Abstract
Introduction
Pain is a global phenomenon encompassing many subtypes that include neuropathic, musculoskeletal, acute postoperative, cancer, and geriatric pain. Traditionally, opioids have been a mainstay pharmacological agent for managing many types of pain. However, opioids have been a subject of controversy with increased addiction, fatality rates, and cost burden on the US healthcare system. Cannabinoids have emerged as a potentially favorable alternative or adjunctive treatment for various types of acute and chronic pain. This narrative review seeks to describe the efficacy, risks, and benefits of cannabinoids as an adjunct or even potential replacement for opioids in the treatment of various subtypes of pain.
Methods
In June of 2022, we performed a comprehensive search across multiple databases for English-language studies related to the use of cannabinoids in the treatment of various types pain: neuropathic pain, musculoskeletal pain, acute postoperative pain, cancer pain, and geriatric pain. Data from meta-analyses, systematic reviews, and randomized control trials (RCTs) were prioritized for reporting. We sought to focus our reported analysis on more recent literature as well as include older relevant studies with particularly notable findings.
Results
There is conflicting evidence for the use of cannabinoids in the management of pain. While cannabinoids have shown efficacy in treating specific chronic pain subtypes such as neuropathic pain, fibromyalgia pain, and geriatric pain, they do not show as clear benefit in acute postoperative and the majority of musculoskeletal pain syndromes. Data trends towards cannabinoids having a positive effect in treating cancer pain, but results are not as conclusive. To date, there is a paucity of data comparing cannabinoids directly to opioids for pain relief. Overall, the side effects of cannabinoids appear to be relatively mild. However, there is still potential for addiction, altered brain development, psychiatric comorbidities, and drug–drug interactions.
Conclusion
Cannabinoids may be effective in specific subtypes of pain, but current evidence and guidelines do not yet support its use as the first-line treatment for any type of acute or chronic pain. Rather, it may be considered a good adjunct or alternative for patients who have failed more typical or conservative measures. Additional studies are needed with standardized forms of cannabinoids, route of delivery, and dosing for greater-powered analysis. Providers must weigh the individualized patient risks, benefits, and concurrent medication list in order to determine whether cannabinoids are appropriate for a patient’s pain treatment plan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cannabinoids are helpful in treating chronic neuropathic pain and chronic geriatric pain. However, additional caution must be exercised when prescribing geriatric patients cannabinoids due to altered pharmacokinetics as well as drug–drug interactions (particularly increased bleeding risks with warfarin). |
The overall data on cannabinoids for the treatment of musculoskeletal pain are inconclusive. However, available data do strongly support the use of cannabinoids in treating fibromyalgia pain. |
The use of cannabinoids as an adjunct in cancer pain is generally supported, but results are less conclusive. Opioids remain the mainstay for the treatment of moderate to severe cancer pain. However, opioids are associated with many serious adverse effects. |
The use of cannabinoids in acute postoperative pain is not supported. One study demonstrated worsening pain in patients treated with cannabinoids postoperatively. |
The side effects of cannabinoids are relatively mild in comparison with opioids. However, cannabis usage is still linked to addiction, especially if it begins in youth. Short-term usage can impair memory, coordination, and judgment. Long-term usage in younger individuals can lead to altered brain development with cognitive impairment, including lower IQ, poor educational performance, and higher psychiatric illnesses. Cannabis smoking also causes airway irritation, cough, and chronic obstructive pulmonary disease (COPD). |
There is a paucity of data comparing cannabinoids directly to opioids for pain relief. Additional studies are needed to determine optimal forms, administration routes, and doses of cannabinoids for each subtype of acute and chronic pain. |
Introduction
According to the World Health Organization (WHO)’s World Mental Health Survey completed in ten developed countries, approximately 37% of adults experience chronic pain conditions. In the USA, at least 116 million people experience chronic pain [1]. This statistic is likely to increase in coming years. Pain places a great burden on the USA’s healthcare system and economy. It is estimated that the arthritis-attributable medical costs alone were $140 billion in the USA in 2013 [2]. A US study from the early 2000s also demonstrated that lost productive time from common pain conditions among the active workforce equated to approximately $61.2 billion a year [3]. Pain conditions have profound, detrimental effects on the overall population.
Over the past few decades, society has seen a pendulum effect with opioid prescriptions, initially with an increase, then followed by a decrease. The initial increase in opioid prescriptions in the 1990s was largely due to several factors, including the growing availability of more advanced, long-acting opioid formulations, stronger marketing strategies employed by pharmaceutical companies, disregard for longer-term results, and a perceived undertreatment of pain [4]. Due to this significant rise in opioid prescriptions, numerous unfavorable consequences emerged. Mean per capita annual health care costs from 1998 to 2002 were estimated to be $16,000 for opioid abusers compared with $1800 for non-abusers [5]. Individuals with opioid abuse also missed more than 2.2 days of work monthly as compared with 0.83 days among non-abusers [6]. Furthermore, increased fatality rates were linked to the increased presence of prescription opioids [7].
More recently, there have been two subsequent waves of opioid-related deaths from overdoses. One of these waves started in 2010 and was due to a rapid rise in deaths caused by heroin overdose [8]. The second wave happened in 2013 with an increase in overdoses from synthetic opioids, primarily fentanyl [9]. The Centers for Disease Control and Prevention (CDC) then reported an increase in opioid-related overdose deaths by 45.2% from 2016 to 2017 [10]. Along with the number of overdoses, opioids also have other serious adverse effects, including addiction, misuse/abuse, constipation, nausea, pruritus, sedation, confusion, respiratory depression, and opioid-induced hyperalgesia [11]. In spite of all of these adverse effects, opioids have noteworthy merits as well. Opioids remain the mainstay for moderate to severe cancer pain because of their rapid onset, lack of ceiling effects, and limited impact on organ function [12, 13]. Additionally, opioids also remain the cornerstone of acute postoperative pain management [14, 15]. This is largely because opioids have the ability to block pain signal transmission at multiple sites in the pain signaling pathway [16].
Given the unfavorable effects resulting from the “opioid epidemic,” there has been a need to find alternative therapies to better manage pain and decrease the use of opioids. Cannabinoids have emerged as a promising alternative or adjunct to opioids in the treatment of pain. The earliest recorded uses of cannabis were about 12,000 years ago in Central Asia, and since that time, they have been used for a wide variety of purposes, both medicinal and recreational [17]. Cannabis has been described to have uses in alleviating pain as well as symptoms of cancer, chemotherapy, multiple sclerosis, osteoarthritis, glaucoma, AIDS, and even depression [18]. Although cannabis was initially legal in the colonial USA, the federal government imposed restrictions from the 1930s onwards. Eventually, the Reagan Administration criminalized cannabis altogether in the 1980s, and it was classified as an illegal Schedule 1 substance [19]. During this period of legal barriers, cannabis still managed to gain traction in the alternative treatment space, given its less dangerous side-effect profile when compared with opioids [20]. California, in 1995, became the first of 16 states to legalize cannabis for medical use, despite federal bans imposed in most of the USA. Soon after, recreational and medical marijuana was entirely legalized in Colorado, Washington, Alaska, Oregon, and the District of Columbia. Twenty-three other states have also decriminalized marijuana for medical use [19].
Given its growing popularity as a treatment option for various ailments including pain, the WHO acknowledged the need for additional research on cannabinoid mechanisms and its medical effectiveness, particularly in regard to cancer pain [21]. It is becoming imperative for healthcare providers to understand and have the ability to address cannabis-related clinical issues, even if a provider does not necessarily support its use [20]. Additionally, when providers authorize cannabinoid medications, it is important that they understand the indications, risks, and benefits, as well as the differences between strains (indica versus sativa), active ingredients [primarily tetrahydrocannabinol (THC) and cannabidiol (CBD)], and routes of ingestion (which include inhalational, oral, and topical). Given the growing abundance of cannabinoid-based pharmaceutical products, clinicians should make every conscious effort to remain aware of the evolving data [22].
