Abstract
Introduction
Complex regional pain syndrome type 1 (CRPS-1) is prevalent after trauma, with intractable pain being the most prominent clinical symptom. The impact of sympathetic block on CRPS is unclear. The goal of this study was to explore the characteristics that predict successful symptom relief with lumbar sympathetic block (LSB) in patients with lower extremity CRPS-1.
Methods
The study was designed as a prospective cohort study. Ninety-eight patients diagnosed with lower extremity CRPS-1 between March 2021 and March 2022 were enrolled as participants. All of the patients received two LSB treatments within a month. Sympthetic skin response (SSR) and numeric rating scale (NRS) were recorded before and after LSB treatment. The procedure was judged as a clinically positive response if the patients a 50% or greater reduction in NRS scores. Patients were divided into positive response and negative response groups after LSB treatment: LSB (+) and LSB (−), and the different characteristics and examination findings of the two groups of patients were compared. Furthermore, a multivariable logistic regression model was utilized to evaluate the predictors of successful symptom relief following LSB treatment.
Results
A total of 43.9% (43/98) of patients experienced successful symptom relief, while 56.1% (55/98) had unsuccessful symptom relief. After LSB treatment of all subjects, the overall NRS score decreased, the SSR amplitude increased, and the SSR latency shortened in the affected extremity (P < 0.05). There was a significant difference in the change in SSR amplitude between the LSB (-) and LSB (+) groups (P = 0.000). A 12-month disease duration had an OR (odds ratio) of 4.477 (P = 0.009), and a 510-µV baseline SSR amplitude of the affected extremity had an OR of 7.508 (P = 0.000) in the multivariable analysis that included these explanatory variables.
Conclusions
Patients with lower extremity CRPS-1 can experience significant pain relief after LSB treatment. The predictors of successful symptom relief after LSB treatment were a baseline SSR amplitude of the affected extremity < 510 µV and a disease duration < 12 months.
Trial Registration
The study was registered in the Chinese Clinical Trial Registry (ID: ChiCTR2000037755, date of registration: September 4, 2020).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? | |
An objective, quantitative test for the therapeutic effectiveness of sympathetic block in complex regional pain syndrome type 1 (CRPS-1) patients is currently lacking. | |
The causes for CRPS-1 patients' clinical positive response to lumbar sympathetic block (LSB) are diverse. We aimed to predict which CRPS-1 features may benefit from LSB treatment. | |
What was learned from the study? | |
Patients with CRPS-1 in the lower extremities may benefit from LSB treatment, which is associated with increased SSR amplitude and shortened SSR latency of ipsilateral extremity. | |
There was a significant difference in the change in SSR amplitude between the LSB (−) and LSB (+) groups. | |
The baseline SSR amplitude of the affected extremity < 510 µV and disease duration < 12 months were predictors of successful symptom relief after LSB treatment. |
Introduction
Complex regional pain syndrome type 1 (CRPS-1) is a progressive and chronic pain disorder characterized by intractable pain with allodynia and hyperalgesia, autonomic dysfunction, and motor impairment that develops after noxious inciting events such as a fracture, sprain, or surgery [1]. Limb edema, fluctuations in skin temperature and color, and abnormal sweating are clinical symptoms and signs of autonomic dysfunction, this indicating that the sympathetic nervous system plays a role in the pathophysiology of CRPS-1 [2]. Therefore, sympathetic dysfunction is assumed to be one of the main mechanisms of CRPS-1, and sympathetic intervention is frequently utilized to alleviate the symptoms of sympathetically maintained pain (SMP). However, because of CRPS's multiple interacting mechanisms, effective sympathetic treatment is unstable, limiting its application [3]. There is currently no efficient method for diagnosing sympathetic dysfunction in CRPS patients. We will be able to identify appropriate patients to improve their symptoms if we can predict which characteristics of CRPS will benefit from sympathetic nerve block.
