Abstract
Introduction
This study aimed to compare the efficacy between patient-controlled caudal epidural analgesia (PCCA) and patient-controlled intravenous analgesia (PCIA) after perianal surgery, to provide a feasible solution to postoperative pain.
Methods
This was a prospective, randomized controlled trial comprising 100 patients who underwent caudal epidural block on perianal surgery at Chengdu Shang Jin Nan Fu Hospital of West China Hospital at Sichuan University between April and August 2020. Patients were randomly divided into the PCCA and PCIA groups. Visual analog scale (VAS) scores were recorded at 2, 4, 6, 24, 48, and 72 h after surgery, and at the first dressing change and first defecation. The lower limb mobility in the post-anesthetic recovery room (PACU) was determined. The analgesic effect, usage amount of patient-controlled analgesia (PCA), usage amount and frequency of remedial analgesic measures, number of individuals who must be catheterized, and incidence of adverse reactions were recorded. Satisfaction of postoperative analgesic effect and convenience of PCA were also assessed.
Results
The patients in the PCCA group had significantly lower VAS scores at 4, 6, 24, 48, 72 h, the first dressing change, and the first defecation compared with the PCIA group. There were more patients receiving postoperative remedial analgesics in the PCIA group than in the PCCA group. The outcome of the number of PCA and catheterization rates did not differ significantly between the groups. There were two cases of sensory numbness below the S3 plane. The major postoperative complications in the PCIA group were pruritus (3/47, 6.4%), nausea, and vomiting (6/47, 12.8%) (one case combined with pruritus). Patients in the PCCA group were more satisfied with the analgesic effect, while those in the PCIA group were more satisfied with the convenience.
Conclusion
In the postoperative analgesia program of perianal surgery, PCCA may provide a better analgesic effect without increasing the incidence of complications.
Trial Registration
Chinese Clinical Trial Registry identifier, ChiCTR2000038425, September 2020, retrospectively registered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Postoperative pain of perianal surgery, particularly during dressing change and defecation, is still severe. |
This study aimed to compare the efficacy between patient-controlled caudal epidural analgesia (PCCA) and patient-controlled intravenous analgesia (PCIA) after perianal surgery. |
What was learned from the study? |
PCCA may provide a better analgesic effect without increasing the incidence of complications. |
PCCA should be encouraged in order to advance the ERAS process of perianal surgery. |
Introduction
Currently, anorectal diseases, such as hemorrhoids, anal fissures, and anal abscesses are very common worldwide. Hemorrhoids are detected in approximately 40% of patient-screening colonoscopies performed in the United Kingdom [1]. The annual incidence of perianal abscess is 16.1–20.2 per 100,000, and the rate of subsequent fistula formation following an abscess is 15.5% [2]. The incidence of anal fistulas is 5.5 and 12.1 per 100,000 women and men, respectively [3]. Perianal surgery is commonly considered as a useful treatment alternative for these anorectal diseases [4, 5]. Several patients generally complain of severe pain after perianal surgery [6]. This may be primarily owing to the dense sensory innervation of the anal canal and the proctologic procedure causing significant postoperative pain [7, 8]. There may be increased complications owing to poor control of perioperative pain, including nausea, ileus, delayed mobilization, prolonged hospital stays, and chronic pain syndromes [9]. Therefore, to alleviate the pain of patients in the perioperative period, better analgesia is required.
Although there are many ways to decrease the postoperative pain of perianal surgery, such as patient-controlled analgesia (PCA); oral analgesics (paracetamol, non-steroidal anti-inflammatory drugs, and opioids), and locally infiltrated anesthetic agents, postoperative pain, particularly during dressing change and defecation, is still severe [8]. The two common pain relief alternatives are patient-controlled epidural analgesia (PCEA) and patient-controlled intravenous analgesia (PCIA) [10]. PCIA is convenient to use with exact analgesic effects [11]. However, the incidence of nausea, vomiting, and other complications is relatively high, owing to the use of opioids [12]. Analgesia using PCEA may provide better pain relief and fewer side effects than using PCIA [13]. However, PCEA was more frequently associated with numbness, motor weakness, and discontinuation of PCA [14]. In patient-controlled caudal epidural analgesia (PCCA), a type of PCEA, the puncture site is in the sacral hiatus, and a tube is placed in the sacral canal cavity with a low block area [15]. Therefore, there may be better pain control, less exercise block, and low side effects induced by opioids [16]. According to Vadhanan et al., ultrasound-guided caudal epidural anesthesia, as an anesthetic technique in perianal surgery, is practical and easy to use [15]. The postoperative analgesic effect of the single-shot caudal epidural block has been demonstrated to be better than that of PCIA after total hip arthroplasty [17]. However, evidence on the use of PCCA for postoperative pain control in perianal surgery is limited. Therefore, we aimed to compare postoperative pain relief and satisfaction and complications in PCCA with PCIA in this prospective randomized controlled trial.
