosteson ANA, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358(8):794–810.
Kreiner DS, Shaffer WO, Baisden JL, Gilbert TJ, Summers JT, Toton JF, Hwang SW, Mendel RC, Reitman CA, North American Spine Society. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update). Spine J. 2013;13(7):734–43.
Watters III, William C, et al. Degenerative lumbar spinal stenosis: an evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J. 2008;8(2):305–10.
Atlas SJ, Delitto A. Spinal stenosis: surgical versus nonsurgical treatment. Clin Orthop Relat Res. 2006;443:198.
Deer TR, Grider JS, Pope JE, Falowski S, Lamer TJ, Calodney A, Provenzano DA, Sayed D, Lee E, Wahezi SE, Kim C, Hunter C, Gupta M, Benyamin R, Chopko B, Demesmin D, Diwan S, Gharibo C, Kapural L, Kloth D, Klagges BD, Harned M, Simopoulos T, McJunkin T, Carlson JD, Rosenquist RW, Lubenow TR, Mekhail N. The MIST guidelines: the lumbar spinal stenosis consensus group guidelines for minimally invasive spine treatment. Pain Pract. 2019;19(3):250–74. https://doi.org/10.1111/papr.12744. (Epub 2018 Dec 2).
Jon L, Christy T-L. Management of lumbar spinal stenosis. BMJ. 2016;352: h6234.
Sengupta DK, Herkowitz HN. Lumbar spinal stenosis. Treatment strategies and indications for surgery. Orthop Clin N Am. 2003;34(2):281–95.
Diwan S, Sayed D, Deer TR, Salomons A, Liang K. An algorithmic approach to treating lumbar spinal stenosis: an evidenced-based approach. Pain Med. 2019;20(Suppl 2):S23–31.
Zaina F, et al. Surgical versus non-surgical treatment for lumbar spinal stenosis. Cochrane Database Syst Rev. 2016;2016(1):CD010264.
Carragee EJ. The increasing morbidity of elective spinal stenosis surgery: is it necessary? JAMA. 2010;303(13):1309–10.
Formica M, Quarto E, Zanirato A, Mosconi L, Vallerga D, Zotta I, Baracchini ML, Formica C, Felli L. Lateral lumbar interbody fusion: what is the evidence of indirect neural decompression? A systematic review of the literature. HSS J. 2020;16(2):143–54. https://doi.org/10.1007/s11420-019-09734-7. (Epub 2020 Mar 20).
Jacobs WCH, Rubinstein SM, Koes B, et al. Evidence for surgery in degenerative lumbar spine disorders. Best Pract Res Clin Rheumatol. 2013;27:673–84.
Lurie JD, Tosteson TD, Tosteson A, et al. Long-term outcomes of lumbar spinal stenosis: eight-year results of the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976). 2015;40(2):63–76.
Malmivaara A, Slätis P, Heliövaara M, Sainio P, Kinnunen H, Kankare J, Dalin-Hirvonen N, Seitsalo S, Herno A, Kortekangas P, Niinimäki T, Rönty H, Tallroth K, Turunen V, Knekt P, Härkänen T, Hurri H, Finnish Lumbar Spinal Research Group. Surgical or nonoperative treatment for lumbar spinal Stenosis? A randomized controlled trial. Spine. 2007;32(1):1–8.
Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the Maine Lumbar Spine Study. Spine (Phila Pa 1976). 2005;30(8):936–43.
Weinstein JN, Tosteson TD, Lurie JD, Tosteson A, Blood E, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine (Phila Pa 1976). 2010;35(14):1329–38.
Hao J. Fusion or not for degenerative lumbar spinal stenosis: a meta-analysis and systematic review. Surgical options for lumbar spinal stenosis. Pain Physician. 2018;21:1–7.
Machado GC, Ferreira PH, Yoo RI, Harris IA, Pinheiro MB, Koes BW, van Tulder MW, Rzewuska M, Maher CG, Ferreira ML. Decompression plus fusion versus decompression alone for degenerative lumbar spondylolisthesis: a systematic review and meta-analysis. Cochrane Database Syst Rev. 2016;1:11.
Liang HF, Liu SH, Chen ZX, Fei QM. Decompression plus fusion versus decompression alone for degenerative lumbar spondylolisthesis: a systematic review and meta-analysis. Eur Spine J. 2017;26(12):3084–95.
Gala RJ, Russo GS, Whang PG. Interspinous implants to treat spinal stenosis. Curr Rev Musculoskelet Med. 2017;10(2):182–8.
Gazzeri R, Galarza M, Alfieri A. Controversies about interspinous process devices in the treatment of degenerative lumbar spine diseases: past, present, and future. BioMed Res Int. 2014;2014:1–15.
Nunley PD, Shamie AN, Blumenthal SL, Orndorff D, Block JE, Geisler FH. Interspinous process decompression: expanding treatment options for lumbar spinal stenosis. Biomed Res Int. 2016;2016:3267307.
