Abstract
Introduction
A significant number of women who undergo neuraxial labor analgesia experience breakthrough pain. Prompt mitigation of breakthrough pain is essential to improve maternal and fetal outcomes. We evaluated epidural chloroprocaine compared with ropivacaine in alleviating labor breakthrough pain.
Methods
We performed a double-blind randomized controlled clinical trial between May and July 2023. Eligible parturients received epidural analgesia with ropivacaine and sufentanil. Those with breakthrough pain were randomized to receive either 0.125% epidural ropivacaine (group R) or chloroprocaine at concentrations of 0.5% (group C1), 1.0% (group C2), or 1.5% (group C3), all in a volume of 6 mL. The primary outcome was the treatment success rate, indicated by a decrease of at least 4 points on the numerical rating scale pain score 9 min after analgesic injection. Secondary outcomes and adverse effects were also recorded.
Results
Out of 323 patients receiving epidural analgesia, 192 experienced breakthrough pain. After exclusion of three patients because of protocol deviation, there were 47, 48, 47, and 47 patients in group R, C1, C2, and C3, respectively. Group C3 demonstrated a higher treatment success rate (39/47, 83.0%) in managing breakthrough pain than group R (26/47, 55.3%), group C1 (12/48, 25.0%), and group C2 (30/47, 63.8%) (p < 0.001). Group C3 had lower numerical rating scale scores at 6 and 9 min after injection and required fewer patient-controlled epidural boluses than other groups. In addition, group C3 reported greater satisfaction than the other groups (p < 0.001). No significant differences were observed in obstetric or neonatal outcomes across these groups.
Conclusion
Parturients experiencing breakthrough pain could receive 1.5% epidural chloroprocaine, rather than lower chloroprocaine concentrations and ropivacaine, to achieve more rapid and better pain relief with higher patient satisfaction.
Trial Registration
Chinese Clinical Trial Registry, ChiCTR2300071069, http://www.chictr.org.cn/index.aspx.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Breakthrough pain affects a significant number of parturients who undergo neuraxial labor analgesia, risking adverse maternal and fetal outcomes if unaddressed. |
There is limited evidence on optimal epidural analgesics for rapid breakthrough pain relief in parturients. |
We hypothesized that epidural chloroprocaine could provide faster and more effective breakthrough pain relief than ropivacaine in parturients. |
What was learned from the study? |
Chloroprocaine at a concentration of 1.5% had greater efficacy (83% success) for breakthrough pain relief than lower concentrations of chloroprocaine and ropivacaine. |
Chloroprocaine at a concentration of 1.5% reduced short-term pain scores and the requirement for patient-controlled epidural boluses compared with lower concentrations. |
For labor breakthrough pain, 1.5% chloroprocaine may improve relief with fewer interventions. |
Introduction
Neuraxial analgesia is broadly accepted for pain relief during labor owing to its rapid onset, preservation of motor function, and minimal adverse effects. However, a significant number of parturients (30–89%) undergoing this analgesia still experience breakthrough pain (BTP) [1]. BTP refers to the onset of pain after a period of effective analgesia. Many factors, such as physiological and psychological stress, as well as genetic factors, can contribute to BTP. Frequent BTP can lead to maternal complications, including prolonged labor and an increased rate of cesarean section [2]. Additionally, unrelieved pain can have physiological consequences, such as maternal hyperventilation and increased oxygen consumption, which results in hypoxemia and acidosis in mother and fetus [3]. Furthermore, pain-induced hypersecretion of catecholamines can further compromise maternal and fetal outcomes, causing increased maternal blood pressure, reduced uterine contractions, and potential fetal distress [4]. Therefore, it is crucial to develop and implement effective strategies for managing BTP to ensure improved pregnancy outcomes.
