Abstract
Introduction
Quadratus lumborum block (QLB) has proven to be an effective analgesic technique in various abdominal surgeries. Magnesium sulfate as an adjuvant in different nerve blocks has been reported. The aim of this study was to assess the efficacy of magnesium sulfate as an adjuvant to ropivacaine in an ultrasound-guided QLB for postoperative analgesia in laparoscopic gynecologic surgery.
Methods
Ninety patients belonging to American Society of Anesthesiologists (ASA) physical status I or II, aged between 40 and 60 years, scheduled for laparoscopic gynecologic surgery were enrolled. Patients were divided into three groups and received bilateral quadratus lumborum block: ropivacaine group (group N, 0.375% ropivacaine 40 ml + normal saline 4 ml), magnesium sulfate group (group M, 0.375% ropivacaine 40 ml + 10% magnesium sulfate 4 ml), and control group (group C, normal saline 44 ml). Visual analogue scale (VAS) at rest and during activity at 4, 6, 12, 24, and 48 h postoperatively, consumption of morphine, the time of first analgesic request, frequency of rescue analgesia, satisfaction with postoperative analgesia, and any side effects were recorded.
Results
VAS scores in groups M and N were significantly lower than in group C at 4 and 6 h postoperatively (P < 0.001). VAS scores were lower in group M at 12 and 24 h postoperatively compared to groups N and C (P < 0.05). The mean total morphine consumption was significantly lower in group M than in groups N and C (P < 0.001). The mean time to the first patient-controlled analgesia (PCA) bolus was significantly prolonged in group M compared to group C (P < 0.05). The satisfaction with postoperative analgesia of group M was superior to that of groups N and C (P < 0.05). There was no significant difference in side effects among the three groups.
Conclusion
Magnesium sulfate as an adjuvant to ropivacaine in ultrasound-guided QLB prolongs the duration of analgesia, decreases analgesic requirements, and improves patient satisfaction without significant side effects.
Trial Registration
Chinese Clinical Trial Registry, ChiCTR1900027066.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Regional anesthesia is a significant determinant influencing prognosis, which not only provides effective postoperative analgesia but also reduces the requirement for postoperative opioids, stress response, and insulin resistance. |
New dose formulations and delivery strategies have been developed for regional nerve blocks in recent years. |
There is a significant prevalence of postoperative nausea and vomiting in women, who are pain-sensitive. In these patients, regional nerve blocks are beneficial. |
The addition of magnesium sulfate to ropivacaine in quadratus lumborum block (QLB) resulted in prolonging analgesia duration, decreasing analgesic requirements, prolonging time to initial analgesia, and improving patient satisfaction without significant side effects. |
Magnesium sulfate’s ideal dosage can be determined in future studies. |
Introduction
Although the application of laparoscopy shortens the incision length, patients will still have unbearable pain after surgery because of peritoneal irritation, traction, and injury of the abdominal wall during surgery. Postoperative pain not only affects the patients’ rest but also produces a series of pathological reactions. Postoperative analgesia is an important factor influencing prognosis, including regional anesthesia, which not only provides effective postoperative analgesia but also reduces the need for postoperative opioids, stress response, and insulin resistance [1, 2]. With the wide application of visualization technology in anesthesia, the safety of ultrasound-guided regional block has been greatly improved. In recent years, various ultrasound-guided regional anesthesia techniques have been extensively used. Quadratus lumborum block (QLB), as reported by Blanco [3], can provide postoperative analgesia of the segmental innervation from T6 to L1. Local anesthetics can spread through the thoracolumbar fascia to the paravertebral space, thereby blocking part of the sympathetic nerves. Currently, it is mainly used in combined anesthesia and postoperative analgesia for abdominal and hip surgeries. Clinical studies have shown that compared with the current widely used transverse abdominal block, QLB provides longer analgesia and wider block levels when the same dose of local anesthetic is used [4, 5]. Unlike traditional trunk block, QLB cannot use the sense of falling during puncture and can only be performed under ultrasound guidance, which has the advantages of being real-time and dynamic. Ultrasound-guided nerve block provides more accurate positioning and benefits for patients with less injury.
Magnesium plays an important role in the physiological function of the human body. A large number of studies have reported the safety and effectiveness of adding magnesium sulfate in various regional anesthesia techniques [6,7,8,9]. Therefore, we designed a prospective randomized controlled trial to compare the analgesic efficacy of combining magnesium sulfate with ropivacaine in QLB. We hypothesized that the addition of magnesium sulfate would prolong the duration of analgesia and reduce 48-h morphine patient-controlled analgesia (PCA) requirements after gynecological laparoscopic surgery.