To date, there is a paucity of randomized control trials (RCTs) comparing cannabinoids directly with opioids in the treatment of pain syndromes. There is also a lack of clear guidelines about the indications for medical cannabis. It has been especially difficult to establish unified guidelines because of varying concentrations of active ingredients and multiple routes of ingestion for cannabinoid-based products. This narrative review aims to describe the endocannabinoid system and clarify the efficacy of cannabinoids as a potential alternative or adjunct to opioids in the management of various types of chronic pain and acute postoperative pain. Outcome measures analyzed by this review include changes in pain scores/intensity, changes in opioid consumption, pain relief scores, and adverse events related to cannabinoid ingestion. Since there is significant variability among cannabinoid-based products, the review focuses on the topic of cannabinoids as a whole, rather than on individual forms of cannabinoid products. In addition, this review seeks to clarify the risks, benefits, and limitations of cannabinoid use among different patient demographics. Thus, the importance of this review is to allow providers to make well-informed decisions about whether individual patients with pain would benefit from cannabinoids instead of, or in addition to, opioid medications.
Endocannabinoid Receptors and Signaling
The human body naturally produces cannabinoids, and endocannabinoid receptors are found in high concentrations in the brain and spinal cord, but can also be found in peripheral organs [23]. Cannabinoids have been thought to have roles in the modulation of pain signaling [24]. Numerous mechanisms have been discussed for endocannabinoid signaling and synaptic function. The primary proposed mechanism has been retrograde signaling, for which endocannabinoids mediate short- and long-term forms of plasticity at excitatory and inhibitory neurons. This primary proposed mechanism can be seen in Fig. 1.
In retrograde signaling, postsynaptic activity leads to the formation of an endocannabinoid that transports backward across the synapse, binds presynaptic cannabinoid type 1 receptors (CB1Rs), and modulates neurotransmitter release (typically by inhibition). This binding typically results in decreased pain pathway signaling in nociceptive neurons [25]. In addition, cannabinoids may disinhibit release of gamma-aminobutyric acid (GABA) and glutamate neurotransmitters in the periaqueductal gray (PAG) area of the midbrain, thereby directly activating descending inhibitory pain pathways and further contributing to its antinociceptive effects [26]. When specifically looking at cannabis, THC is an analog to endogenous cannabinoids and has a high binding affinity at CB1Rs. CBD, on the other hand, has low binding affinity for cannabinoid receptors but can regulate pain sensation by regulating the activity of other G-protein-coupled receptors (GPCRs), ion channels, and peroxisome-proliferator-activated receptors (PPARs) [27].
There is growing evidence that supports endocannabinoid participation in non-retrograde signaling as well. Endocannabinoids modulate neural function and synaptic transmission by engaging transient receptor potentials, vanilloid receptor type 1, and CB1Rs found on or within postsynaptic cells. Furthermore, endocannabinoid signaling through astrocytes can indirectly modulate presynaptic and postsynaptic functions [24]. Synthetic cannabinoids can also affect signaling by acting on these same discussed receptors.
The endocannabinoid system may also be related to stress-induced analgesia (SIA). SIA is an adaptive response that refers to a decrease in pain sensation due to a stressor. This adaptive mechanism is dependent on the recruitment of brain pathways projecting from the amygdala to the midbrain periaqueductal grey matter and down to the rostroventromedial medulla and posterior column spinal cord [28]. Current literature suggests that endogenous opioids have functions in SIA, but endocannabinoids may also play an important role [29].
Methods
In June of 2022, a comprehensive search was performed on English-language studies focusing on cannabis, opioids, and pain management. We searched the following databases: PubMed, Medline, SciHub (not used for initial search), Cochrane Database of Systematic Reviews, and Google Scholar using the following Medical Subject Headings (MeSH) terms: “Cannabinoids,” “Cannabis,” “cannabis-based medicines,” “chronic pain,” and “opioids,” in conjunction with each of the following specific types of pain: “neuropathic pain,” “musculoskeletal pain,” “postoperative pain,” “cancer pain,” and “geriatric pain”. For PubMed, Medline, and Cochrane Database of Systematic Reviews, the MeSH terms were searched in the abstract, title, and keywords. For Google Scholar, these MeSH terms were searched specifically in the title, as including results with MeSH terms “anywhere in the article” identified over 50,000 results (which could not be feasibly analyzed). SciHub was only used to search and access full-text articles after the initial screening and exclusion criteria had already been applied.
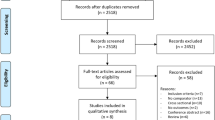
The initial search of these databases identified 943 records from PubMed, 296 records from Medline, 199 records from the Cochrane Database, and 924 records from Google Scholar.
The search was then refined to only include meta-analyses, systematic reviews, and RCTs published within the last two decades (from 2002 to 2022). At this time, 16 duplicates were removed and the remaining articles were sorted by category of pain. This yielded 57 unique article titles with respect to neuropathic pain, 18 unique article titles with respect to musculoskeletal pain, 16 unique article titles with respect to acute postoperative pain, 56 unique article titles with respect to cancer pain, and 6 unique article titles with respect to geriatric pain. Articles were excluded if they were not in English, did not contain human subjects, or whose primary focus was not on the efficacy of cannabinoids in treating one or more of the predetermined subtypes of pain. Studies focusing on topical cannabinoid products were also excluded, as it was determined that there are too many confounding factors with additional active ingredients present in topical products.
After applying the exclusion criteria, 14 focused on neuropathic pain, 6 focused on musculoskeletal pain, 3 focused on acute postoperative pain, 12 focused on cancer pain, and 1 focused on geriatric pain. These articles were then briefly evaluated by the authors for design, patient population, outcome measurement, and significance of findings. On the basis of subjective evaluation, the following were selected for inclusion in our final review: six articles focusing on neuropathic pain, three articles focusing on musculoskeletal pain, two articles focusing on acute postoperative pain, two articles focusing on cancer pain, and one article focusing on geriatric pain. The process for selecting articles included in our narrative review can be seen in Fig. 2, presented using a modified version of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram [30].
Additionally, a total of four older articles with unique or landmark findings were selected for inclusion in our final review: one article focusing on acute postoperative pain and three articles focusing on cancer pain. Each of the articles selected for our final review was analyzed according to design and setting, patient population and control group, specific cannabinoid selected for intervention (including dose and route of administration, if available), pain outcomes assessed along with timepoints of assessment, statistically significant pain outcomes, and adverse events related to cannabinoid ingestion/administration. General prioritization for analysis and reporting of data was placed on meta-analyses followed by systematic reviews, and RCTs, but consideration was also made for quality, nonrandomized data if we felt there were insufficient data on cannabinoids for the treatment of a specific pain subtype. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. It complies with all national and institutional ethical standards.
Results
Neuropathic Pain
The International Association for the Study of Pain (IASP) defined neuropathic pain in 2011 as “pain caused by a lesion or disease of the somatosensory system” [31]. Neuropathic pain is often associated with sensory loss or sensory gain due to peripheral or central sensitization and is characterized by burning pain [32]. This type of pain is frequently caused by nerve injuries. The most common neuropathic pain etiologies include diabetic neuropathy, postherpetic neuralgia, phantom limb pain, postsurgical neuropathic pain, and HIV neuropathy [33]. The prevalence of neuropathic pain in the general population is estimated at around 7–10% [34]. Neuropathic pain has proven difficult to treat effectively, and few individuals experience clinically significant benefits from a single pharmacologic intervention [35]. There are numerous existing studies examining the efficacy of cannabinoids for the treatment of neuropathic pain. To date, they have generally yielded positive results. The majority of the analyzed articles suggest that cannabinoids are safe, effective, and well tolerated for the treatment of neuropathic pain, but a few yielded somewhat less promising results [36,37,38,39,40,41].