Ropivacaine, with its favorable sensorimotor differential block, reduced accumulation potential, and superior cardiovascular safety profile, has been used for epidural analgesia during labor and post-cesarean section pain management [5]. However, its delayed onset and limited spread may render it less practical for immediate relief from sudden BTP. Chloroprocaine, a benzoyl ester local anesthetic, has the advantages of rapid onset, efficient spread, and safety profile [6, 7]. It can provide immediate pain relief without significant maternal or neonatal complications in emergency cesarean sections following epidural labor analgesia [8]. Previous research has highlighted the efficacy of chloroprocaine in epidural labor analgesia, even at a concentration as low as 0.43%, and its enhanced efficacy when combined with adjuncts such as 3 µg/mL fentanyl [9]. Recent studies also reported the benefits of 1.5% chloroprocaine in labor, especially in terms of rapid onset and preservation of motor function [10, 11]. Most studies have concentrated on the analgesic duration or pain scores at specific intervals after chloroprocaine administration, disregarding the direct assessment of BTP. There is a lack of comprehensive data on how different chloroprocaine doses impact the BTP during labor analgesia.
Considering this, we hypothesized that chloroprocaine might offer rapid and potent relief from BTP without increased maternal or fetal risks. We have structured a prospective, double-blind, randomized study comparing 0.125% ropivacaine with graded concentrations of chloroprocaine (0.5%, 1.0%, and 1.5% at 6 mL) for epidural labor analgesia. Our primary objective was to explore the onset and efficacy of these chloroprocaine concentrations on BTP during labor analgesia.
Methods
Study Design, Ethical Approval, and Registration
This prospective, double-blind randomized controlled trial took place at the Anhui Provincial Maternal and Child Health Hospital, China, between May and July 2023. The study adhered to the Declaration of Helsinki and received the approval from the hospital Ethics Committee (Approval No. YYLL2023-04-01-01, April 1, 2023). The study protocol was registered in the Chinese Clinical Trial Registry (ChiCTR2300071069, http://www.chictr.org.cn/index.aspx). All participants provided the informed consent.
Research Participants and Inclusion/Exclusion Criteria
Recruitment occurred after the parturients entered the delivery room before the initiation of labor analgesia. Eligible patients were primiparas with singleton, full-term pregnancy, normal fetal position, aged between 20 and 36 years, a body mass index (BMI) of 21.0–35.0 kg/m2, American Society of Anesthesiology (ASA) physical status I or II, scheduled for vaginal delivery under epidural analgesia, and experienced BTP after epidural analgesia. BTP was defined as a numerical rating scale (NRS) score > 3 after initial pain relief following at least one patient-controlled epidural analgesia (PCEA) bolus administration. Exclusion criteria included patient refusal of additional analgesic, high-risk pregnancies (placental abruption, severe preeclampsia, and placenta previa), known fetal abnormalities, history of chronic pain or prenatal psychotropic drug use, poor mental cooperation, allergies to opioids or local anesthetics, delivery within 1 h post-analgesia, and cases where labor analgesia required an emergency cesarean section.
Randomization and Blinding
A computer-generated random number generator was used to randomize study participants on a 1:1:1:1 ratio to receive different concentrations of local anesthetics for labor analgesia.
- Group R (control):
-
1.5 mL of 0.5% ropivacaine + 4.5 mL of normal saline (0.125% ropivacaine).
- Group C1:
-
1 mL of 3% chloroprocaine + 5 mL of normal saline (0.5% chloroprocaine).
- Group C2:
-
2 mL of 3% chloroprocaine + 4 mL of normal saline (1.0% chloroprocaine).
- Group C3:
-
3 mL of 3% chloroprocaine + 3 mL of normal saline (1.5% chloroprocaine).
The final volume of the study drugs was 6 mL. There were no intravenous drugs administrated perioperatively. A nonparticipating nurse prepared these drugs. Group allocations were concealed from patients, obstetricians, and research staff involved in patient enrollment and data collection. All patients were accompanied by one of the investigators (LH or CZ) throughout the labor. Data were recorded until the end of labor.