Currently, the main issue is the lack of an objective, quantitative test for the therapeutic efficacy of sympathetic nerve block, which results in the lack of standardization of CRPS prognostic indicators. A precise method to measure sympathetic activity in CRPS patients is still needed. The sympathetic skin response (SSR) is a transient change in the electrical skin potential caused by a variety of internal and external stimuli. It is evaluated utilizing a simple, noninvasive test that indicates an interaction between the surrounding epidermal tissue and sweat glands [4]. Because SSR is a multisynaptic reflex, its amplitude and latency are variable. The SSR latency reflects the conduction duration of the nerve impulse and the SSR amplitude reflects the excitability of sympathetic postganglionic fibers and sweat glands [5]. Generally, amplitude and latency are measured in response to a stimulus, and the values after treatment and before treatment are compared. Due to the large variation in SSR, it should be considered in combination with amplitude and latency when determining whether a single test is abnormal. SSR has recently been used to evaluate patients suffering somatic and autonomic neuropathies [6,7,8], with abnormal SSR results such as prolonged SSR latency or decreased SSR amplitude described in both central and peripheral nervous system illnesses. However, until now, there has been little debate and practical application of SSR in CRPS.
We evaluated the successful symptom relief rates of 98 CRPS-1 patients with lower extremities who received lumbar sympathetic block (LSB) at our institution and analyzed their prognostic value based on clinical characteristics. We discovered that a baseline SSR amplitude of < 510 µV and a disease duration of < 12 months were predictors of successful symptom relief after LSB treatment using multivariate logistic regression analysis.
Methods
Participants
This study was designed as a prospective cohort study. From March 2021 to March 2022, CRPS-1 patients with lower extremity pain who were diagnosed using the Budapest criteria were included in the Department of Pain Management at Shanghai Sixth People's Hospital. A total of 145 patients were chosen based on the following eligibility criteria: newly diagnosed CRPS-1 patients with unilateral lower extremity following a fracture, trauma, or surgery; NRS score of 4 or more on a scale of 0–10 (with 0 indicating no pain and 10 indicating the worst imaginable pain); disease duration greater than 3 months after the initiating noxious stimulus; and age 20–70 years. The exclusion criteria were as follows: SSR failed to be exported; history of diabetes; history of alcoholism; history and signs of central nervous system involvement; history of oral anticholinergic drugs; pregnancy; coagulation abnormalities; infection; or any other condition that may cause signs and symptoms similar to CRPS.
Eventually, of the 145 patients who were included, the study enrolled 98 patients whose eligibility was guaranteed (Fig. 1). This trial (No. 2019–119) was approved by the Shanghai Sixth People's Hospital's ethics committee and was registered in the Chinese Clinical Trial Registry. The registration number is ChiCTR2000037755. The trial was registered at https://www.chictr.org.cn/on September 4, 2020. All procedures involving individuals were carried out in accordance with the ethical guidelines set out by the National Research Council. In accordance with the Declaration of Helsinki, the researchers explained the significance of the study to all participants, who signed a written informed consent form.
Treatment Protocols
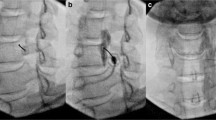
Each patient received two LSB treatments in a month in accordance with our standard protocol. NRS scores and lower extremity SSR of patients were recorded before enrollment and then recorded again 1 week after the second LSB treatment. Throughout the procedure and for at least 30 min later, the patients were continuously monitored by continuous noninvasive blood pressure monitoring and pulse oximetry. Just prior to the LSB, an intravenous line was established for safety purposes. Physicians with considerable experience performed the LSB procedure on all CRPS participants in the trial. Based on the previous literature [9], each patient was lying on his side during the procedure. By combining ultrasonography and fluoroscopy, the target of LSB was located in the upper third of L3 or the lower third of L2. A 15-cm, 20-gauge needle was inserted with real-time ultrasound guidance until the anterolateral side of the vertebra was reached after needle insertion. The anterior fascia of the psoas major muscle was penetrated by the ultrasound through guidance medially over the transverse process of the lumbar spine. The psoas major muscle on the anterolateral side of the lumbar vertebral body was the target of the needle tip, which was positioned anteriorly and medially. To avoid harming the vascular system and to create the needle trajectory, color Doppler was used. Before placing the needle, the kidney was examined to ensure that it would not get in the way. Using an in-plane technique, the needle was inserted from the lateral to the medial direction. After confirming the final position of the needle tip, the injection of 2–3 ml of contrast agent was then monitored using the C-arm in the anterior posterior (AP) and lateral projection. In a successful LSB, the sympathetic diffusion of the contrast agent was straight throughout the longitudinal axis with no lateral or posterior extension and no apparent psoas shadow. Following the confirmation that the contrast dye had diffused appropriately, 15 ml of 1% lidocaine was injected [10].