A combined systematic review and meta-analysis of cannabinoids for various medical conditions was performed in 2015. Twelve of the studies included in this meta-analysis examined the use of cannabinoids specifically for neuropathic pain. When comparing cannabinoids with placebo, the odds ratio was 1.41 (95% CI 0.99–2.00) for patients reporting a reduction in pain of at least 30% [36]. Another reviewed study performed an individual patient data Bayesian meta-analysis of 178 participants among five RCTs studying the use of cannabinoids in treating chronic neuropathic pain. This meta-analysis found that cannabis led to short-term reductions in neuropathic pain for one in every five to six patients (NNT 5.6, Bayesian 95% credible interval of 3.4–14) [37]. The odds ratio of inhaled cannabis improving pain was 3.2, and this study concluded that cannabis had “at least moderate benefit” in treating chronic neuropathic pain. In addition, these effects were similar across various etiologies of chronic neuropathic pain. An additional 2015 systematic review also demonstrated promising results supporting the use of cannabinoids for chronic neuropathic pain. This review included 13 studies and demonstrated that cannabinoids provide statistically significant pain reduction for neuropathic pain in short-term and longer-term follow-up without significant adverse effects [38].
Although the results have generally been statistically significant and positive when using cannabinoids to treat neuropathic pain, the clinical effect appears to be relatively small [39,40,41]. A 2011 qualitative systematic review of 18 RCTs examined the use of cannabinoids in the treatment of non-cancer pain. Of the 18 included trials, 11 were studies focused on chronic neuropathic pain. The analysis concluded that cannabis-based medications are “modestly” effective in neuropathic pain treatment. Similarly, a 2016 systematic review also found that cannabinoids were “marginally superior” to placebo in pain alleviation for chronic neuropathic pain and inferior in regard to tolerability. This review’s authors recommended only considering cannabinoid treatment when patients have failed first-line and second-line treatment options [40]. Furthermore, one 2015 meta-analysis struggled to find positive results when examining the use of cannabinoids for chronic neuropathic pain. This meta-analysis analyzed various pharmacological treatments for neuropathic pain in adults, including nine trials with nabiximols (an oromucosal spray from extracts of plant Cannabis sativa). Out of these nine trials, only two yielded positive results using cannabinoids [41]. The findings of the neuropathic pain studies are summarized in Appendix Table A.
Given the overall data from the meta-analyses and systematic reviews, the available literature suggests that cannabinoids are effective in treating chronic neuropathic pain. However, the effect size is relatively modest. Management from a more conservative perspective suggests that traditional treatment methods, including tricyclic antidepressants, serotonin–noradrenaline reuptake inhibitors, pregabalin, and gabapentin, should still be used for chronic neuropathic pain [41]. Cannabinoids should be a treatment consideration only in refractory neuropathic pain or as an adjunct when first-line and second-line options alone are insufficient.
Musculoskeletal Pain
Chronic musculoskeletal pain is described as chronic pain arising from musculoskeletal structures such as bones or joints [42]. This broad subset comprises the greatest number of chronic pain conditions and includes diseases such as osteoarthritis, inflammatory arthritis, fibromyalgia, and connective tissue diseases. Chronic musculoskeletal pain can be further broken down into primary and secondary musculoskeletal pain [43]. Primary musculoskeletal pain can be characterized by significant emotional distress or functional disability without clear attribution to a damaging disease process. One of the most common ailments in this category is nonspecific lower back pain. On the contrary, secondary musculoskeletal pain results from another underlying disease, such as musculoskeletal pain associated with multiple sclerosis [43].
There has been a limited number of high-quality RCTs exploring the effectiveness of cannabinoids in the treatment of chronic musculoskeletal pain conditions. The existing literature suggests minimal efficacy. A systematic review in 2019 examined 33 studies looking at the efficacy of cannabis in treating musculoskeletal pain conditions such as post-trauma pain, postsurgical pain, back pain, and osteoarthritis. The studies show that cannabis is effective when compared with placebo or in studies with no comparator. However, did not demonstrate superior efficacy when compared with another active treatment [44]. While cannabis may have some efficacy in treating musculoskeletal pain, it likely is not better than current treatment options for most musculoskeletal pain conditions.
One specific pain condition where cannabinoids have overwhelmingly positive treatment results is fibromyalgia. A study occurred from 2015 to 2017 following 367 fibromyalgia patients over 6 months to study the effect of medical cannabis in decreasing fibromyalgia pain intensity. The 6-month response rate from this study was 70.8%, with pain intensity (from 0 to 10) reduced from a median of 9.0 to 5.0 (p < 0.001) and 81.1% of patients achieving treatment response. There was also a significant increase in the percent of these patients reporting “good” or “very good quality of life” (from 2.7% at initiation to 61.9% at 6 months). On the basis of evidence from this study, medical cannabis offers a very promising role in the treatment of fibromyalgia pain [45]. However, studies on the use of cannabinoids in treating other chronic musculoskeletal pain conditions including generalized rheumatologic disorders have proven far less conclusive, so the efficacy of cannabinoids may be limited to fibromyalgia [46, 47].
A particularly important area of study is the use of cannabinoids for lower back pain [48]. As one of the primary causes of chronic musculoskeletal pain, finding alternative, effective methods of managing lower back pain is crucial. Search of existing literature did not yield any larger-scale RCTs investigating the efficacy of medical cannabis in the treatment of chronic lower back pain. However, a systematic review of six studies did examine the relationship of cannabis and cannabinoid products to lower back pain. The results of this systematic review are largely inconclusive, with the authors of this review also noting a lack of quality studies utilizing medical cannabis for the treatment of lower back pain [49]. The findings of the musculoskeletal pain studies are summarized in Appendix Table B.
On the basis of reviewed data, cannabinoids do not appear to have significantly beneficial effects in chronic musculoskeletal pain conditions, with the exception of fibromyalgia pain. Cannabinoids appear to be very effective in treating fibromyalgia pain specifically, but unfortunately, this effect is not generalizable to all musculoskeletal pain conditions [44, 45, 49]. The available data indicate that cannabinoids may be a good first-line treatment option for fibromyalgia in the future, but remain a weaker adjunct option for the majority of primary and secondary musculoskeletal pain conditions.
Acute Postoperative Pain
Intentional or trauma during surgery can produce inflammatory bodily reactions and often causes nociceptive pain in patients. This surgical stimulus results in postoperative pain. Acute postoperative pain is experienced immediately following surgery and lasts up to 7 days [50]. Cannabinoids have been studied as a possible pharmacologic agent in reducing acute postoperative pain since the 1980s, but there have been few ground-breaking studies performed since that time. One of the first studies on this topic was performed in 1981 and examined the efficacy of intramuscular levonantradol (a synthetic cannabinoid analog of dronabinol) in alleviating moderate to severe postoperative or trauma pain in 56 patients. Compared with placebo, patients receiving levonantradol had significant pain reduction. Surprisingly, there was not a dose-dependent response across intramuscular doses ranging from 1.5 to 3.0 mg. Side effects did increase with dosage up to the 2.5 mg group (but no further increased side effects were seen with the 3.0 mg group), suggesting that 1.5 mg may be the optimal dose for analgesic effect with minimum side effects [51].