Labor Analgesia Protocol
In the labor room, every parturient was monitored by pulse oximetry, noninvasive blood pressure measurement, and fetal tocodynamometry. An intravenous access was established. The parturient was placed in the left lateral position. At the estimated L2–3 interspace, the epidural procedure was performed using a 16-gauge needle and the loss of resistance to air technique. Subsequently, a 19-gauge multi-orifice epidural catheter was advanced approximately 4 cm into the epidural space. After the administration of a test dose of 3 mL of 1.0% lidocaine, epidural analgesia was initiated and maintained using a solution of 0.09% ropivacaine combined with 0.4 µg/mL sufentanil (10 mL). The sensory block level was assessed using an alcohol-soaked cotton swab. If the sensory block level was below T10 after 15 min, an additional 5 mL of the analgesic solution was administered. Thirty minutes later, if the analgesia was deemed insufficient (NRS score > 3), the epidural catheter placement was considered unsuccessful, and the parturient was excluded from the study.
After successful epidural analgesia initiation, the labor analgesia was continued by PCEA. Analgesia via programmed intermittent epidural bolus (PIEB) commenced with a 10-mL bolus 30 min after pump initiation. It was sustained with an hourly 10-mL programmed bolus and complemented by 7-mL PCEA boluses, with a 20-min lockout interval. The hourly epidural volume reached a maximum of 31 mL.
Breakthrough Pain Management Protocol
Patients undergoing epidural labor analgesia were randomly assigned to analgesic treatment with different study drugs once they experienced BTP during labor. Once the BTP occurred, every parturient received the experimental analgesic and was evaluated every 3 min for 15 min using the maternal NRS (0–10, where 0 and 10 indicated no pain and severe pain, respectively) for pain. If the experimental randomized treatment did not provide adequate pain relief, the alternative treatment was administered. Subsequent evaluation occurred 15 min post-administration of the second solution. When the pain score did not decrease by at least 4 points from the baseline 15 min after the second drug administration, 5 mL of 2% epidural lidocaine was administered. If the third dose failed to decrease the visual analog scale pain score by at least 4 points below the baseline within 15 min (45 min post-initial study drug), the epidural catheter was deemed nonfunctional, and the case was excluded from further analysis.
Outcome Measurements
The primary outcome was the treatment’s success rate for BTP. This randomized, double-blind trial aimed to investigate the onset of action and analgesic effect of chloroprocaine compared with ropivacaine in breakthrough pain relief. Unlike a previous study [12], we defined treatment success as a decrease in the NRS score by at least 4 from the baseline during active uterine contraction observed 9 min post-drug administration.
Secondary outcomes included the following: (a) number of patients with effective analgesia (decrease in the pain score by at least 4 from the baseline at 15 min post-administration); (b) maternal NRS pain scores assessed every 3 min for 15 min after injection, with the NRS scores recorded every 15 min for 15–60 min after the initial injection and then every hour until delivery; (c) modified Bromage scores (1 = complete motor block; 2 = almost complete, allowing only foot movement; 3 = partial, allowing knee movement; 4 = detectable hip flexion weakness, allowing leg raise but not sustained; and 5 = no weakness, allowing leg raise for 10 s; 6 = no weakness), evaluated at 15 min post-injection; (d) ropivacaine and sufentanil consumption; and (e) maternal satisfaction gauged at 1 and 24 h post-delivery using a 10-cm visual analog scale.
Additional secondary outcomes encompassed the following: (1) stages leading to delivery; (2) potential adverse effects such as postpartum headache, itching, nausea, and vomiting; (3) hypotension incidence, marked by systolic blood pressure decreasing below 90 mmHg or declining more than 20% from the baseline; (4) outcomes concerning neonates; and (5) obstetric outcomes.