The SSR test uses electrodiagnostic equipment (Focus, Dantec Keypoint) to monitor an induced change in the electrical potential of the skin to evaluate sudomotor function. Electrical stimulus was used to detect SSR in the lower extremities, with surface electrodes containing an active electrode and a reference electrode. In a room with a temperature of approximately 25 °C, the active potential changes were recorded on the soles of the feet, and the dorsum of the feet was used as a reference. The stimulus current has a wave width of 0.2 ms, a current intensity of 20–30 mA, and an interval of more than 60 s between electrical stimuli, which can minimize the inaccuracy caused by the adaptation of the stimulus site. The SSR parameters to be measured include latency (the time when the baseline deviates for the first time following an electrical stimulus) and amplitude (the distance between negative wave peak and positive wave peak) (Fig. 2). That SSR failure to export was defined as no reaction to the stimulus at either extremity; that is, no consistent change in the baseline was detected in either of the 2 s following the stimulus recordings. Finally, the last SSR test was conducted no less than 1 week after LSB treatment.
Data Recording
Baseline characteristics, including age, body mass index (BMI), disease duration, LSB results (LSB−/LSB+), baseline NRS scores, baseline SSR latency of the affected side and healthy side, and baseline SSR amplitude of the affected side and healthy side, were collected from the patients’ medical records. The NRS was used to assess the severity of limb pain, which ranged from 0 (no pain) to 10 (severe pain). After the procedure, we will assess the efficacy of the LSB treatment. The percentage of pain relief was calculated as (NRS at baseline—NRS after block)/NRS at baseline × 100%. The procedure was judged as a clinically positive response if the patients exhibited a 50% or greater reduction in their NRS score. Otherwise, the procedure was considered to have a negative response.
During the procedure, any adverse symptoms (such as abnormally elevated pain, numbness, paresthesia, back discomfort, and motor weakness) were noted.
Statistical Analysis
The mean ± standard deviation (SD) was reported for normally distributed continuous data on patient demographics and baseline characteristics, whereas the median (interquartile range) was reported for nonnormally distributed continuous data. Student’s t test was used to assess the effect of baseline demographics and clinical symptoms on the response to LSB, while Pearson’s chi-square test was used to compare categorical variables. To compare the changes in NRS scores and SSR parameter values before and after LSB, the paired Wilcoxon test or the signed rank-sum test was utilized.
Binomial logistic regression analysis was used to determine covariates that were associated with the clinical response of LSB. SSR parameter baseline values and other potential prognostic variables that exhibited a significance level of less than 0.20 were entered into the logistic regression. Univariate regression was run first, using one covariate at a time. Next, the covariates that were significant predictors at a P < 0.2 level were considered together in the multivariate regression model to adjust the SSR predictive effect and rule out any confounder effects. Multicollinearity was checked before we performed the multivariate logistic regression analysis. The variance inflation factor (VIF) was calculated, and a VIF above 10 indicates that the model has multicollinearity.
All statistical analyses were performed with SPSS software (version 22.0 IBM Corp., Armonk, NY, USA). P < 0.05 was considered statistically significant.
Results
Demographic and Baseline Characteristics of Participants
This study enrolled a total of 98 participants. Patient demographics and initial baseline NRS scores and SSR parameter values are shown in Table 1. The baseline SSR latency of affected limbs was much longer than that of the healthy limbs, and the baseline SSR amplitude of the affected limbs was significantly lower than that of the healthy limbs (P < 0.05) (Table 1).
The Clinical Response of LSB and the Change in SSR Measures
Each patient's symptom relief rates were evaluated after two LSB treatments. The procedure was considered a clinical positive response if the patients' NRS scores were reduced by at least 50%. In contrast, the procedure was considered a negative response for symptom relief. As a result, the patients were divided into positive and negative groups: LSB (+) and LSB (−), which represented successful and unsuccessful LSB treatment, respectively. Statistics after calculation show that the number of CRPS patients in the LSB (−) group is 55, and the number of patients in the LSB (+) group is 43. Between the LSB (−) and LSB (+) groups, there were no significant differences in age, sex, baseline NRS score, SSR latency of affected and healthy limbs, or baseline SSR amplitude of healthy limbs (P > 0.05). However, there were significant differences in BMI, disease duration and baseline SSR amplitude of the affected limbs between the two groups (P = 0.044, P = 0.000 and P = 0.000, respectively). According to Budapest criteria, some specific CRPS features were observed between the LSB (−) and LSB (+) groups. There was no significant difference in the subjective categories of sensory and motor/trophic disturbances between the two groups (P = 1.000 and P = 0.702, respectively), but there were significant differences in the subjective categories of vasomotor and sudomotor/edema disturbances between the two groups (P = 0.014 and P = 0.001, respectively) (Table 2).