Methods
Study Design
This single-center randomized superiority trial included adult patients undergoing perianal surgery at the Chengdu Shang Jin Nan Fu hospital of the West China Hospital, Sichuan University. The Ethics Committee of Chengdu Shang Jin Nan Fu hospital provided ethical approval (No. 2019042504) for the study protocol. The latter was registered in the Chinese Clinical Trial Registry (ChiCTR2000038425). All participants provided informed consent before enrollment.
Patient Recruitment
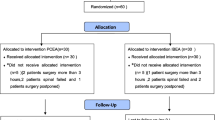
Eligible patients aged 18–65 years with American Society of Anesthesiologists (ASA) functional status of I–III, who were diagnosed with mixed hemorrhoids, anal fissures, anal fistulas, or perianal abscesses, and who underwent perianal surgery, were evaluated for inclusion between April 1 and August 31, 2020. The following were the exclusion criteria: (1) patients who participated in other clinical trials; (2) those who refused to cooperate with this study owing to communication difficulties or other reasons; (3) those with contraindications to epidural anesthesia (patients with coagulation dysfunction and taking anticoagulants); (4) allergies to any drugs used; (5) history of spinal trauma or surgery; (6) history of chronic pain or long-term use of analgesics before surgery; (7) pregnancy and lactation or using oral contraceptives; and (8) ultrasound screening revealing the sacral cavity to be too narrow (< 0.3 cm), or the shape of the sacral cavity to be irregular, and so difficult to puncture the sacral canal. The exit criteria were patients with ineffective blocks or those refusing to be followed after surgery. The number of each group is reported in Fig. 1.
Randomization
Using a computer-generated list of random numbers to receive PCCA or PCIA for pain control by SPSS for Windows, v.22 (IBM, USA), the patients were randomized in a 1:1 ratio. An investigator (L.W.) prepared sealed opaque envelopes for each patient. After ultrasound screening of the sacral cavity, the patients were assigned to the PCCA group or PCIA group by the investigator based on the number in the sealed envelopes.
Anesthesia and Intraoperative Care
Peripheral venous access was established, and an infusion of Ringer’s lactate solution was initiated in the operating room. On entering the preparation room, all patients were routinely monitored, including electrocardiography, non-invasive blood pressure, pulse oximetry, and supplemental oxygen. After administering midazolam 1 mg intravenously, the patients were placed in a left lateral position, and ultrasound-guided caudal epidural block was performed by the attending anesthesiologist. First, the probe was placed in the middle of the sacrum and transverse view, illustrating the superficial sacrococcygeal ligament (SL) in between two sacral cornua and the deeper sacral bone base. Between the SL and the sacral bone is the sacral hiatus, where the needle was inserted. The distance from the anterior edge of SL to the sacrum and from the skin to the anterior edge of the sacral ligament were measured. Following this, to obtain a longitudinal view, the probe was turned 90°. A 20-G intravenous catheter with an inner stylet was inserted through the SL into the sacral hiatus. The caudal space was identified via the loss of resistance technique using saline. To keep the advancement of the needle tip beyond the apex of sacral hiatus limited to 5 mm to avoid a dural puncture, the block needle was visualized in real-time. To identify the successful caudal block, the unidirectional flow on color Doppler was utilized. After negative aspiration, 1 mL of a solution containing 5 µg epinephrine was administrated as a test dose. Ropivacaine (100 mg/10 mL Naropin; AstraZeneca, Sodertalje, Sweden) diluted with 0.9% w/v saline to achieve 0.5% concentration without epinephrine was injected at the rate of 0.2 mL/s, when no evidence of intravascular injection was found after 2 min. After injection, the patient in the PCIA group was turned to a supine position for further assessment [18, 19]. In the PCCA group, a 16-G intravenous catheter with an inner stylet was again inserted through the SL into the sacral hiatus. After negative aspiration, the anesthetist placed an epidural catheter into the sacral cavity (3–4 cm) and used a 16-G venipuncture needle to make a 5-cm subcutaneous tunnel along the spine. The epidural catheter passes through the subcutaneous tunnel, which was fixed using tape.