Sample Size Calculation
The sample size was estimated using PASS v11.0 (PASS, NCSS, USA). The primary outcome of this study was the “success” rate of epidural injection analgesia for BTP. In our preliminary study involving four patient groups (10 patients/group), the success rates of epidural analgesia for BTP in groups R, C1, C2, and C3 were 40%, 50%, 60%, and 80%, respectively. With a power of 0.90 and an α level of 0.05, a minimum of 38 participants per group, with a total of 152, were required. Considering a 20% attrition rate, we decided to enroll 48 participants in each group, with a total of 192 participants.
Statistical Analysis
A per-protocol analysis was conducted. Data were presented as either mean ± standard deviation or median (interquartile range) unless stated otherwise. The Kolmogorov–Smirnov test was used to assess the normality of continuous data. Variables were categorized and analyzed on the basis of their standard or skewed distribution. For continuous variables, either analysis of variance (ANOVA) or the Kruskal–Wallis H test was used. Categorical data were presented as proportions and analyzed using the chi-squared or Fisher’s exact test. Odds ratio (OR) and 95% confidence intervals (CI) were reported. The linear mixed model was used to compare pain scores across groups at specified time points, adjusting for baseline scores. A Bonferroni correction accounted for multiple comparisons. The initial experimental randomized drug loading dose had an endpoint recorded at 0 min. Only scores from the first 3 h were included in the analysis. All data were analyzed using an intention-to-treat principle. Statistical significance was set at a two-sided p value of less than 0.05. Statistical analyses were performed in SPSS 26.0 (Chicago, IL, USA).
Results
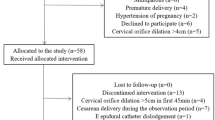
The Consolidated Standards of Reporting Trials flow diagram details the number of participants in this clinical trial (Fig. 1). A total of 323 patients were assessed for eligibility. After exclusion of 131 patients, 192 patients were randomized, with 48 allocated to each group. There were three protocol deviations. One participant in each of groups C2 and C3 gave birth within 1 h. One participant in group R had a unilateral block. The remaining study participants were analyzed in the groups to which they were randomized. The primary outcome was recorded for all 189 participants randomized. However, four participants (one in group R, two in group C1, and one in group C2) were switched to cesarean section, and their secondary outcome data were not recorded. There were no statistically significant differences in baseline characteristic comparisons among four groups (Table 1).
Primary Outcome
The study’s primary outcome, the treatment’s success rate for BTP, exhibited significant differences among the four groups (Fig. 2). The success rate of the treatment in group C3 was significantly higher (39/47 (83.0%)) than those in the other three groups (p < 0.01). Compared with group R [26/47 (55.3%)], group C1 exhibited a significantly lower success rate [12/48 (25.0%)] [odds ratio (OR) 0.27; 95% confidence interval (CI) 0.11–0.64], whereas group C3 had a significantly higher success rate (OR 3.94; 95% CI 1.52–10.22) (p < 0.001). No significant difference was observed between group C2 [30/47 (63.8%)] and group R (OR 1.43; 95% CI 0.62–3.26) (Fig. 2).
Primary outcome. a The proportion of patients in the four groups with successful breakthrough pain treatment. b The absolute difference (95% CIs) in success rates between chloroprocaine (groups C1, C2, and C3) and ropivacaine (group R). Group R, 0.125% ropivacaine; group C1, 0.5% chloroprocaine; group C2, 1.0% chloroprocaine; group C3, 1.5% chloroprocaine
Secondary Outcomes
Pain scores exhibited similarity among the four groups when BTP treatment was requested. At 6 min post-initial drug injection, the NRS pain scores were lower in group C3 (Fig. 3). Significant differences were noted at specific time points (linear mixed model: 6 min, p < 0.01; 9 min, p < 0.01). There was no statistical difference in the four groups after 15 min of treatment, indicating similar effective analgesia among the groups (p = 0.05). Participants in group C3 received a smaller number of PCEA bolus than those in the other groups (Table 2). Patient satisfaction in groups C2 and C3 was significantly higher than in groups C1 and R at 1 and 24 h post-delivery (Table 2).