Studies since that time have not found nearly as promising results when using cannabinoids for the treatment of acute postoperative pain. A 2003 study found no significant analgesic effects when trialing the use of orally administered δ-9-THC in women undergoing elective abdominal hysterectomy. Forty women were randomized into two groups: one receiving δ-9-THC and the other receiving a placebo upon requesting analgesia on postoperative day 2, when patient-controlled analgesia had already been stopped. The primary outcome measure in this study was summed pain intensity difference (SPID) at 6 h after administration of the study medication, and there was no statistically significant difference found between these groups. However, given the small sample size utilized in this study, it is possible that the power was insufficient to detect a difference. It is also possible that this specific type of cannabinoid and route of administration had a lesser effect when compared with intramuscular levonantradol [52].
Interestingly, some studies have found negative, anti-analgesic effects when administering higher-dose cannabinoids following surgery [53, 54]. One study found negative, anti-analgesic effects when administering nabilone (a synthetic cannabinoid) in higher doses following major surgery. Forty-one study patients were divided into four groups receiving various study medications given at 8-h intervals for 24 h following surgery: 1 mg nabilone, 2 mg nabilone, ketoprofen 50 mg, or placebo. Although cumulative 24-h morphine consumption was not meaningfully different between these groups, the pain scores at rest and upon movement were significantly higher in the 2 mg nabilone group [55]. Similarly, a 2019 retrospective study with propensity-matched cohorts demonstrated that recreational and medical cannabis use before surgery was associated with higher pain scores in the acute postoperative period [54]. Taken together, the results of these studies suggest that cannabinoid use in the perioperative period may actually increase pain instead of decreasing it.
Thus, the existing data on the use of cannabinoids for acute postoperative pain have been limited and do not currently support its use. As of 2007, only 202 patients had been involved in studies looking at cannabinoids as an analgesic agent in the acute postoperative setting [55]. With few supplementary double-blinded RCTs since that time, it has been particularly difficult to make clear conclusions. Although a few studies have suggested against the use of cannabinoids for postoperative pain management, definitive recommendations on this topic will require additional studies [56]. The findings of the reviewed acute postoperative pain studies are summarized in Appendix Table C.
Cancer Pain
In patients with advanced cancer, chronic pain is highly prevalent. Surveys have shown that more than 75% of patients with advanced cancer have moderate to severe chronic pain [57]. Tumor invasion and metastatic tumor formation represent approximately 75% of cancer-related pain, while the other 25% is related to cancer treatments [58]. Opioids remain a mainstay for treating moderate to severe cancer pain, but often have significant side effects at the higher doses required to relieve cancer pain. The treatment and management of cancer-related chronic pain are important to understand in order to create a comprehensive palliative care plan for patients with cancer.
There have been studies performed as early as the 1970s aiming to investigate the analgesic effect of THC at varying oral doses in patients with pain from various types of cancer [59, 60]. One of these early studies evaluated the analgesic effect and side effects of THC at doses ranging from 5 to 20 mg as compared with placebo. This study found a dose-dependent decrease in pain with increasing doses of THC, showing the potential of THC as an analgesic agent [59]. A subsequent, larger study with 36 subjects sought to compare the analgesic effect of 10 mg and 20 mg of THC versus 60 mg and 120 mg of codeine. They found that the low and high doses of THC showed similar levels of pain relief to the low and high doses of codeine. However, only the high doses of THC and codeine showed a statistically significant difference in pain relief compared with placebo [60]. These studies noted a number of side effects including sedation, dizziness, ataxia, blurred vision, mental cloudiness, and social withdrawal. These side effects were significant enough in higher doses of THC to deem it prohibitive of therapeutic use.
These side effects of THC soon prompted the search for THC derivatives that would have similar analgesic value with minimal adverse reactions. Studies performed in 1977 and 1978 focused on the THC analogs benzopyranoperidine and a nitrogen-containing benzopyran derivative. In these studies, the THC derivatives were found to either have poorly tolerated side effects, or, in the case of benzopyranoperidine, augmented pain sensation [61, 62].
Given that cannabinoids did not show promise as a primary treatment for cancer pain early on, more recent studies have sought to investigate the analgesic properties of cannabinoids as adjuvant analgesics for cancer pain. A 2010 study performed in the UK sought to compare the efficacy of nabiximols (THC in combination with CBD, THC:CBD) against THC extract and placebo in relieving advanced cancer pain. A total of 177 patients with cancer pain who experienced inadequate analgesia in spite of chronic opioid dosing were randomized into three groups, each receiving an assigned medication prescribed in addition to their baseline opioid regiment. Patients were allowed to self-titrate their prescribed medication according to their response and tolerance to the medication. The THC:CBD group showed a statistically significant reduction in the numerical rating scale score (NRS) when compared with placebo (−1.37 versus −0.69), whereas the THC extract group did not show significant change. Twice as many patients taking THC:CBD showed a reduction of more than 30% from baseline pain NRS scores compared with a placebo alone. Despite these results, it is important to note that there were no changes in the baseline median dose of opioid background medication, nor in the median number of doses for breakthrough pain medications for any of the patients in this study [63].
Other derivatives of cannabinoids have also shown some promise in the treatment of cancer pain. A 2012 study investigated the dose–response of nabiximols (an oral spray containing cannabis extract) in patients with cancer with opioid-refractory pain. In analyzing mean average and worst pain scores, they found that nabiximols at lower doses (one to ten sprays per day) showed a significant analgesic effect and an overall 26% reduction in pain compared with baseline. However, higher doses of nabiximols were not found to have any analgesic effects and also had poorly tolerated adverse effects [64]. The most common adverse effects with higher-dose nabiximols were nausea, vomiting, dizziness, drowsiness, and dry mouth, and these were found to be correlated with increased dosages. On the other hand, lower-dose nabiximols were found to be relatively safe with a lesser side-effect profile [63, 64].
Standardized combinations of THC with CBD, such as nabiximols, have been shown to have some analgesic efficacy, specifically as adjuvant analgesics for pain from advanced cancer. However, they do not suffice as lone treatments for cancer pain. It is also important to highlight that, in spite of decreased pain scores when utilized as an adjunct treatment to opioids, cannabinoids did not decrease overall opioid consumption. Therefore, cannabinoids have not been shown to reduce the overall opioid burden in cancer patients. The findings of the reviewed cancer pain studies are summarized in Appendix Table D.
Geriatric Pain
Demographic shifts have led to an increased number of elderly patients worldwide and, as a result, an increase in the prevalence of geriatric medical conditions. Chronic pain is very common in the geriatric population. Chronic pain in the elderly has been shown to lead to adverse outcomes such as depression, falls, or overall functional impairment. In addition, it tends to be underreported due to the misconception that chronic pain is a normal part of aging [65].
Pain relief is often cited as a reason for cannabis usage among older individuals. In the Colorado Medical Cannabis registry, 89.7% of patients over the age of 61 listed pain as their primary or secondary condition [66]. Additionally, epidemiological studies have shown an increase in cannabis usage among older adults with an increase in cannabis use from 2.4% in 2015 to 4.2% in 2018 in adults over the age of 65 [67]. Despite the increasing use, the evidence for the efficacy of cannabinoids in geriatric patients remains limited. Many studies evaluating cannabinoids for pain have included older adults as part of their inclusion criteria. Even still, they are not a large portion of the study population, and no study reviewed by the authors had performed a subgroup analysis for geriatric patients [68]. Even recent guidelines for the medical use of cannabinoids in chronic pain provided minimal discussion in regard to older patients [69].