Block onset was evaluated by pinpricking around the perineal area (S3 dermatome) and the existence of a flaccid anal sphincter. An effective caudal block was defined only if a lax anal sphincter was present 15 min after the caudal injection and the patient underwent pain-free surgery. After the assessment was completed at 15 min and indicated the success of CEB, 0.5 μg/kg/h dexmedetomidine was administered intravenously for anesthesia maintenance. If necessary, propofol 1–2 mg/kg was administered to maintain the depth of anesthesia. If the CEB was ineffective, 0.5% ropivacaine 3–8 mL was locally infiltrated, and sufentanil 5 µg was simultaneously administered intravenously. When the perianal relaxation effect was still not sufficient for the surgery, the anesthesia method was modified to general anesthesia.
Implementation of Postoperative Analgesia
After the surgery, the surgeon used 4–8 mL of a mixture of 1% ropivacaine and methylene blue (9:1) around the perianal incision for immediate local infiltration. Five milligrams each of dezocine and tropisetron hydrochloride were routinely injected intravenously for postoperative analgesia and prevention of nausea and vomiting. Following this, the patients were observed in PACU after the operation. The patient’s lower limb mobility and VAS score were assessed.
The medication administered through the epidural catheter in the PCCA group was a continuous infusion of ropivacaine (2 mg/mL) at a rate of 4 mL/h. Patients could self-administer 4 mL boluses with a lockout time of 60 min via a pump (ZZB-300; APON, Jiangsu Province, China).
The medication administered via intravenous PCA in the PCIA group was a 200 mL mixture (200 µg Sufentanil + 200 µg Dexmedetomidine + 400 mg Tramadol + 20 mg Granisetron + normal saline) at 2 mL/h. Patients could self-administer 0.5 mL boluses with a lockout time of 15 min via a pump (ZZB-300; APON).
If the VAS scores were ≥ 4 when the patients were resting, dezocine 5 mg intravenously or Ibuprofen and Codeine Phosphate Tablets were given orally as rescue analgesia.
Measurements and Outcomes
The VAS scores recorded at the first dressing change were the primary outcome. The first dressing change was carried out by a ward nurse or surgeon 48 h after surgery. Secondary outcomes included VAS scores at rest at 2 h, 4 h, 6 h, 24 h, 48 h, and 72 h after surgery, as well as the rate of remedial analgesia and the number of times PCA was used within 72 h of the operation. Other secondary outcomes included the movement of the lower limbs when leaving the PACU (Bromage scale), the incidence of adverse reactions during the perioperative period, such as nausea and vomiting, itching, urine retention, numbness of the lower limbs or movement disorders), and satisfaction level (satisfied, moderately satisfied, unsatisfied), and the convenience of carrying and using analgesic pumps (convenient, moderately convenient, inconvenient).
Sample Size
The VAS scores of the PCIA group (3.60 ± 2.33) and the PCCA group (2.40 ± 1.43) were recorded at the first dressing change in a preliminary study of 20 patients. Based on the data of the pilot study, a sample size of 100 patients (50/group) was calculated, with a two-sided 5% significance level, a power of 80%, and an anticipated 15% group drop-out rate.
Statistical Analyses
The modified intention to treat approach was used for statistical analyses, which excluded patients who were deemed ineligible after enrollment. For statistical analysis, all data were checked for normal distribution using the Kolmogorov–Smirnov test in SPSS22.0 software (IBM). Continuous data are presented as means, standard deviations (SD), and medians for normally distributed variables, and medians for nonnormally distributed data. Proportions were used to summarize categorical variables. Student's t test or Mann–Whitney U test was used to analyze continuous variables. Pearson's χ2 test or Fisher's exact test was used to compare categorical variables, as appropriate. To account for repeated measures of pain scores, the least significant difference test was used. P < 0.05 was considered statistically significant for all tests.