Most participants scored between 4 and 6 on the modified Bromage scale. Therefore, the proportion of participants scoring 4 or less was compared across the four groups. Although group C3 more frequently experienced motor block, no significant differences were observed (Table 2). No differences were observed in obstetric and neonatal outcomes or adverse effects across the four groups (Table 3).
Discussion
This study compared the onset time and analgesic effects of ropivacaine and different concentrations of chloroprocaine for the treatment of BTP in labor analgesia. The results indicated that 1.5% chloroprocaine had a more rapid onset than 0.125% ropivacaine and 0.5%/1.0% chloroprocaine for treating BTP during labor analgesia, although there was no significant difference in the final analgesic effect. The epidural administration of 1.5% chloroprocaine resulted in a reduced requirement for PCEA boluses when compared with 0.5%/1.0% chloroprocaine or 0.125% ropivacaine during labor analgesia. Nevertheless, higher concentrations of chloroprocaine might increase the motor block incidence compared with lower concentrations of chloroprocaine or ropivacaine, although without statistically significant differences. We concluded that 1.5% epidural chloroprocaine could be a superior choice for treating BTP during labor analgesia, owing to its rapid onset, decreased short-term NRS scores, decreased requirement for PCEA bolus adjustments, and increased patient satisfaction compared with the other groups.
Managing BTP during labor analgesia is crucial. Inadequate pain relief causes maternal discomfort and can also affect fetal health and the delivery process [13]. Delays in the onset of pain relief are common with certain analgesics and can be distressing because of the unpredictable nature of childbirth pain [14]. Our research indicated that 1.5% chloroprocaine could provide a rapid and efficient treatment. Its fast action can not only improve the pain relief but also significantly enhance the maternal childbirth experience. Additionally, the persistent pain relief, indicated by fewer requirements for PCEA boluses, suggested a more efficient way to treat BTP.
There are a wide choice of local anesthetics for epidural labor analgesia [15]. Because of its distinct pharmacological characteristics, chloroprocaine has always attracted research attention in many prospective trials [7, 16]. Our research, which focused on 1.5% chloroprocaine, revealed findings consistent with, yet differing from, earlier studies. Historically, many studies have reported the fast onset of action with 3% chloroprocaine administration during elective cesarean section [7, 17, 18]. However, a lower dose of chloroprocaine might be adequate to provide epidural analgesia. Our results confirmed this, suggesting fast and effective BTP relief at the 1.5% concentration of chloroprocaine. This was in line with the study by Zhu et al., who advocated 6 mL of 1.5% chloroprocaine for activating labor analgesia [11]. Earlier studies implied a trade-off between onset speed and relief duration, implying that faster agents might last for less time [7, 19, 20]. We challenged this idea, showing that 1.5% chloroprocaine could provide fast and lasting BTP relief because the PIEB used in the current study might have produced a better analgesic effect. The PIEB’s high-volume (7–12 mL) bolus doses improved the distribution of local anesthetic solution in the epidural space [21, 22], whereas the majority of PCEA regimens used reduced bolus volumes (4–6 mL) [23].
Our research exclusively involved women in labor experiencing BTP. Differing from previous studies [12, 24], we specifically examined pregnant women exhibiting BTP, namely, those with an NRS > 3, requiring additional epidural after PCEA bolus use in the first labor stage. Our method offered a more focused and clinically pertinent perspective, unlike previous studies that included labor analgesia with BTP incidence or total analgesic consumption [24, 25]. Moreover, our BTP protocol granted patients pain control, a crucial factor for satisfaction. Thus, further research on this subject remains necessary for additional validation.