Methods
This prospective randomized controlled, double-blind study was registered with the Chinese Clinical Trial Registry (ChiCTR1900027066) and approved by the Ethics Committee of Shengjing Hospital of China Medical University (2019PS188K). The clinical research was conducted in accordance with the Declaration of Helsinki, and all patients and their families provided informed consent. From December 2019 to June 2021, after informed consent was obtained, 90 patients aged 40–60 years with American Society of Anesthesiology (ASA) physical status I or II who were scheduled for laparoscopic total hysterectomy or total hysterectomy plus accessory resection were enrolled into the study. Exclusion criteria were as follows: body mass index (BMI) greater than 32 kg/m2, allergy or contraindication to the study drug, long-term use of calcium channel blockers, known abuse of alcohol or medication, puncture site infection, peripheral neuropathy, uncooperative or psychiatric patients.
Computer-generated randomization numbers were used to randomly assign patients into three groups. All patients were monitored by continuous electrocardiogram, pulse oximetry, and non-invasive blood pressure measuring. General anesthesia was induced by using etomidate 0.15–0.3 mg/kg and sufentanil 0.2–0.4 µg/kg. Cisatracurium 0.2 mg/kg was given to facilitate endotracheal intubation.
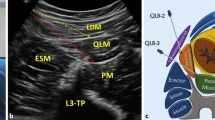
After induction, an ultrasound scanner was used in all cases (GE Healthcare, Venue50), with a curvilinear transducer 5–2 MHz for posterior QLB. All blocks were performed in a supine position by an anesthetist with extensive experience in regional blockade using a needle-in-plane technique. The probe was placed at the level of the anterior axillary line between the anterior superior iliac spine and the 12th rib to obtain the transversus abdominis plane (TAP) image. The external oblique muscle was followed posterolaterally until the transversus abdominis muscle disappeared, and the quadratus lumborum muscle was visualized. The probe was tilted down to identify a bright hyperechoic line corresponding with the middle layer of the thoracolumbar fascia. The needle (22-gauge × 90 mm, Tuoren, Henan, China) was inserted from the ventral side to the dorsal side. Injections were performed posteriorly to the quadratus lumborum muscle in the plane between quadratus lumborum and erector spinae muscles (Fig. 1). In the ropivacaine group (group N), patients received 0.375% ropivacaine 20 ml + 2 ml normal saline at each side. The ropivacaine combined with magnesium sulfate group (group M) received a QLB with 0.375% ropivacaine 20 ml + 10% magnesium sulfate 2 ml. Patients in the control group (group C) received 22 ml of normal saline at each side.
Maintenance of anesthesia was achieved by 1 MAC of sevoflurane and remifentanil of 2–5 µg/(kg h). Before surgery, ondansetron 4 mg and flurbiprofen axetil 50 mg were given intravenously. At the end of the surgery, patients were extubated after adequate reversal of the muscle relaxant with neostigmine 0.05 mg/kg and atropine 0.02 mg/kg. After extubation, patients were admitted to a postanesthesia care unit (PACU). Postoperatively, all patients were connected to a morphine PCA pump programmed to deliver an intravenous bolus of 0.02 mg/kg, with a lockout of 10 min and no background infusion. The rescue analgesia on the ward was intravenously administered flurbiprofen axetil if the visual analog scale (VAS) at rest was greater than 4.
The primary outcomes measured were VAS scores at rest and during activity at admission to the PACU and 4, 6, 12, 24, and 48 h postoperatively (0, no pain; 10, worst pain imaginable). The secondary outcomes included the total number of PCA morphine demands, time to the first PCA bolus (min), and frequency of rescue analgesia, nausea, vomiting, or any other adverse effects. Patient satisfaction was also recorded at 48 h (0, unsatisfactory; 5, very satisfactory). Each patient was assessed by a blinded ward nurse observer trained to evaluate the outcomes.
Data were analyzed using SPSS 21 (IBM Corp, Armonk, NY, USA). Quantitative data were described as mean ± standard deviation (SD), and analysis of variance (ANOVA) was used for comparison. This was followed by post hoc tests if a difference between the groups had been detected. Pain scores were presented as median (interquartile range) and analyzed by Kruskal–Wallis H test. If there was a statistical difference, the comparison between groups was performed by using post hoc tests after rank transform analysis. Categorical data were compared using the chi-square test. The time to first PCA administration was analyzed by Kaplan–Meier survival analysis and log rank statistics together with an evaluation of the hazard ratio by Cox proportional hazards model. Differences were considered significant for P values less than 0.05.