Results
From April 1 to August 31, 2020, a total of 100 patients were evaluated for eligibility, and randomly assigned to either the PCCA (n = 50) or the PCIA (n = 50) groups (Fig. 1). Seven patients were excluded from this study. Because of poor anesthesia effects, the anesthesia methods of five patients (two in the PCCA group and three in the PCIA group) were changed. Furthermore, two PCCA patients were excluded from this study due to a postoperative catheter that fell out and severe hip numbness. In the end, 93 patients (46 in the PCCA group and 47 in the PCIA group) were included in the statistical analysis. The groups' demographic, surgical, and anesthesia characteristics were comparable (Table 1).
Primary Outcome
The PCCA group had significantly lower VAS scores at the first dressing change than the PCIA group (2.30 ± 1.68 vs. 3.77 ± 1.72, P < 0.001) (Table 2).
Important Secondary Outcomes
The VAS scores of the two groups of patients at rest were 0 at 2 h after surgery, and the pain scores gradually increased until 24 h. Patients in the PCCA group consistently scored lower than those in the PCIA group. In both groups, patients reported the most pain 24 h after surgery. Patients in the PCCA group had significantly lower pain scores than patients in the PCIA group at 4 h (0.09 ± 0.46vs 0.68 ± 1.36, P < 0.001), 6 h (0.54 ± 1.28 vs. 1.98 ± 2.01, P < 0.001), 24 h (1.65 ± 1.33 vs. 2.79 ± 0.93, P < 0.001), 48 h (0.98 ± 1.10 vs. 2.40 ± 1.23, P < 0.001), 72 h (0.72 ± 0.95 vs. 1.87 ± 1.17, P < 0.001) and first defecation (2.24 ± 1.63 vs. 3.83 ± 1.56, P < 0.001) than those in the PCIA group (Table 2).
The number of patients who used oral rehabilitative measures after surgery was lower in the PCCA group (14/46, 32.6%) than in the PCIA group (39/47, 78.7%) (P < 0.001). The PCCA group used fewer oral Ibuprofen and Codeine Phosphate Tablets than the PCIA group (P < 0.001). The number of patients receiving intravenous remedial analgesia in the PCCA group (5/46,11.9%) was lower than in the PCIA group (33/47, 70.2%) (P < 0.001). The dose of intravenous analgesics was still lower in the PCCA group than in the PCIA group (P < 0.001). The number of times of patient-controlled analgesia (PCA) in the PCCA group was more than that in the PCIA group (P = 0.023)(Table 3).
Other Secondary Outcomes
The rate of postoperative catheterization did not differ statistically between the groups (P = 0.686). In the PCCA group, two patients reported lower limb numbness after surgery, while three patients in the PCIA group developed pruritus. Six patients experienced postoperative gastrointestinal reactions, one of which was pruritus, which improved with symptomatic treatment. As shown in Table 3, there were no significant differences in lower extremity mobility when patients left the PACU postoperatively, nor in complications related to anesthesia.
Patients in the PCCA group were more satisfied with their pain control than those in the PCIA group (P < 0.001). However, the PCIA group found the analgesia pump to be more convenient than the PCCA group (P < 0.001).
Discussion
In our study, patients in the PCCA group had lower VAS scores than those in the PCIA group at 4, 6, 12, 24, 48, and 72 h after surgery, particularly at the first dressing change and defecation. Meanwhile, the PCCA group received less remedial analgesics and had fewer patients receive them than the PCIA group. There were no significant differences in side effects when compared to the PCIA group. Furthermore, PCCA provides better analgesia satisfaction than PCIA.