Another distinction is the decreased requirement for PCEA boluses in our findings. The analgesic effects of chloroprocaine were well documented [26, 27]. Our study demonstrated persistent BTP relief by chloroprocaine during labor analgesia, leading to fewer interventions. This could impact patient comfort and could also lessen the workload of healthcare professionals in labor monitoring and interventions. However, patient individual factors, clinical contexts, and evolving techniques should still be considered during the personalized pain managements. Our study contributes to the chloroprocaine literature, but more research is required.
Several limitations warrant consideration before applying our study’s findings to clinical settings. First, the findings may not be universally applicable because of stringent inclusion criteria, especially for patients with conditions such as obesity or shoulder dystocia. Second, using preliminary experiments instead of a sequential method to determine chloroprocaine’s dosage may impact the data’s accuracy. Third, because we only observed the short-term outcomes with analgesics given during the first stage of labor, our findings might not apply to the second stage. Patients in the second stage of labor may require increased epidural analgesia doses. Future studies can investigate the long-term impacts of 1.5% chloroprocaine on the maternal and neonatal outcomes. The combination effects of chloroprocaine with other analgesics also require further research. In addition, pain experience is highly individualized. The patient-specific genetic, metabolic, and psychological factors could also influence the personalized pain management approaches.
Conclusion
Compared with 0.5%/1.0% chloroprocaine or 0.125% ropivacaine, 1.5% chloroprocaine could accelerate the onset of analgesia, reduce additional PCEA consumption, and improve patient satisfaction without increasing the incidences of adverse effects in parturients with breakthrough pain after epidural labor analgesia. Epidural administration of 1.5% chloroprocaine could be beneficial for parturients experiencing the breakthrough pain.
Data Availability
De-identified data are available from the corresponding author upon reasonable request.
References
Diez-Picazo LD, Guasch E, Brogly N, Gilsanz F. Is breakthrough pain better managed by adding programmed intermittent epidural bolus to a background infusion during labor epidural analgesia? A randomized controlled trial. Minerva Anestesiol. 2019;85(10):1097–104. https://doi.org/10.23736/S0375-9393.19.13470-0.
Tzeng YL, Yang YL, Kuo PC, et al. Pain, anxiety, and fatigue during labor: a prospective, repeated measures study. J Nurs Res. 2017;25(1):59–67. https://doi.org/10.1097/jnr.0000000000000165.
Reynolds F. The effects of maternal labour analgesia on the fetus. Best Pract Res Clin Obstet Gynaecol. 2010;24(3):289–302. https://doi.org/10.1016/j.bpobgyn.2009.11.003.
Shnider SM, Abboud TK, Artal R, et al. Maternal catecholamines decrease during labor after lumbar epidural anesthesia. Am J Obstet Gynecol. 1983;147(1):13–5. https://doi.org/10.1016/0002-9378(83)90076-5.
Song Y, Du W, Zhou S, et al. Effect of dural puncture epidural technique combined with programmed intermittent epidural bolus on labor analgesia onset and maintenance: a randomized controlled trial. Anesth Analg. 2021;132(4):971–8. https://doi.org/10.1213/ANE.0000000000004768.
Kuhnert BR, Kuhnert PM, Philipson EH, et al. The half-life of 2-chloroprocaine. Anesth Analg. 1986;65(3):273–8.
Reschke MM, Monks DT, Varaday SS, et al. Choice of local anaesthetic for epidural caesarean section: a Bayesian network meta-analysis. Anaesthesia. 2020;75(5):674–82. https://doi.org/10.1111/anae.14966.
Ituk U, Wong CA. Anesthetic choices for intrapartum cesarean delivery in patients with epidural labor analgesia. Adv Anesth. 2020;38:23–40. https://doi.org/10.1016/j.aan.2020.07.002.
Polley LS, Columb MO, Lyons G, et al. The effect of epidural fentanyl on the minimum local analgesic concentration of epidural chloroprocaine in labor. Anesth Analg. 1996;83(5):987–90. https://doi.org/10.1097/00000539-199611000-00015.