Results
A total of 94 patients were recruited for this study. Four patients were excluded, and 90 were included and randomized (Fig. 2). Three patients were excluded because of not meeting the inclusion criteria (one patient was 61 years old, and two patients had a BMI greater than 32 kg/m2), and one patient declined to participate. There were no statistically significant differences in characteristics among the three groups (Table 1).
Table 2 shows the VAS scores at rest and during activity. VAS scores did not differ significantly among the three groups immediately after recovery from anesthesia. However, VAS scores were significantly higher for both pain at rest and during activity in group C compared to groups N and M at 4 and 6 h postoperatively (P < 0.001). At 12 h postoperatively, the VAS scores at rest and during activity were significantly lower in group M than in groups N and C (P < 0.001). The VAS scores during activity were also lower in group M than in groups N and C (P < 0.05) at 24 h postoperatively. At 48 h, VAS scores were not significantly different among the three groups. Analgesia duration was significantly longer in group M compared to group N (Fig. 3).
Postoperative 48-h morphine demands in group M were significantly lower than in groups C and N (P < 0.001). No patient in group M required postoperative rescue analgesia compared to six patients in group C (P < 0.05). Patient satisfaction for postoperative analgesia in group M was significantly higher than that in groups N and C (P < 0.05). There were no statistically significant differences in the incidence of postoperative nausea or vomiting among the three groups (Table 3).
Figure 4 shows the Kaplan–Meier curve for the time to the first PCA bolus. Group M had a longer time to first morphine demand compared with group C (P < 0.05). In a Cox proportional hazards model, the hazard ratio of first PCA administration in group C patients was two times greater than in group M patients (hazard ratio 2; 95% confidence interval [CI] 1.208–4.137; P < 0 0.05).
Discussion
The results of the trial showed that local use of magnesium as an adjuvant for QLB resulted in lower VAS scores at both 12 h and 24 h postoperatively, longer analgesia duration, and lesser morphine requirement and rescue analgesia.
The use of QLB as a part of a multimodal regimen for postoperative analgesia by virtue of simplicity and effectiveness has succeeded as an analgesic technique in various abdominal surgeries. The quadratus lumborum muscle is a posterior abdominal wall muscle originating from the posterior part of the iliac crest and the lower part of the 12th rib and ending at the upper edge of the iliac crest. The middle layer of the thoracolumbar fascia (TLF) lies between the erector spinae and quadratus lumborum muscles. The sympathetic nerve fibers and mechanoreceptors pass through the TLF, which plays an important role in the mechanism of action of the QLB. Moreover, several investigations have shown that local anesthetics can spread through the TLF to the paravertebral space; hence, they can relieve visceral pain.
According to the anatomic location of needle tip placement in relation to the quadratus lumborum muscle, there are three injection pathways for QLB, namely lateral QLB (QL1), posterior QLB (QL2), and transmuscular QLB, also called anterior QLB, (QL3). We chose QL2 for the study because this approach is in close proximity to the surface; therefore, it is safer and quicker to perform. With a more superficial approach, the needle tip is separated from the peritoneum, reducing the risk of intraperitoneal injection and visceral injury.
Several studies have shown that QLB plays an important role in the treatment of postoperative pain after lower abdominal surgery, demonstrating that QLB reduces postoperative pain and provides a significant reduction in postoperative opioid consumption and side effects [10,11,12]. However, in a recent trial, the addition of QLB to intrathecal morphine after cesarean section has not reduced postoperative opioid consumption or provided additional analgesic benefit beyond 6 h [13]. Our results showed that VAS scores at rest and during activity were reduced 6 h after surgery but not at any other time point up to 48 h. Additionally, we did not find that the addition of QLB could reduce morphine consumption in 48 h or reduce the frequency of nausea and vomiting.
We found that magnesium sulfate augmented the postoperative analgesic effect of QLB. This result coincides with multiple previous studies that have investigated magnesium sulfate as an adjuvant to local anesthetics in various regional techniques. The mechanism of action by which magnesium sulfate potentiates the analgesic effect of local anesthetics is still unclear. Magnesium is an N-methyl-d-aspartate (NMDA) receptor and calcium antagonist. Some studies have shown that the analgesic effects of magnesium are primarily based on the inhibition of calcium influx and the excitability of NMDA receptors, thus reducing the sensitivity to central or peripheral pain stimulation [14, 15]. A meta-analysis has proven that the combination of magnesium sulfate and local anesthetics in nerve blocks could result in longer postoperative analgesia [6]. Ammar et al. [7] reported that the addition of magnesium sulfate to bupivacaine during transversus abdominis plane (TAP) block significantly prolonged the duration of analgesia and reduced postoperative morphine requirements and frequency of nausea and vomiting. In another trial, adding 150 mg magnesium sulfate as an adjuvant to 0.5% ropivacaine during subclavian brachial plexus nerve block resulted in a longer duration of sensory and motor block and lesser demands for rescue analgesics without significant side effects [8].