After LSB treatment of all subjects, the overall NRS score of CRPS patients significantly decreased, the SSR latency of the affected limbs was significantly shortened, and the SSR amplitude of the affected limbs was significantly increased (P < 0.05) (Fig. 3).
In CRPS patients before and after treatment, between the LSB (−) and LSB (+) groups, there was no significant difference in the change in SSR latency (103.98 ± 79.07 ms and 122.33 ± 91.34 ms) (P = 0.290); however, there was a significant difference in the change in SSR amplitude (54.73 ± 41.18 and 145.58 ± 61.81 µV) (P = 0.000) (Fig. 4).
SSR and Other Factors Influencing the Effectiveness of LSB
The univariable analysis revealed that age, BMI, sex, disease duration, and SSR amplitude of the affected limb were significant explanatory variables (P < 0.20) (Table 3). As all VIF values were < 1.5, there was no multicollinearity in our model. The multivariable logistic analysis showed that a < 510-µV SSR amplitude of the affected limb had an OR of 7.508, and a < 12-month duration of illness had an OR of 4.477 (Table 4).
Discussion
Clinically, sympathetic activity is frequently observed to significantly enhance the degree of pain in CRPS patients, which is mediated by the coupling of sympathetic postganglionic C fibers with peripheral afferent sensory neurons to produce SMP [11, 12]. Sympathetic block is a typical treatment for CRPS pain and other symptoms, and its therapeutic effect varies greatly [13, 14]. It is difficult to determine which CRPS-1 features are required for effective sympathetic block. In this study, we employed a multivariate logistic regression model to explore predictors of successful symptom relief following LSB treatment. It was discovered that a baseline SSR amplitude of the affected extremity < 510 µV and disease duration < 12 months were predictors of successful LSB treatment.
Currently, the clinical diagnosis of CRPS mainly depends on the reported symptoms, presence of signs, and exclusion of alternative causes [15, 16], and there is still a lack of objective and quantitative diagnostic or prediction tests. Despite substantial autonomic dysfunction, CRPS-1 patients, unlike CRPS-2 patients, do not have impairments in motor and major sensory nerve fiber function [17]. As a consequence, nerve conduction velocity (NCV) is ineffective for diagnosis since it cannot detect thinly myelinated or unmyelinated small nerve fiber injury [18]. The SSR is the only autonomic test widely available on routine EMG equipment [19], which can substitute for the deficiencies of NCV by detecting the conduction function of sympathetic sudomotor fibers [18, 20, 21]. The SSR latency and amplitude can be changed in response to sympathetic hyperactivity of CRPS because it alters the sweat fibers, which in turn influences skin resistance [22]. Additionally, SSR has been shown to have good consistency with other autonomic function detection methods in diabetic neuropathy [23], uremic peripheral neuropathy [24], chemotherapy-induced peripheral neuropathy [25] and other diseases and can well evaluate the autonomic function impairment of these diseases.
There are very few articles investigating the role of SSR in CRPS diagnosis and treatment. In one study [26], CRPS patients showed prolonged latency of SSR. In another study [27], it was found that the mean amplitude was higher on the affected side, while the mean latency was shortened. However, after sympathetic block, amplitude was reduced, and latency was prolonged. The two studies had contradictory results, most likely because they were all restricted by a small number of patients. Lee et al. [28]. compared the results of sympathetic blocks to those of 263 people who had completed combined autonomic nervous system (ANS) testing to identify CRPS. Although the incidences of abnormal SSR tests were not substantial, they were considerably higher in the CRPS group. However, this study did not conduct quantitative stratification of SSR parameters and instead filtered out SSR-positive patients. As the pathogenic mechanism of the sympathetic nerve in CRPS has become more apparent in recent years [29], the application of sympathetic block treatment has increased again. As a result, choosing the most appropriate patients for individualized treatment is essential. According to our findings, following LSB to alleviate pain, the mean SSR amplitude increased, and the mean SSR latency shortened on the affected side (Fig. 3). These findings support LSB's effective treatment of pain symptoms in CRPS patients and clarify the range of changes in SSR parameters.