A pain score of < 4 was considered satisfactory analgesia [20]. Inadequate treatment of postoperative pain during the first dressing change and defecation is a challenging problem in the perioperative care of perianal surgery; thus, choosing the VAS scores recorded at the first dressing change as our primary outcome for postoperative pain assessment has clinical significance [8, 21]. Sufentanil and tramadol were selected as major analgesics in PCIA analgesic pumps. This analgesic strategy resulted in a significant reduction in the total sufentanil requirement without increasing the incidence of adverse effects [22]. Ropivacaine was selected as the primary analgesic in the PCCA analgesic pump. Since PCCA and PCIA use different drugs, we cannot rule out differences in drugs, but the purpose of this article is not to prove that PCCA administration routes are superior to intravenous routes. Drugs and routes of administration should be considered part of analgesia regimens, and this article compares the two analgesic regimens PCIA and PCCA, the purpose of which is to explore an analgesic regimen that can achieve satisfactory analgesic effects while reducing opioid consumption. Notably, despite the fact that both PCCA and PCIA can provide adequate analgesia in postoperative pain management, PCCA has a superior analgesic effect. According to a meta-analysis published by Salivate et al., PCEA has better analgesic effects than PCIA in intra-abdominal surgery [13]. Patients in the PCCA group had lower VAS scores, a lower dosage of remedial analgesics, and a higher number of patients receiving remedial analgesics than patients in the PCIA group, which is consistent with our findings.
In this study, two patients in the PCCA group experienced lower limb numbness, which was similar to the incidence of complications in a previous PCEA study [14], and no other serious complications occurred. The main complications in the PCIA group were opioid side effects, with three cases of pruritus and six cases of nausea and vomiting, which was consistent with previous literature [13]. The incidence of urinary retention following surgery is comparable to previous literature, which reported a rate of 21.9% [23]. Although there was no statistically significant difference in the sex ratio between the two groups, there were more female patients in the PCIA group, and it has been reported in the literature that more women than men reported adverse reactions to at least one opioid, and that women were more likely to report adverse reactions to tramadol [24]. Therefore, the higher incidence of adverse reactions in the PCIA group may also be related to sex distribution. In addition, because the remedial analgesics are opioids, and multiple drugs might bring combined action, the cause of opioid adverse reactions in PCIA cannot be determined due to complexity of opioid receptor function [25]. The analgesic properties of opioids make them valuable pharmacologic options for patients with severe postoperative pain, but opioid-related adverse reaction must be cautious [26]. Obviously, the present study was trying to explore whether PCCA would reduce the use of opioids postoperation to achieve better analgesic effect and higher patient satisfaction with less remedial opioid use.
Patients in the PCCA group reported better analgesic effects and higher levels of satisfaction. The epidural catheters, on the other hand, may limit the patient's postoperative activity. There are specific requirements for postoperative sitting baths because the dressing must be kept clean and dry. Furthermore, regular drug replacement around epidural catheters is required. Because of these factors, people are dissatisfied with the convenience. Further advancements in PCCA analgesic technology may aid in improving patient perioperative experience, which will be useful in the development of patient self-control analgesia. Although the PCIA group's analgesic effect is not as good as the PCCA group's, patients' postoperative activities are less affected, and analgesic management is more convenient, saving human resources.
There are a few limitations to this study. First and foremost, this was a one-center study with small sample size, and the adverse reactions in the PCIA group were also affected by sex distribution and multiple-drug confounding factors, which leads to the need for future multicenter large-sample clinical trials to evaluate the safety of these two analgesia regimens. Second, in the PCCA group, adjuvants such as dexamethasone or dexmedetomidine were not added to the analgesic pump to extend the duration of postoperative analgesia and improve the analgesic effect and time. As a result, future research may further optimize the formulation of PCCA analgesic drugs and adjuvants, extending analgesic time, reducing complications, and improving analgesia satisfaction. Third, only the VAS score at rest was measured during the data collection, while the score during movement was not.
Conclusion
In patients undergoing perianal surgery, PCCA may have a better analgesic effect, especially during dressing changes and defecation. Patient satisfaction with postoperative analgesia is higher in the PCCA group than in the PCIA group. As a result, PCCA should be encouraged in order to advance the ERAS process of perianal surgery.
References
Jacobs DO. Hemorrhoids: what are the options in 2018? Curr Opin Gastroenterol. 2018;34(1):46–9.
Hsieh MH, Lu YA, Kuo G, et al. Epidemiology and outcomes of anal abscess in patients on chronic dialysis: a 14-year retrospective study. Clinics (Sao Paulo, Brazil). 2019;74: e638.
Cirocchi R, Trastulli S, Morelli U, et al. The treatment of anal fistulas with biologically derived products: is innovation better than conventional surgical treatment? An update. Tech Coloproctol. 2013;17(3):259–73.