In our study, we added 400 mg of magnesium sulfate to 0.375% ropivacaine during QLB, and there were significant differences in postoperative pain scores among the three groups. The duration of the QLB was unknown. We found that pain scores at rest and during activity were reduced at 4 h and 6 h postoperatively in groups M and N. Lu et al. [16] studied QLB on volunteers, and they have found that the duration of analgesia with ropivacaine for QL2 was about 18.5 h. We observed that VAS scores at rest and during activity were significantly lower in group M compared to group N at 12 and 24 h postoperatively, which was consistent with previous results reported by Ammar et al. [7]. We believe that patients in group M had a low VAS score because in 6 h postoperatively, the effect of ropivacaine gradually disappeared, while the analgesic action remained after the combination with magnesium sulfate.
The optimal dose of magnesium sulfate for a peripheral nerve block is also unclear. From the current research, the minimum dose is 100 mg [17], and the maximum dose is 1000 mg [18]. Intrathecal magnesium administered at a dose of 200 mg has increased the incidence of nausea by two to three times within 12 h after surgery [19]. However, this side effect has not been reported with magnesium at an equal or even higher dose in several studies. Animal studies have shown that intrathecal administration of magnesium can cause nerve damage [20, 21]. However, no related side effects have been observed clinically. In our study, 400 mg magnesium sulfate was used, and there were no recorded side effects. The potential neurotoxicity and side effects of intrathecal magnesium have not been adequately studied. Therefore, further high-quality clinical studies are needed.
There are some limitations to our study. We lacked the measurement of serum magnesium level or adding intravenous administration of magnesium sulfate to detect if the enhancement effect is related to the systemic action of magnesium. Like transversus abdominis plane block, QLB is a fascial plane block. Local anesthetics are not directly injected near large nerves, but, rather, into the surrounding areas that have a high density of small nerve endings. There have been no reports of nerve damage [22]. All the patients in the study were women, who are sensitive to pain [23]. QLB under sedation may create additional pain and diminish patient satisfaction. We performed QLB under general anesthesia. It is easier to evaluate the level of analgesia in an awake state.
Conclusion
The addition of magnesium sulfate at a dose of 400 mg to ropivacaine in QLB resulted in prolonging analgesia duration, decreasing analgesic requirements, prolonging time to first analgesia, and improving patient satisfaction without significant side effects.
References
Helander EM, Webb MP, Bias M, Whang EE, Kaye AD, Urman RD. Use of regional anesthesia techniques: analysis of institutional enhanced recovery after surgery protocols for colorectal surgery. J Laparoendosc Adv Surg Tech A. 2017;27(9):898–902.
Uchida I, Asoh T, Shirasaka C, Tsuji H. Effect of epidural analgesia on postoperative insulin resistance as evaluated by insulin clamp technique. Br J Surg. 1988;75(6):557–62.
Blanco R. 271: Tap block under ultrasound guidance: the description of a “no pops” technique. Reg Anesth Pain Med. 2007;32(5):130.
Murouchi T, Iwasaki S, Yamakage M. Quadratus lumborum block: analgesic effects and chronological ropivacaine concentrations after laparoscopic surgery. Reg Anesth Pain Med. 2016;41(2):146–50.
Öksüz G, Bilal B, Gürkan Y, et al. Quadratus lumborum block versus transversus abdominis plane block in children undergoing low abdominal surgery: a randomized controlled trial. Reg Anesth Pain Med. 2017;42(5):674–9.
Pascual-Ramírez J, Gil-Trujillo S, Alcantarilla C. Intrathecal magnesium as analgesic adjuvant for spinal anesthesia: a meta-analysis of randomized trials. Minerva Anestesiol. 2013;79(6):667–78.
Ammar AS, Mahmoud KM, Kasemy ZA. Comparison between adenosine and magnesium sulphate as adjuvants for transversus abdominis plane block: a prospective randomized controlled trial. Minerva Anestesiol. 2018;84(3):304–10.