Barbagallo GM, Olindo G, Corbino L, Albanese V. Analysis of complications in patients treated with the X-Stop Interspinous Process Decompression System: proposal for a novel anatomic scoring system for patient selection and review of the literature. Neurosurgery. 2009;65(1):111–9 (discussion 119-20).
Patel VV, Whang PG, Haley TR, Bradley WD, Nunley PD, Davis RP, Miller LE, Block JE, Geisler FH. Superion interspinous process spacer for intermittent neurogenic claudication secondary to moderate lumbar spinal stenosis: two-year results from a randomized controlled FDA-IDE pivotal trial. Spine (Phila Pa 1976). 2015;40(5):275–82.
Patel VV, Nunley PD, Whang PG, Haley TR, Bradley WD, Davis RP, et al. Superion® interspinous spacer for treatment of moderate degenerative lumbar spinal stenosis: durable three-year results of a randomized controlled trial. J Pain Res. 2015;8:657–62.
Deer TR, Kim C, Wahezi SE, et al. Objective real-world outcomes of patients suffering from painful neurogenic claudication treated with the MILD procedure: interim 6-month report of a randomized controlled trial. J Pain Res. 2021;14:1687–97.
Deer TR, Gride JS, Pope JE, et al. Best practices for minimally invasive lumbar spinal stenosis treatment 2.0 (MIST): consensus guidance from the American Society of Pain and Neuroscience (ASPN). J Pain Res. 2022;15:1325–54.
Staats P, Chafin T, Golovac S, Kim C, Li S, et al. Long-term safety and efficacy of minimally invasive lumbar decompression procedure for the treatment of lumbar spinal stenosis with neurogenic claudication. Reg Anesth Pain Med. 2018;43:789–94.
Mekhail NA, Costandi SJ, et al. The impact of age on the outcomes of minimally invasive lumbar decompression for lumbar spinal stenosis. Med Devices Evid Res. 2020;13:151–61.
Jain S, Deer T, Sayed D, et al. Minimally invasive lumbar decompression: a review of indications, techniques, efficacy and safety. Pain Manag. 2020;10:331–48 (epub ahead of print).
Postacchini F, Postacchini R, Menchetti PP, Sessa P, Paolino M, Cinotti G. Lumbar interspinous process fixation and fusion with stand-alone interlaminar lumbar instrumented fusion implant in patients with degenerative spondylolisthesis undergoing decompression for spinal stenosis. Asian Spine J. 2016;10(1):27–37.
Schmidt S, Franke J, Rauschmann M, Adelt D, Bonsanto M, Sola S. Prospective, randomized, multicenter study with 2-year follow-up to compare the performance of decompression with and without interlaminar stabilization. J Neurosurg Spine. 2018;28:406–15.
Musacchio MJ, Lauryssen C, Davis RJ, Bae HW, Peloza JH, Guyer RD, Zigler JE, Ohnmeiss DD, Leary S. Evaluation of decompression and interlaminar stabilization compared with decompression and fusion for the treatment of lumbar spinal stenosis: 5-year follow-up of a prospective, randomized, controlled trial. Int J Spine Surg. 2016;26(10):6.
Xu W, Ran B, Luo W, Li Z, Gu R. Is lumbar fusion necessary for chronic low back pain associated with degenerative disk disease? A meta-analysis. World Neurosurg. 2021;146:298–306. https://doi.org/10.1016/j.wneu.2020.11.121.
Wang X, Wanyan P, Tian JH, Hu L. Meta-analysis of randomized trials comparing fusion surgery to non-surgical treatment for discogenic chronic low back pain. J Back Musculoskelet Rehabil. 2015;28(4):621–7. https://doi.org/10.3233/BMR-140571.
Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. Four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Jt Surg Am. 2009;91(6):1295–304.
Nunley PD, Patel VV, Orndorff DG, Lavelle WF, Block JE, Geisler FH. Five-year durability of stand-alone interspinous process decompression for lumbar spinal stenosis. Clin Interv Aging. 2017;12:1409–17.
Schenck CD, Terpstra SES, Moojen WA, van Zwet E, Peul W, Arts MP, Vleggeert-Lankamp CLA. Interspinous process device versus conventional decompression for lumbar spinal stenosis: 5-year results of a randomized controlled trial. J Neurosurg Spine. 2021. https://doi.org/10.3171/2021.8.SPINE21419. (Epub ahead of print).
Meyer B, Baranto A, Schils F, Collignon F, Zoega B, Tan L, LeHuec JC, NICE Trial Study Group. Percutaneous interspinous spacer vs. decompression in patients with neurogenic claudication: an alternative in selected patients? Neurosurgery. 2018;82(5):621–9. https://doi.org/10.1093/neuros/nyx326.
Pope JE, Deer TR, Falowski SM. A retrospective, single-center, quantitative analysis of adverse events in patients undergoing spinal stenosis with neurogenic claudication using a novel percutaneous direct lumbar decompression strategy. J Pain Res. 2021;24(14):1909–13.