The current study used a cohort of unilateral CRPS-1 lower extremity patients from our institution, regardless of disease severity. The value of SSR has been widely reported for several autonomic nerve disorders [23, 30], but the treatment of CRPS, particularly the prediction of successful symptom relief, has received relatively less attention. In our research, 98 CRPS-1 patients had two LSB treatments in 1 month. A total of 43.9% of the patients (43/98) experienced successful symptom relief, whereas 56.1% of the patients (55/98) had unsuccessful symptom relief. This result is better than that of one prior study [31], which found that just 31% of CRPS-1 patients responded well to sympathetic block. In contrast to our study, the subjects involved upper and lower extremities. Another study [32] discovered that 61% of CRPS patients effectively reacted to LSB, making it the most effective study of LSB in recent literature and yielding a higher rate than our findings. It is worth noting that there was a significant difference in the SSR amplitude of the affected limbs between the two groups in our study but not in the SSR latency. It is generally believed that latency is a relatively stable parameter that does not change markedly with different stimuli, and it reflects the conduction duration of the nerve impulse that generates perspiration throughout the reflex arc [33]. However, the amplitude is a reliable response of the excitability of sympathetic postganglionic fibers and sweat glands, which is more able to reflect peripheral sympathetic nerve activity [34]. It is also worth mentioning that, according to the Budapest criteria, vasomotor and sweating/edema, which are CRPS symptoms, were more prevalent in the LSB (+) group. It could be that these two types of symptoms are most closely linked to sympathetic activity [35].
The reasons for the clinical positive response to LSB are multifaceted. Our results showed a significant difference in the change in SSR amplitude between the LSB (−) and LSB (+) groups before and after treatment. The effectiveness of LSB could potentially be predicted by changes in SSR amplitude (Fig. 4). Therefore, we analyzed age, BMI, sex, disease duration, baseline NRS, baseline SSR latency and amplitude. In accordance with previous research [36] on predictors, our results show that disease duration appears to be a factor determining the success of LSB, with patients having less than 12 months exhibiting a fourfold higher efficiency than patients with a longer duration. This indicates that CRPS with a duration ≥ 12 months is a poor prognostic factor for successful LSB treatment. Patients with CRPS may develop central sensitization, which can explain why sympathetic block is less effective over time [37]. SSR is thought to be triggered by synchronized sweat gland activity. The afferent part of the SSR is composed of large myelinated peripheral sensory fibers, whereas the efferent part is composed of sympathetic postganglionic unmyelinated C fibers that terminate in sweat glands. SSR is considered to be altered in CRPS due to increased sympathetic activity (4). In our results, an SSR amplitude < 510 µV was a good prognostic factor for successful LSB treatment.
This study has a number of limitations. First, this study lacked healthy controls and relied entirely on the healthy lower limbs of CRPS patients for control, which is known as contralateral control. As normal SSR parameter values have not yet formed a unified standard, our judgment of values in this study is based on neurologists' interpretation. Second, the LSB's efficacy was judged by short-term pain alleviation with no long-term follow-up. Although we could not find a long-term effect of LSB in our investigation, we managed to record the NRS following treatment 1 week after two LSB sessions. Third, we did not examine inflammatory cytokines or pain-related mediators to determine whether there were any changes in biomarkers. Finally, as we conducted a single-center study, it is possible hard to generalize our findings.
Conclusions
Collectively, we found that individuals with lower extremity CRPS-1 may significantly relieve pain with LSB treatment, which is accompanied by increased SSR amplitude and shortened SSR latency of ipsilateral extremity. There was a significant difference in the change in SSR amplitude between the LSB (−) and LSB (+) groups. We attempted a quantitative analysis of the SSR parameter and identified a cutoff value that might effectively instruct the therapeutic effects. Finally, we discovered that a baseline SSR amplitude of the affected extremity < 510 µV and disease duration < 12 months were predictors of successful symptom relief after LSB treatment.