Ross EL, Reiter PD, Murphy ME, et al. Evaluation of prolonged epidural chloroprocaine for postoperative analgesia in infants. J Clin Anesth. 2015;27(6):463–9. https://doi.org/10.1016/j.jclinane.2015.05.022.
Zhu HJ, He Y, Wang SY, et al. A randomized clinical trial comparing different concentrations of chloroprocaine with lidocaine for activating epidural analgesia during labor. Int J Gen Med. 2022;15:1307–17. https://doi.org/10.2147/IJGM.S351030.
Lee A, Landau R, Lavin T, Goodman S, Menon P, Smiley R. Comparative efficacy of epidural clonidine versus epidural fentanyl for treating breakthrough pain during labor: a randomized double-blind clinical trial. Int J Obstet Anesth. 2020;42:26–33. https://doi.org/10.1016/j.ijoa.2019.11.003.
Sun S, Guo Y, Wang T, et al. Analgesic effect comparison between nalbuphine and sufentanil for patient-controlled intravenous analgesia after cesarean section. Front Pharmacol. 2020;11:574493. https://doi.org/10.3389/fphar.2020.574493.
Wilson SH, Wolf BJ, Bingham K, et al. Labor analgesia onset with dural puncture epidural versus traditional epidural using a 26-gauge whitacre needle and 0.125% bupivacaine bolus: a randomized clinical trial. Anesth Analg. 2018;126(2):545–51. https://doi.org/10.1213/ANE.0000000000002129.
Zhou SQ, Wang J, Du WJ, et al. Optimum interval time of programmed intermittent epidural bolus of ropivacaine 0.08% with sufentanyl 0.3 μg/mL for labor analgesia: a biased-coin up-and-down sequential allocation trial. Chin Med J (Engl). 2020;133(5):517–22. https://doi.org/10.1097/CM9.0000000000000669.
Coppens M, Anssens S, Parashchanka A, et al. Determination of the median effective dose (ED50) of spinal chloroprocaine in labour analgesia. Anaesthesia. 2017;72(5):598–602. https://doi.org/10.1111/anae.13808.
Sharawi N, Williams M, Athar W, et al. Effect of dural-puncture epidural vs standard epidural for epidural extension on onset time of surgical anesthesia in elective cesarean delivery: a randomized clinical trial. JAMA Netw Open. 2023;6(8):e2326710. https://doi.org/10.1001/jamanetworkopen.2023.26710.
Lee LO, Ramirez-Chapman AL, White DL, Zhang X, Lu M, Suresh MS. Postcesarean analgesia with epidural morphine after epidural 2-chloroprocaine: a randomized noninferiority trial. Anesth Analg. 2023;136(1):86–93. https://doi.org/10.1213/ANE.0000000000006109.
Kasson B, Hledin V, Clayton B, Pellegrini J, Reede L. Considerations for management of bupivacaine formulation shortage affecting obstetric anesthesia services. AANA J. 2018;86(4):76–8.
Hillyard SG, Bate TE, Corcoran TB, Paech MJ, O’Sullivan G. Extending epidural analgesia for emergency Caesarean section: a meta-analysis. Br J Anaesth. 2011;107(5):668–78. https://doi.org/10.1093/bja/aer300.
Epsztein Kanczuk M, Barrett NM, Arzola C, Downey K, Ye XY, Carvalho JC. Programmed intermittent epidural bolus for labor analgesia during first stage of labor: a biased-coin up-and-down sequential allocation trial to determine the optimum interval time between boluses of a fixed volume of 10 mL of bupivacaine 0.0625% with fentanyl 2 μg/mL. Anesth Analg. 2017;124(2):537–41. https://doi.org/10.1213/ane.0000000000001655.