Among the rare studies that do specifically investigate the efficacy of cannabinoids on chronic pain in the elderly, the data can often be misleading and of poor quality. A 2018 prospective study on recruited patients over the age of 65 who received medical cannabis from a specialized cannabis clinic followed their pain intensity, quality of life, and adverse events for 6 months. The mean age of the patients was 74.5 ± 7.5 years, with the most common cannabis indications being for pain (66.6%) and cancer (60.8%). Treatment with cannabis was found to significantly reduce the intensity of reported pain, with the median pain score of 8 decreasing to 4 on an 11-point scale and with the percentage of respondents reporting high pain intensity of 8–10 decreasing from 66.8% to 7.6%. Of the study patients, 18.1% stopped using their opioid analgesics altogether or reduced their dose. In spite of these overwhelmingly positive results, it is important to note that only 33% of the patients responded to the 6-month questionnaire, leading to a strong possibility of selection bias. In addition, this study was funded by a commercial cannabis supplier that used their own cannabis as the exclusively tested treatment [70].
Given the small percentage of elderly participants in most cannabinoid-related pain studies, it is difficult to elucidate how much pain relief this population truly receives from the use of cannabinoids. However, based on the limited available evidence, cannabinoids do appear to be beneficial in treating geriatric pain. The findings of the discussed geriatrics pain study are summarized in Appendix Table E.
Risks
The regulatory landscape regarding the use of cannabis is rapidly changing. As cannabis and cannabinoid-based products gain further legalization and acceptance, it is probable that use among patients will also increase. There is a popular misconception that cannabis is a harmless medication without significant side effects. However, the use of cannabis has been associated with numerous detrimental effects, as summarized in Appendix Table F. Other forms of cannabinoids share similar risks and side effect profiles, but depending on route of administration, these forms may not have as many lung-related adverse events.
The most common short-term side effects of cannabis use appear to be nausea, vomiting, dizziness, drowsiness, and impaired attention, memory, and motor function [71]. However, there are also longer-term side effects from cannabis usage. Long-term cannabis use also predisposes younger individuals to altered brain development with cognitive impairment including lower IQ, poor educational outcomes, and paranoia and psychosis in those with a baseline predisposition towards psychiatric disorders. Furthermore, smoking cannabis releases carcinogens that cause airway inflammation, chronic cough, or even chronic obstructive lung disease [72].
Cannabis use may lead to addiction, particularly when usage starts early in adolescence [73, 74]. Cannabis abuse and dependence have been combined in the DSM-5 into a single entity termed “cannabis use disorder.” Diagnosis requires “a problematic pattern of cannabis use leading to clinically significant impairment or distress” with two further inclusion criteria within 12 months. The inclusion criteria consists of unsuccessful efforts to cut down, increased time spent obtaining cannabis, and symptoms of tolerance and withdrawal when stopping cannabis use [75]. One epidemiological survey from 2012 to 2013 found the prevalence of cannabis use disorder to be 2.5% within that single year and a lifetime prevalence of 6.3% [76].
Cannabis use disorder can also be associated with other substance use disorders and psychiatric conditions, particularly anxiety [77]. In fact, one-third of individuals with cannabis use disorder may also have a personality or mood disorder [78]. Unfortunately, only 13.7% of adults with cannabis use disorder ever seek treatment or intervention [79]. Thus, it is particularly important that providers prescribing medical cannabis to patients aggressively screen these patients for cannabis use disorder.
Recent data have shown that cannabis withdrawal syndrome is physiologically valid and clinically important. As early as 2004, there have been proposed criteria for cannabis withdrawal, including symptoms of anger, aggression, decreased appetite, irritability, restlessness, and sleep difficulty [80]. Cannabis dependence criteria were subsequently announced in the DSM-5, which required three of seven potential symptoms within 1 week of ceasing or reducing cannabis use [81]. Most of these symptoms are true, transient withdrawal symptoms with a similar time course to other withdrawal syndromes. Additional evidence has shown that cannabis withdrawal is quite common in heavy users. One study found that, among people using cannabis more than three times per week in the past year, 44% of the subjects reported at least two withdrawal symptoms, with 34% of the subjects experiencing at least three withdrawal symptoms [82].
The increased adverse events associated with cannabis use could be related to increased cannabis potency. Since the 1980s, the THC content in confiscated cannabis samples has steadily increased from 3% to 12% in 2012 [59]. Given a dose-dependent side effect profile, this trend suggests increased future risks of adverse effects with cannabis consumption [60]. Contamination of cannabis is another concern, especially in patients with cancer who may already be immunocompromised. There is evidence that cannabis can contain naturally occurring molds and fungi that could cause respiratory problems in healthy individuals and detrimental lung disease in immunocompromised populations. Other illicit synthetic substances may also be added to make the cannabis feel more potent to consumers. No reviewed systematic studies have yet addressed this contamination issue, and further research is required in this area of interest [83].
The pharmacokinetics of cannabinoids can vary greatly on the basis of route of administration, with inhalation being significantly faster than oral ingestion, with peak plasma levels within 3–10 min [84]. The active ingredients THC and CBD are primarily metabolized in the liver by the cytochrome P450 (CYP)3A4 and CYP2C9 enzymes [85]. Thus, inhibitors of the CYP3A4 enzyme can cause significant increases of systemic THC and CBD concentrations [86]. Furthermore, CBD is a known inhibitor of the CYP2C19 enzyme, which is important in metabolizing medications like warfarin. Because of this, cannabinoids have been reported to cause significantly increased international normalized ratio (INR) and risk of bleeding in patients concurrently taking warfarin [87]. Patients on warfarin should be advised not to use cannabinoids. In addition, all patients should be advised about increased risks when taking any concurrent medications which induce, inhibit, or serve as a substrate for the CYP3A4, CYP2C9, and CYP2C19 enzymes.
Specific to geriatric patients, cannabinoids, like other drugs in the elderly, have different pharmacokinetics due to decreased hepatic clearance and renal elimination in older adults. Elderly patients also have increased body fat, increasing the volume of lipophilic substances such as cannabinoids [88]. The most common adverse events related to cannabinoid use, which include dizziness, drowsiness, confusion, and disorientation, could be particularly detrimental to the geriatric population, who often experience vision problems, frequent falls, and cognitive decline at baseline. Although the effects of cannabinoids on cardiovascular diseases are not well established, prior case reports have reported arrhythmias and myocardial infarction in young, healthy cannabinoid users, implying that caution should be taken in geriatric populations where cardiovascular disease is much more prevalent. In addition, the polypharmacy often associated with geriatric patients further complicates the use of cannabinoids in this population [68, 70, 88].
Additional research needs to be done and guidelines established on the safety and efficacy of cannabinoid use in the geriatric patient population. Because of conflicting evidence for cannabinoid use in various pain conditions, many different institutional treatment protocols exist for cannabinoid use in geriatric patients. On the basis of these protocols, it appears that one of the commonalities is to adhere to the lowest, tolerable doses with special attention paid to adverse events if cannabinoids are to be used in the elderly [68, 88].
Discussion
On the basis of our narrative review, there is conflicting evidence for the use of cannabinoids in the management of acute post-operative and chronic pain syndromes. While cannabinoids have shown positive results in treating specific pain types such as fibromyalgia pain, neuropathic pain, and geriatric pain, the data reviewed are less promising in regard to treating acute postoperative pain, musculoskeletal pain syndromes (with the exception of fibromyalgia), and cancer pain. It is also important to note that, while there was an overall benefit when used as an analgesic adjunct in cancer pain, it did not reduce the overall opioid burden. This failure to reduce opioids was similarly seen in treating geriatric pain with cannabinoids [62, 69]. Furthermore, it is important to note that the geriatric study reviewed did contain conflicts of interest (funded by a commercial cannabis supplier using their own cannabis for treatment) [70].