References
Bruehl S. Complex regional pain syndrome. BMJ. 2015;351: h2730.
Kortekaas MC, Niehof SP, Stolker RJ, Huygen FJ. Pathophysiological mechanisms involved in vasomotor disturbances in complex regional pain syndrome and implications for therapy: a review. Pain Pract. 2016;16:905–14.
Kessler A, Yoo M, Calisoff R. Complex regional pain syndrome: an updated comprehensive review. NeuroRehabilitation. 2020;47:253–64.
Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13:256–70.
Toyokura M. Sympathetic skin responses: the influence of electrical stimulus intensity and habituation on the waveform. Clin Auton Res. 2006;16:130–5.
Lin X, Chen C, Liu Y, Peng Y, Chen Z, Huang H, Xu L. Peripheral nerve conduction and sympathetic skin response are reliable methods to detect diabetic cardiac autonomic neuropathy. Front Endocrinol (Lausanne). 2021;12: 709114.
Lizarraga AA, Rammohan KW, Weinstock-Guttman B, Sharma K. Peripheral nervous system electrodiagnostic abnormalities in predominantly Hispanic Multiple Sclerosis patients. Mult Scler Relat Disord. 2021;56: 103254.
Idiaquez J, Casar JC, Fadic R, Iturriaga R. Sympathetic and electrochemical skin responses in the assessment of sudomotor function: a comparative study. Neurophysiol Clin. 2023;53: 102840.
Ryu JH, Lee CS, Kim YC, Lee SC, Shankar H, Moon JY. Ultrasound-assisted versus fluoroscopic-guided lumbar sympathetic ganglion block: a prospective and randomized study. Anesth Analg. 2018;126:1362–8.
Xu Y, Jiang Q, and Xu X. The tourniquet ischemia test effectively predicts the efficacy of lumbar sympathetic block in patients with lower extremity complex regional pain syndrome type 1. 2022;15: 1659–67
Bruehl S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 2010;113:713–25.
Baron R, Schattschneider J, Binder A, Siebrecht D, Wasner G. Relation between sympathetic vasoconstrictor activity and pain and hyperalgesia in complex regional pain syndromes: a case-control study. Lancet. 2002;359:1655–60.
Shim H, Rose J, Halle S, Shekane P. Complex regional pain syndrome: a narrative review for the practising clinician. Br J Anaesth. 2019;123:e424–33.
Urits I, Shen AH, Jones MR, Viswanath O, Kaye AD. Complex regional pain syndrome, current concepts and treatment options. Curr Pain Headache Rep. 2018;22:10.
Harden NR, Bruehl S, Perez R, Birklein F, Marinus J, Maihofner C, Lubenow T, et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for complex regional pain syndrome. Pain. 2010;150:268–74.
Birklein F, Ajit SK, Goebel A, Perez R, Sommer C. Complex regional pain syndrome—phenotypic characteristics and potential biomarkers. Nat Rev Neurol. 2018;14:272–84.
Bruehl S, Harden RN, Galer BS, Saltz S, Backonja M, Stanton-Hicks M. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain. 2002;95:119–24.
Sopacua M, Hoeijmakers JGJ, Merkies ISJ, Lauria G. Small-fiber neuropathy: expanding the clinical pain universe. 2019;24: 19–33
Zeidman LA. Advances in the management of small fiber neuropathy. Neurol Clin. 2021;39:113–31.
Aramaki S, Kira Y, Hirasawa Y. A study of the normal values and habituation phenomenon of sympathetic skin response. Am J Phys Med Rehabil. 1997;76:2–7.
Tyvaert L, Laureau E, Hurtevent JP, Hurtevent JF, Derambure P, Monaca C. A-delta and C-fibres function in primary restless legs syndrome. Neurophysiol Clin. 2009;39:267–74.
Drory VE, Korczyn AD. The sympathetic skin response in reflex sympathetic dystrophy. J Neurol Sci. 1995;128:92–5.
Sun PC, Lin HD, Jao SH, Chan RC, Kao MJ, Cheng CK. Thermoregulatory sudomotor dysfunction and diabetic neuropathy develop in parallel in at-risk feet. Diabet Med. 2008;25:413–8.
Robles NR, Alvarez-Lobato VC, Caravaca F, Roncero F, Solis J, Sanchez-Casado E. Sympathetic skin response in peritoneal dialysis patients. Asaio J. 2003;49:88–90.