Mukherjee K, Das A, Basunia SR, Dutta S, Mandal P, Mukherjee A. Evaluation of magnesium as an adjuvant in ropivacaine-induced supraclavicular brachial plexus block: a prospective, double-blinded randomized controlled study. J Res Pharm Pract. 2014;3(4):123–9.
Li M, Jin S, Zhao X, et al. Does magnesium sulfate as an adjuvant of local anesthetics facilitate better effect of perineural nerve blocks? A meta-analysis of randomized controlled trials. Clin J Pain. 2016;32(12):1053–61.
Blanco R, Ansari T, Riad W, Shetty N. Quadratus lumborum block versus transversus abdominis plane block for postoperative pain after cesarean delivery: a randomized controlled trial. Reg Anesth Pain Med. 2016;41(6):757–62.
Krohg A, Ullensvang K, Rosseland LA, Langesæter E, Sauter AR. The analgesic effect of ultrasound-guided quadratus lumborum block after cesarean delivery: a randomized clinical trial. Anesth Analg. 2018;126(2):559–65.
Ishio J, Komasawa N, Kido H, Minami T. Evaluation of ultrasound-guided posterior quadratus lumborum block for postoperative analgesia after laparoscopic gynecologic surgery. J Clin Anesth. 2017;41:1–4.
Irwin R, Stanescu S, Buzaianu C, et al. Quadratus lumborum block for analgesia after caesarean section: a randomised controlled trial. Anaesthesia. 2020;75(1):89–95.
Mert T, Gunes Y, Guven M, Gunay I, Ozcengiz D. Effects of calcium and magnesium on peripheral nerve conduction. Pol J Pharmacol. 2003;55(1):25–30.
Shahi V, Verma AK, Agarwal A, Singh CS. A comparative study of magnesium sulfate vs dexmedetomidine as an adjunct to epidural bupivacaine. J Anaesthesiol Clin Pharmacol. 2014;30(4):538–42.
Lu Y, Zhang J, Xu X, et al. Sensory assessment and block duration of transmuscular quadratus lumborum block at L2 versus L4 in volunteers: a randomized controlled trial. Minerva Anestesiol. 2019;85(12):1273–80.
Gunduz A, Bilir A, Gulec S. Magnesium added to prilocaine prolongs the duration of axillary plexus block. Reg Anesth Pain Med. 2006;31(3):233–6.
Imani F, Rahimzadeh P, Faiz HR, Abdullahzadeh-Baghaei A. An evaluation of the adding magnesium sulfate to ropivacaine on ultrasound-guided transverse abdominis plane block after abdominal hysterectomy. Anesth Pain Med. 2018;8(4):e74124.
Lee AR, Yi HW, Chung IS, et al. Magnesium added to bupivacaine prolongs the duration of analgesia after interscalene nerve block. Can J Anaesth. 2012;59(1):21–7.
Saeki H, Matsumoto M, Kaneko S, et al. Is intrathecal magnesium sulfate safe and protective against ischemic spinal cord injury in rabbits. Anesth Analg. 2004;99(6):1805–12.
Ozdogan L, Sastim H, Ornek D, Postaci A, Ayerden T, Dikmen B. Neurotoxic effects of intrathecal magnesium sulphate. Braz J Anesthesiol. 2013;63(1):139–43.
Yuan L, Zhang Ye, Chengshi Xu, Anshi Wu. Postoperative analgesia and opioid use following hip arthroscopy with ultrasound-guided quadratus lumborum block: a randomized controlled double-blind trial. J Int Med Res. 2020;48(5):300060520920996.
Pieretti S, Di Giannuario A, Di Giovannandrea R, et al. Gender differences in pain and its relief. Ann Ist Super Sanita. 2016;52(2):184–9.
Acknowledgements
We thank our colleagues for their help in this study.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
All authors contributed to the study conception, design, material preparation, data collection and analysis. The first draft of the manuscript was written by Qinxue Peng; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Qinxue Peng, Xue Yang, Jingya Li, Yuqing You and Xiaochun Zhao declare that they have no conflict of interest.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee of Shengjing Hospital of China Medical University (2019PS188K). The clinical research was conducted in accordance with the Declaration of Helsinki, and all patients and their families provided informed consent.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Peng, Q., Yang, X., Li, J. et al. The Effect of the Magnesium Sulfate in Ultrasound-Guided Quadratus Lumborum Block on Postoperative Analgesia: A Randomized Controlled Trial. Pain Ther 12, 141–150 (2023). https://doi.org/10.1007/s40122-022-00436-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00436-3