Benyamin RM, Staats PS, MiDAS EI. MILD® is an effective treatment for lumbar spinal stenosis with neurogenic claudication: MiDAS ENCORE randomized controlled trial. Pain Physician. 2016;19(4):229–42.
Brown LL. A double-blind, randomized, prospective study of epidural steroid injection vs. the mild® procedure in patients with symptomatic lumbar spinal stenosis. Pain Pract. 2012;12(5):333–41.
Sclafani JA, Liang K, Ohnmeiss DD, Gordon C. Clinical outcomes of a polyaxial interspinous fusion system. Int J Spine Surg. 2014;8:35.
Kim HJ, Bak KH, Chun HJ, et al. Posterior interspinous fusion device for one-level fusion in degenerative lumbar spine disease: comparison with pedicle screw fixation—preliminary report of at least one year follow up. J Korean Neurosurg Soc. 2012;52(4):359–64.
Grinberg SZ, Simon RB, Dowe C, Brecevich AT, Cammisa FP, Abjornson C. Interlaminar stabilization for spinal stenosis in the Medicare population. Spine J. 2020;20(12):1948–59. https://doi.org/10.1016/j.spinee.2020.06.015.
Simon RB, Dowe C, Grinberg S, Cammisa FP Jr, Abjornson C. The 2-level experience of interlaminar stabilization: 5-year follow-up of a prospective, randomized clinical experience compared to fusion for the sustainable management of spinal stenosis. Int J Spine Surg. 2018;12(4):419–27. https://doi.org/10.14444/5050.
Acknowledgements
The authors would like to thank Dr. Chris Bovinet for his contributions and support over the past 3 years spent writing this manuscript.
Funding
PSPS received an unrestricted educational grant as part of participation in supporting the PSPS Minimally Invasive Spine Cadaveric Training Lab in Seattle in 2021, to further support spine and pain interventions, collaboration, education, and research, with funding from Avanos, Relievant, Vertos, Boston Scientific, PainTEQ, Stryker, Spinal Simplicity, Aurora, and Vivex Biologics. PSPS is funding the journal’s Rapid Service and Open Access Fees.
Author information
Authors and Affiliations
Contributions
Michael Dorsi, Jason E Pope, Raman Naidu, and Stephen M Falowski conceived the review. Michael J Dorsi, Patrick Buchanan, Chau Vu, Harjot S Bhandal, David W Lee, Samir Sheth, Phil M Shumsky, Nolan J Brown, Alexander Himstead, and Ryan Mattie reviewed and critically appraised studies and drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Michael J Dorsi serves as a consultant for Abbott, Camber Spine, Life Spine, Nevro, Nuvasive. Patrick Buchanan serves as a consultant and principal investigator for Abbott and PainTEQ. Chau Vu serves as a consultant for Saluda Medical; consultant and principal investigator for PainTEQ. Harjot S Bhandal serves as a consultant for Saluda Medica and principal investigator for Aurora Spine. David W Lee serves as a consultant for Medtronic, Boston Scientific, Mainstay Medical, Petal Surgical; speakers bureau for Abbott. Samir Sheth serves as consultant for SPR, Boston Scientific, Medtronic, and Vertos. Phil M Shumsky serves as a consultant for Saluda Medical. Nolan J Brown has no disclosures or conflicts of interest. Alexander Himstead has no disclosures or conflicts of interest. Ryan Mattie serves as a consultant for SPR therapeutics and Sutton Pierce. Steven M Falowski serves as a consultant for Saluda Medical, SPR Therapeutics, CornerLoc, PaintTEQ, SpineThera, and Aurora Spine and has relevant financial relationships with Abbott, Medtronic, Saluda Medical, CornerLoc and Mainstay. Ramana Naidu serves as a consultant for Avanos, Abbott, Bicycle Health, Biotronik, Bioventus, Boston Scientific, Cerevu, DoctorPlan, ExerAI, KarunaLabs, Medtronic, Nalu, Omnia Medical, PainTEQ, Relievant, Sonosite, SPR Therapeutics, and Vivex. Jason E Pope serves as a consultant for Abbott, Medtronic, Saluda, Flowonix, SpineThera, Vertos, Vertiflex, SPR Therapeutics, Tersera, Aurora, Spark, Ethos, Biotronik, Mainstay, WISE, Boston Scientific, Thermaquil; has received grant and research support from: Abbott, Flowonix, Saluda, Aurora, PainTEQ, Ethos, Muse, Boston Scientific, SPR Therapeutics, Mainstay, Vertos, AIS, Thermaquil; and is a shareholder of: Vertos, SPR Therapeutics, PainTEQ, Aurora, Spark, Celeri Health, Neural Integrative Solutions, Pacific Research Institute, Thermaquil and Anesthetic Gas Reclamation.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Dorsi, M.J., Buchanan, P., Vu, C. et al. Pacific Spine and Pain Society (PSPS) Evidence Review of Surgical Treatments for Lumbar Degenerative Spinal Disease: A Narrative Review. Pain Ther 13, 349–390 (2024). https://doi.org/10.1007/s40122-024-00588-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-024-00588-4