To date, there is a paucity of RCTs directly comparing cannabinoids to opioids for pain relief. However, a 2018 survey including 2841 respondents who were using cannabis to replace prescription medications did demonstrate that 38.1% of the respondents terminated prescription drug use and 45.9% substantially decreased prescription drug use [89]. Additionally, in a California survey of 2897 patients consuming medical cannabis, 97% of respondents “strongly agree” or “agree” that they were able to decrease opioid consumption when also using cannabis and 81% of respondents “strongly agree” or “agree” that the sole use of cannabis was more effective at treating their condition than taking cannabis combined with opioids [90]. In spite of the lack of RCTs comparing cannabis directly with opioids in various pain syndromes, these surveys strongly suggest that cannabinoids may be a good alternative to opioids in various pain syndromes. On the other hand, it is still important to conduct non-inferiority studies comparing cannabinoids directly with opioids for all types of pain in order to establish valid and generalizable guidelines. One potential hindrance to completing large-scale studies in the USA on this topic is the controversial legalization of cannabis throughout all states. As of now, about one-third of the states still have not legalized cannabis [91].
One significant limitation in furthering cannabinoid research as a treatment for select types of pain is the lack of intervention standardization between studies. The two main subspecies of the cannabis plant are Cannabis indica and Cannabis sativa. The indica strains have a higher CBD content, while the sativa strains have a higher THC concentration. Traditionally, THC is the type of cannabinoid associated with the “high” sensation individuals associate with recreational use. Thus, the greater THC concentration in Cannabis sativa makes it a preferred choice among users [92]. Numerous routes of administration have been described, including smoking, vaping, edibles, dabbing, intramuscular injection, and oil concentrate use. Of all these mentioned administration methods, smoking is the most common form [93]. Over the past few years, synthetic cannabinoids (SCs) have had a growing presence in the market, and more recently, liquid formulations can now be vaped through electronic cigarettes [73].
The many specific forms of cannabinoids combined with multiple routes of administration and variable dosing regimens make it incredibly difficult to standardize and aggregate intervention data across multiple RCTs for greater-power analysis. This lack of standardization also renders it incredibly difficult to provide detailed recommendations regarding optimal form, route of administration, and dosing for each specific subtype of pain. The matter is further complicated by concurrent use of many different baseline pain medications among study participants [71].
Overall, the side effects of cannabinoids and risks associated with use are still far milder than the associated adverse effects of opioid medications. Drugs overdose still leads as the number one cause of accidental death in the USA, and opioids remain the most common drug causing overdose. Although it is important not to be dismissive of adverse effects associated with cannabinoids (particularly in the geriatric population), cannabinoids do continue to have a much safer pharmacological profile with no reported deaths due to overdose [94]. Also, given the effect of cannabinoids and opioids on separate receptors within the body, they may function synergistically to provide greater analgesia when used concurrently.
Conclusions
This narrative review of currently available evidence suggests that cannabinoids are effective analgesic medications in treating specific pain conditions such as chronic neuropathic pain, fibromyalgia pain, and geriatric pain. While cannabinoids did improve pain scores among chronic neuropathic pain patients, the effect was relatively modest. Thus, cannabinoids should be viewed largely as an alternative analgesic for patients who either have failed first-line therapies or have refractory chronic neuropathic pain. On the other hand, cannabinoids appeared to be quite effective for both fibromyalgia and geriatric pain and potentially could be considered for first-line treatment. Additional studies would be needed before this can be conclusively determined. Unfortunately, cannabinoids showed less promising results in ameliorating cancer pain and acute postoperative pain. On the basis of the data reviewed, cannabinoids should be recommended only as an adjuvant analgesic in cancer pain (with opioids being the mainstay of cancer pain treatment) and should not be recommended for the treatment of acute postoperative pain at this time.
In the future, non-inferiority RCTs would be helpful to compare cannabinoids directly with opioids in the treatment of these various pain subtypes. In addition, clinical researchers should attempt to standardize specific forms, routes of administration, and dosing for cannabinoids utilized between clinical trials. This uniformity would allow better aggregation of interventional data across multiple RCTs for greater-power analysis. By examining more sufficiently powered data, better guidelines may be established on the indications for cannabinoids in treating all types of pain.
Overall, cannabinoids have a safer side-effect profile when compared with opioids and may potentially be used in conjunction with opioids to provide even greater analgesia. However, it is still important to be aware of the risks associated with cannabinoid consumption. Among the most noteworthy risks are cognitive impairment, addiction, and drug–drug interactions. Providers must take into account each patient’s individualized risks, benefits, and concurrent medications when determining whether cannabinoids may be a suitable treatment option for pain.
References
Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for Transforming Prevention, care, education, and research. National Academies Press (US); 2011. http://www.ncbi.nlm.nih.gov/books/NBK91497/. Accessed 28 Sep 2020.
Murphy LB, Cisternas MG, Pasta DJ, Helmick CG, Yelin EH. Medical expenditures and earnings losses among US adults with arthritis in 2013. Arthritis Care Res (Hoboken). 2018;70(6):869–76. https://doi.org/10.1002/acr.23425.
Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290(18):2443–54. https://doi.org/10.1001/jama.290.18.2443.
Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician. 2012;15(3 Suppl):ES67-92.
Strassels SA. Economic burden of prescription opioid misuse and abuse. J Manag Care Pharm. 2009;15(7):556–62. https://doi.org/10.18553/jmcp.2009.15.7.556.
Ruetsch C. Empirical view of opioid dependence. J Manag Care Pharm. 2010;16(1 Suppl B):9–13. https://doi.org/10.18553/jmcp.2010.16.s1-b.9.
Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657–9. https://doi.org/10.1001/jama.2013.272.
Rudd RA, Paulozzi LJ, Bauer MJ, et al. Increases in heroin overdose deaths—28 states, 2010 to 2012. MMWR Morb Mortal Wkly Rep. 2014;63(39):849–54.
O’Donnell JK, Gladden RM, Seth P. Trends in deaths involving heroin and synthetic opioids excluding methadone, and law enforcement drug product reports, by census region—United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(34):897–903. https://doi.org/10.15585/mmwr.mm6634a2.
Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419–27. https://doi.org/10.15585/mmwr.mm675152e1.
Dalal S, Bruera E. Pain management for patients with advanced cancer in the opioid epidemic era. Am Soc Clin Oncol Educ Book. 2019;39:24–35. https://doi.org/10.1200/EDBK_100020.
Virgen CG, Kelkar N, Tran A, et al. Pharmacological management of cancer pain: novel therapeutics. Biomed Pharmacother. 2022;156: 113871. https://doi.org/10.1016/j.biopha.2022.113871.
Wiffen PJ, Wee B, Derry S, Bell RF, Moore RA. Opioids for cancer pain—an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;7: CD012592. https://doi.org/10.1002/14651858.CD012592.pub2.
Baker DW. History of the joint commission’s pain standards: lessons for today’s prescription opioid epidemic. JAMA. 2017;317(11):1117–8. https://doi.org/10.1001/jama.2017.0935.
El Moheb M, Mokhtari A, Han K, et al. Pain or no pain, we will give you opioids: relationship between number of opioid pills prescribed and severity of pain after operation in US vs non-US patients. J Am Coll Surg. 2020;231(6):639–48. https://doi.org/10.1016/j.jamcollsurg.2020.08.771.
Cohen B, Ruth LJ, Preuss CV. Opioid analgesics. In: StatPearls. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK459161/. Accessed 25 Oct 2022.
Crocq MA. History of cannabis and the endocannabinoid system. Dialogues Clin Neurosci. 2020;22(3):223–8. https://doi.org/10.31887/DCNS.2020.22.3/mcrocq.
Grinspoon L, Bakalar JB. Marijuana, the forbidden medicine. Rev. and exp. edn. Yale University Press; 1997.
Bestrashniy J, Winters KC. Variability in medical marijuana laws in the United States. Psychol Addict Behav. 2015;29(3):639–42. https://doi.org/10.1037/adb0000111.
Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clin Proc. 2012;87(2):172–86. https://doi.org/10.1016/j.mayocp.2011.10.003.
World Health Organization. Division of Mental Health and Prevention of Substance Abuse & WHO Expert Working Group on Health Effects of Cannabis Use (1996: Geneva, Switzerland). (1997). Cannabis : a health perspective and research agenda. World Health Organization. https://apps.who.int/iris/handle/10665/63691.
Savage SR, Romero-Sandoval A, Schatman M, et al. Cannabis in pain treatment: clinical and research considerations. J Pain. 2016;17(6):654–68. https://doi.org/10.1016/j.jpain.2016.02.007.
Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20(Suppl 1):10–4. https://doi.org/10.1111/j.1365-2826.2008.01671.x.
Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76(1):70–81. https://doi.org/10.1016/j.neuron.2012.09.020.
Bouchet CA, Ingram SL. Cannabinoids in the descending pain modulatory circuit: role in inflammation. Pharmacol Ther. 2020;209: 107495. https://doi.org/10.1016/j.pharmthera.2020.107495.
Starowicz K, Finn DP. Cannabinoids and pain: sites and mechanisms of action. Adv Pharmacol. 2017;80:437–75. https://doi.org/10.1016/bs.apha.2017.05.003.
Vučković S, Srebro D, Vujović KS, Vučetić Č, Prostran M. Cannabinoids and pain: new insights from old molecules. Front Pharmacol. 2018;9:1259. https://doi.org/10.3389/fphar.2018.01259.
Hohmann AG, Suplita RL, Bolton NM, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435(7045):1108–12. https://doi.org/10.1038/nature03658.
Valentino RJ, Van Bockstaele E. Endogenous opioids: the downside of opposing stress. Neurobiol Stress. 2014;1:23–32. https://doi.org/10.1016/j.ynstr.2014.09.006.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3): e1003583. https://doi.org/10.1371/journal.pmed.1003583.
Jensen TS, Baron R, Haanpää M, et al. A new definition of neuropathic pain. Pain. 2011;152(10):2204–5. https://doi.org/10.1016/j.pain.2011.06.017.
Gierthmühlen J, Baron R. Neuropathic pain. Semin Neurol. 2016;36(5):462–8. https://doi.org/10.1055/s-0036-1584950.
Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3: CD012182. https://doi.org/10.1002/14651858.CD012182.pub2.
Van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–62. https://doi.org/10.1016/j.pain.2013.11.013.
Kalso E, Aldington DJ, Moore RA. Drugs for neuropathic pain. BMJ. 2013;347: f7339. https://doi.org/10.1136/bmj.f7339.
Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–73. https://doi.org/10.1001/jama.2015.6358.
Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: a meta-analysis of individual patient data. J Pain. 2015;16(12):1221–32. https://doi.org/10.1016/j.jpain.2015.07.009.
Boychuk DG, Goddard G, Mauro G, Orellana MF. The effectiveness of cannabinoids in the management of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7–14. https://doi.org/10.11607/ofph.1274.
Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735–44. https://doi.org/10.1111/j.1365-2125.2011.03970.x.
Petzke F, Enax-Krumova EK, Häuser W. Efficacy, tolerability and safety of cannabinoids for chronic neuropathic pain: a systematic review of randomized controlled studies. Schmerz. 2016;30(1):62–88. https://doi.org/10.1007/s00482-015-0089-y.
Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–73. https://doi.org/10.1016/S1474-4422(14)70251-0.
El-Tallawy SN, Nalamasu R, Salem GI, LeQuang JAK, Pergolizzi JV, Christo PJ. Management of musculoskeletal pain: an update with emphasis on chronic musculoskeletal pain. Pain Ther. 2021;10(1):181–209. https://doi.org/10.1007/s40122-021-00235-2.
Perrot S, Cohen M, Barke A, et al. The IASP classification of chronic pain for ICD-11: chronic secondary musculoskeletal pain. Pain. 2019;160(1):77–82. https://doi.org/10.1097/j.pain.0000000000001389.
Madden K, George A, van der Hoek NJ, Borim FM, Mammen G, Bhandari M. Cannabis for pain in orthopedics: a systematic review focusing on study methodology. Can J Surg. 2019;62(6):369–80. https://doi.org/10.1503/cjs.001018.
Sagy I, Bar-Lev Schleider L, Abu-Shakra M, Novack V. Safety and efficacy of medical cannabis in fibromyalgia. J Clin Med. 2019. https://doi.org/10.3390/jcm8060807.
Cameron EC, Hemingway SL. Cannabinoids for fibromyalgia pain: a critical review of recent studies (2015–2019). J Cannabis Res. 2020;2(1):19. https://doi.org/10.1186/s42238-020-00024-2.
Fitzcharles MA, Baerwald C, Ablin J, Häuser W. Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): a systematic review of randomized controlled trials. Schmerz. 2016;30(1):47–61. https://doi.org/10.1007/s00482-015-0084-3.
Ehrlich GE. Low back pain. Bull World Health Organ. 2003;81(9):671–6.
First L, Douglas W, Habibi B, Singh JR, Sein MT. Cannabis use and low-back pain: a systematic review. Cannabis Cannabinoid Res. 2020;5(4):283–9. https://doi.org/10.1089/can.2019.0077.
Gupta A, Kaur K, Sharma S, Goyal S, Arora S, Murthy RSR. Clinical aspects of acute post-operative pain management & its assessment. J Adv Pharm Technol Res. 2010;1(2):97–108.
Jain AK, Ryan JR, McMahon FG, Smith G. Evaluation of intramuscular levonantradol and placebo in acute postoperative pain. J Clin Pharmacol. 1981;21(S1):320S-326S. https://doi.org/10.1002/j.1552-4604.1981.tb02610.x.
Buggy DJ, Toogood L, Maric S, Sharpe P, Lambert DG, Rowbotham DJ. Lack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain. Pain. 2003;106(1–2):169–72. https://doi.org/10.1016/s0304-3959(03)00331-2.
Beaulieu P. Effects of nabilone, a synthetic cannabinoid, on postoperative pain. Can J Anesth. 2006;53(8):769–75. https://doi.org/10.1007/BF03022793.
Liu CW, Bhatia A, Buzon-Tan A, et al. Weeding out the problem: the impact of preoperative cannabinoid use on pain in the perioperative period. Anesth Analg. 2019;129(3):874–81. https://doi.org/10.1213/ANE.0000000000003963.
Beaulieu P. Cannabinoids for postoperative pain. Anesthesiology. 2007;106(2):397–397. https://doi.org/10.1097/00000542-200702000-00028.
Meeker JD, Ayrian E, Mariano ER. Daring discourse—no: cannabinoids should not be used for acute postoperative pain management. Reg Anesth Pain Med. 2020;45(7):520–3. https://doi.org/10.1136/rapm-2020-101475.
Wiffen PJ, Wee B, Moore RA. Oral morphine for cancer pain. Cochrane Database Syst Rev. 2013. https://doi.org/10.1002/14651858.cd003868.pub3.
Portenoy RK. Treatment of cancer pain. Lancet. 2011;377(9784):2236–47. https://doi.org/10.1016/s0140-6736(11)60236-5.
Noyes R, Brunk SF, Baram DA, et al. Analgesic effect of delta-9-tetrahydrocannabinol. J Clin Pharmacol. 1975;15(2–3):139–43. https://doi.org/10.1002/j.1552-4604.1975.tb02348.x.
Noyes R, Brunk SF, Avery DA, et al. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther. 1975;18(1):84–9. https://doi.org/10.1002/cpt197518184.