Argyriou AA, Koutras A, Polychronopoulos P, Papapetropoulos S, Iconomou G, Katsoulas G, Makatsoris T, et al. The impact of paclitaxel or cisplatin-based chemotherapy on sympathetic skin response: a prospective study. Eur J Neurol. 2005;12:858–61.
Kim HJ, Yang HE, Kim DH, Park YG. Predictive value of sympathetic skin response in diagnosing complex regional pain syndrome: a case-control study. Ann Rehabil Med. 2015;39:116–21.
Bolel K, Hizmetli S, Akyüz A. Sympathetic skin responses in reflex sympathetic dystrophy. Rheumatol Int. 2006;26:788–91.
Lee HJ, Lee KH, Moon JY. Prevalence of autonomic nervous system dysfunction in complex regional pain syndrome. 2021;46: 196–202
Misidou C, Papagoras C. Complex regional pain syndrome: an update. Mediterr J Rheumatol. 2019;30:16–25.
Revanappa KK, Moorthy RK, Alexander M, Rajshekhar V. Recovery of sympathetic skin response after central corpectomy in patients with moderate and severe cervical spondylotic myelopathy. Br J Neurosurg. 2017;31:199–204.
van Eijs F, Geurts J, van Kleef M, Faber CG, Perez RS, Kessels AG, Van Zundert J. Predictors of pain relieving response to sympathetic blockade in complex regional pain syndrome type 1. Anesthesiology. 2012;116:113–21.
Cheng J, Salmasi V, You J, Grille M, Yang D, Mascha EJ, Cheng OT, et al. Outcomes of sympathetic blocks in the management of complex regional pain syndrome: a retrospective cohort study. Anesthesiology. 2019;131:883–93.
Tzeng SS, Wu ZA, Chu FL. The latencies of sympathetic skin responses. Eur Neurol. 1993;33:65–8.
Hoeldtke RD, Davis KM, Hshieh PB, Gaspar SR, Dworkin GE. Autonomic surface potential analysis: assessment of reproducibility and sensitivity. Muscle Nerve. 1992;15:926–31.
Donadio V, Lenzi P, Montagna P, Falzone F, Baruzzi A, Liguori R. Habituation of sympathetic sudomotor and vasomotor skin responses: neural and non-neural components in healthy subjects. Clin Neurophysiol. 2005;116:2542–9.
Dev S, Yoo Y, Lee HJ, Kim DH, Kim YC, Moon JY. Does temperature increase by sympathetic neurolysis improve pain in complex regional pain syndrome? A retrospective cohort study. World Neurosurg. 2018;109:e783–91.
Birklein F, Dimova V. Complex regional pain syndrome-up-to-date. Pain Rep. 2017;2: e624.
Acknowledgements
Funding
This research was funded by the Science Technology Department of School of Medicine, Shanghai Jiaotong University (NO. TM202111). The Rapid Service Fee was funded by the authors.
Medical Writing, Editorial, and Other Assistance
We also thank Springer Nature (www.springernature.com) for its linguistic assistance during the preparation of this manuscript. The authors provided funding for this assistance.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Dongping Du and Yongming Xu designed, conducted, and approved the final version of the study. Yongming Xu, Junzhen Wu, and Qingqing Jiang contributed acquire and evaluate data, composed the article, revised it, and approved the final version. Junzhen Wu performed the data analysis and offered critical review of the article. Yingying Lv, Shaofeng Pu, and Chen Li contributed significantly to the study design, research conduct, and confirmation of the final version.
Disclosures
Yongming Xu, Junzhen Wu, Qingqing Jiang, Yingying Lv, Shaofeng Pu, Chen Li, and Dongping Du have nothing to disclose.
Compliance with Ethics Guidelines
The protocol was approved by the ethics committee of the Shanghai Sixth People's Hospital (No. 2019–119). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
The datasets generated during/analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Xu, Y., Wu, J., Jiang, Q. et al. Prediction of the Efficacy of Lumbar Sympathetic Block in Patients with Lower Extremity Complex Regional Pain Syndrome Type 1 Based on the Sympathetic Skin Response. Pain Ther 12, 785–796 (2023). https://doi.org/10.1007/s40122-023-00499-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00499-w