Zakus P, Arzola C, Bittencourt R, Downey K, Ye XY, Carvalho JC. Determination of the optimal programmed intermittent epidural bolus volume of bupivacaine 0.0625% with fentanyl 2 μg/mL at a fixed interval of forty minutes: a biased coin up-and-down sequential allocation trial. Anaesthesia. 2018;73(4):459–65. https://doi.org/10.1111/anae.14159.
Halpern SH, Carvalho B. Patient-controlled epidural analgesia for labor. Anesth Analg. 2009;108(3):921–8. https://doi.org/10.1213/ane.0b013e3181951a7f.
Roofthooft E, Filetici N, Van Houwe M, et al. High-volume patient-controlled epidural vs programmed intermittent epidural bolus for labour analgesia: a randomized controlled study. Anaesthesia. 2023;78(9):1129–38. https://doi.org/10.1111/anae.16060.
Roofthooft E, Barbé A, Schildermans J, et al. Programmed intermittent epidural bolus vs patient-controlled epidural analgesia for maintenance of labour analgesia: a two-centre, double-blind, randomized study. Anaesthesia. 2020;75(12):1635–42. https://doi.org/10.1111/anae.15149. (Published correction appears in Anaesthesia. 2021 Apr;76(4):567).
Sharawi N, Bansal P, Williams M, Spencer H, Mhyre JM. Comparison of chloroprocaine versus lidocaine with epinephrine, sodium bicarbonate, and fentanyl for epidural extension anesthesia in elective cesarean delivery: a randomized, triple-blind, noninferiority study. Anesth Analg. 2021;132(3):666–75. https://doi.org/10.1213/ANE.0000000000005141.
Maes S, Laubach M, Poelaert J. Randomised controlled trial of spinal anaesthesia with bupivacaine or 2-chloroprocaine during caesarean section. Acta Anaesthesiol Scand. 2016;60(5):642–9. https://doi.org/10.1111/aas.12665.
Acknowledgements
We thank all the patients who participated in this study.
Medical Writing and Editorial Assistance.
Initial study design, ethical approval, and recruitment of the study participants were conducted by Tianzhen Ji (Anhui Provincial Maternal and Child Health Hospital) and Cheng Xu (Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine).
We thank LetPub (www.letpub.com) for linguistic assistance and pre-submission expert review, which was funded by National Natural Science Foundation of China (No. 82100315) and the Research Project of Anhui Provincial Maternal and Child Health Hospital (No. zd2021-2-1)
Authorship.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have provided their approval for this version to be published.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82100315) and the Research Project of Anhui Provincial Maternal and Child Health Hospital (No. zd2021-2-1). The Rapid Service Fee was funded by Hefei Maternal and Child Health Hospital.
Author information
Authors and Affiliations
Contributions
Study conception/design: Cheng Xu and Lei Xie. Data acquisition/analysis/interpretation: Tianzhen Ji and Can Jiang. Drafting/revising of paper critically for important intellectual content: all authors (Tianzhen Ji, Can Jiang, Hongxia Liu, Zhehao Cai, Rongrong Liu, Lei Xi and Cheng Xu). Approval of final version of the paper: all authors. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors (Tianzhen Ji, Can Jiang, Hongxia Liu, Zhehao Cai, Rongrong Liu, Lei Xi and Cheng Xu) declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
This prospective randomized controlled study was approved by the Ethics Committee of Anhui Provincial Maternal and Child Health Hospital (No. YYLL2023-04-01-01, dated 1 April 2023). The study protocol was registered in the Chinese Clinical Trial Registry (registration no. ChiCTR2300071069). The clinical research was conducted in accordance with the Declaration of Helsinki, and all patients provided informed consent.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ji, T., Jiang, C., Liu, H. et al. Efficacy and Safety of Epidural Chloroprocaine for Breakthrough Pain During Labor Analgesia: A Prospective, Double-Blind, Randomized Trial. Pain Ther 13, 227–239 (2024). https://doi.org/10.1007/s40122-024-00577-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-024-00577-7