Jochimsen PR, Lawton RL, VerSteeg K, et al. Effect of benzopyranoperidine, a Δ-9-THC congener on pain. Clin Pharmacol Ther. 1978;24(2):223–7. https://doi.org/10.1002/cpt1978242223.
Staquet M, Gantt C, Machin D. Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Clin Pharmacol Ther. 1978;23(4):397–401. https://doi.org/10.1002/cpt1978234397.
Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010;39(2):167–79. https://doi.org/10.1016/j.jpainsymman.
Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13(5):438–49. https://doi.org/10.1016/j.jpain.2012.01.003.
Ali A, Arif AW, Bhan C, et al. Managing chronic pain in the elderly: an overview of the recent therapeutic advancements. Cureus. 2018. https://doi.org/10.7759/cureus.3293.
Kaskie B, Ayyagari P, Milavetz G, et al. The increasing use of cannabis among older Americans: a public health crisis or viable policy alternative? Gerontologist. 2017. https://doi.org/10.1093/geront/gnw166.
Han BH, Palamar JJ. Trends in cannabis use among older adults in the United States, 2015–2018. JAMA Intern Med. 2020;180(4):609. https://doi.org/10.1001/jamainternmed.2019.7517.
Abuhasira R, Ron A, Sikorin I, et al. Medical cannabis for older patients—treatment protocol and initial results. J Clin Med. 2019;8(11):1819. https://doi.org/10.3390/jcm8111819.
Häuser W, Finn DP, Kalso E, et al. European pain federation (EFIC) position paper on appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. Eur J Pain. 2018;22(9):1547–64. https://doi.org/10.1002/ejp.1297.
Abuhasira R, Schleider LB, Mechoulam R, et al. Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly. Eur J Intern Med. 2018;49:44–50. https://doi.org/10.1016/j.ejim.2018.01.019.
Aviram J, Samuelly-Leichtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20(6):E755–96.
Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014;371(9):878–9. https://doi.org/10.1056/nejmc1407928.
Lafaye G, Karila L, Blecha L, Benyamina A. Cannabis, cannabinoids, and health. Dialogues Clin Neurosci. 2017;19(3):309–16. https://doi.org/10.31887/DCNS.2017.19.3/glafaye.
Lopez-Quintero C, de losCobos JP, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Dependence. 2011;115(1–2):120–30. https://doi.org/10.1016/j.drugalcdep.2010.11.004.
Hasin DS, O’Brien CP, Auriacombe M, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170(8):834–51. https://doi.org/10.1176/appi.ajp.2013.12060782.
Hasin DS, Kerridge BT, Saha TD, et al. Prevalence and correlates of DSM-5 cannabis use disorder, 2012–2013: findings from the national epidemiologic survey on alcohol and related conditions–iii. Am J Psychiatry. 2016;173(6):588–99. https://doi.org/10.1176/appi.ajp.2015.15070907.
Flórez-Salamanca L, Secades-Villa R, Hasin DS, et al. Probability and predictors of transition from abuse to dependence on alcohol, cannabis, and cocaine: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Am J Drug Alcohol Abuse. 2013;39(3):168–79. https://doi.org/10.3109/00952990.2013.772618.
Urits I, Gress K, Charipova K, et al. Cannabis use and its association with psychological disorders. Psychopharmacol Bull. 2020;50(2):56–67.
Kerridge BT, Mauro PM, Chou SP, et al. Predictors of treatment utilization and barriers to treatment utilization among individuals with lifetime cannabis use disorder in the United States. Drug Alcohol Depend. 2017;181:223–8. https://doi.org/10.1016/j.drugalcdep.2017.09.032.
Budney AJ. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004;161(11):1967–77. https://doi.org/10.1176/appi.ajp.161.11.1967.
Katz G, Lobel T, Tetelbaum A, Raskin S. Cannabis withdrawal—a new diagnostic category in DSM-5. Isr J Psychiatry Relat Sci. 2014;51(4):270–5.
Hasin DS, Keyes KM, Alderson D, et al. Cannabis withdrawal in the United States. J Clin Psychiatry. 2008;69(9):1354–63. https://doi.org/10.4088/jcp.v69n0902.
McLaren J, Swift W, Dillon P, Allsop S. Cannabis potency and contamination: a review of the literature. Addiction. 2008;103(7):1100–9. https://doi.org/10.1111/j.1360-0443.2008.02230.x.
Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327–60. https://doi.org/10.2165/00003088-200342040-00003.
Antoniou T, Bodkin J, Ho JMW. Drug interactions with cannabinoids. CMAJ. 2020;192(9):E206. https://doi.org/10.1503/cmaj.191097.
Stott C, White L, Wright S, Wilbraham D, Guy G. A phase I, open-label, randomized, crossover study in three parallel groups to evaluate the effect of rifampicin, ketoconazole, and omeprazole on the pharmacokinetics of THC/CBD oromucosal spray in healthy volunteers. Springerplus. 2013;2(1):236. https://doi.org/10.1186/2193-1801-2-236.
Cox EJ, Maharao N, Patilea-Vrana G, et al. A marijuana-drug interaction primer: precipitants, pharmacology, and pharmacokinetics. Pharmacol Ther. 2019;201:25–38. https://doi.org/10.1016/j.pharmthera.2019.05.001.
Minerbi A, Häuser W, Fizcharles M. Medical cannabis for older patients. Drugs Aging. 2018;36(1):39–51. https://doi.org/10.1007/s40266-018-0616-5.
Kvamme SL, Pedersen MM, Rømer Thomsen K, Thylstrup B. Exploring the use of cannabis as a substitute for prescription drugs in a convenience sample. Harm Reduct J. 2021;18(1):72. https://doi.org/10.1186/s12954-021-00520-5.
Reiman A, Welty M, Solomon P. Cannabis as a substitute for opioid-based pain medication: patient self-report. Cannabis Cannabinoid Res. 2017;2(1):160–6. https://doi.org/10.1089/can.2017.0012.
Perlman AI, McLeod HM, Ventresca EC, et al. Medical cannabis state and federal regulations: implications for United States health care entities. Mayo Clin Proc. 2021;96(10):2671–81. https://doi.org/10.1016/j.mayocp.2021.05.005.
Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol. 2012;2(6):241–54. https://doi.org/10.1177/2045125312457586.
Meacham MC, Paul MJ, Ramo DE. Understanding emerging forms of cannabis use through an online cannabis community: an analysis of relative post volume and subjective highness ratings. Drug Alcohol Depend. 2018;1(188):364–9. https://doi.org/10.1016/j.drugalcdep.2018.03.041.
MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12–9. https://doi.org/10.1016/j.ejim.2018.01.004.
Acknowledgements
Funding
No funding was received for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Study concept and design: SA SS WL NH KVP AG OH ADK VO. Analysis and interpretation of data: SA SS WL NH KVP AG OH ADK VO. Drafting of the manuscript: SA SS WL NH KVP AG OH ADK VO. Critical revision of the manuscript for important intellectual content: SA SS WL NH KVP AG OH ADK VO.
Disclosures
Dr. Amitabh Gulati is a consultant for Medtronic, Flowonix, SPR Medical, Nalu Medical and an advisor for Spark Medical and AIS Healthcare. Drs. Samuel Ang, Shawn Sidharthan, Wilson Lai, Nasir Hussain, Kiran V. Patel, Onyeaka Henry, Alan D. Kaye, and Vwaire Orhurhu have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. It complies with all national and institutional ethical standards.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ang, S.P., Sidharthan, S., Lai, W. et al. Cannabinoids as a Potential Alternative to Opioids in the Management of Various Pain Subtypes: Benefits, Limitations, and Risks. Pain Ther 12, 355–375 (2023). https://doi.org/10.1007/s40122-022-00465-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00465-y