Cohee MW, Hurff A, Gazewood JD. Benign anorectal conditions: evaluation and management. Am Fam Physician. 2020;101(1):24–33.
Gardner IH, Siddharthan RV, Tsikitis VL. Benign anorectal disease: hemorrhoids, fissures, and fistulas. Ann Gastroenterol. 2020;33(1):9–18.
Leinicke JA, Carbajal V, Senders ZJ, et al. Opioid prescribing patterns after anorectal surgery. J Surg Res. 2020;255:632–40.
Ivatury SJ, Swarup A, Wilson MZ, Wilson LR. Prospective evaluation of a standardized opioid reduction protocol after anorectal surgery. J Surg Res. 2020;256:564–9.
Ceulemans A, De Looze D, Van de Putte D, Stiers E, Coppens M. High post-operative pain scores despite multimodal analgesia in ambulatory anorectal surgery: a prospective cohort study. Acta Chir Belg. 2019;119(4):224–30.
Tang C, Xia Z. Dexmedetomidine in perioperative acute pain management: a non-opioid adjuvant analgesic. J Pain Res. 2017;10:1899–904.
Deflandre E, Jaucot J. Patient-controlled analgesia: past, present and future. Minerva Anestesiol. 2016;82(8):811–3.
Zafar SU, Hamid M, Hoda MQ. Patient controlled intravenous analgesia (PCIA) in postoperative surgical patients: an audit. JPMA J Pakistan Med Assoc. 2004;54(7):353–6.
McNicol ED, Ferguson MC, Hudcova J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst Rev. 2015;2015(6):Cd00348.
Salicath JH, Yeoh EC, Bennett MH. Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults. Cochrane Database Syst Rev. 2018;8(8):Cd010434.
Koh JC, Song Y, Kim SY, Park S, Ko SH, Han DW. Postoperative pain and patient-controlled epidural analgesia-related adverse effects in young and elderly patients: a retrospective analysis of 2435 patients. J Pain Res. 2017;10:897–904.
Vadhanan P, Rajendran I, Rajasekar P. Ultrasound-guided caudal epidural anesthesia in adults for anorectal procedures. Anesth Essays Res. 2020;14(2):239–42.
Bagheri H, Govsa F. Anatomy of the sacral hiatus and its clinical relevance in caudal epidural block. Surg Radiol Anat SRA. 2017;39(9):943–51.
Nishio S, Fukunishi S, Juichi M, et al. Comparison of continuous femoral nerve block, caudal epidural block, and intravenous patient-controlled analgesia in pain control after total hip arthroplasty: a prospective randomized study. Orthop Rev. 2014;6(1):5138.
Kao SC, Lin CS. Caudal epidural block: an updated review of anatomy and techniques. Biomed Res Int. 2017;2017:9217145.
Chen CP, Wong AM, Hsu CC, et al. Ultrasound as a screening tool for proceeding with caudal epidural injections. Arch Phys Med Rehabil. 2010;91(3):358–63.
Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804.
Wang ZG, Zhang Y, Zeng XD, et al. Clinical observations on the treatment of prolapsing hemorrhoids with tissue selecting therapy. World J Gastroenterol. 2015;21(8):2490–6.
Cao X, Zhang X. Comparison of different sufentanil-tramadol combinations for pain relief within the first 24 hours after cesarean section: a retrospective study. J Pain Res. 2018;11:2445–51.
Toyonaga T, Matsushima M, Sogawa N, et al. Postoperative urinary retention after surgery for benign anorectal disease: potential risk factors and strategy for prevention. Int J Colorectal Dis. 2006;21(7):676–82.
Lopes GS, Bielinski S, Moyer AM, et al. Sex differences in type and occurrence of adverse reactions to opioid analgesics: a retrospective cohort study. BMJ Open. 2021;11(6): e044157.
Valentino RJ, Volkow ND. Untangling the complexity of opioid receptor function. Neuropsychopharmacology. 2018;43(13):2514–20.
Barnett T, Denke L. Managing postoperative pain with opioid-sparing therapies. Nursing. 2020;50(12):60–3.
Acknowledgements
The authors would like to thank all of the patients, doctors and assessors who took part in the study.
Funding
This study were funded by from the National Key Research and Development Program of China (No. 2021YFC2009100) and from National Natural Science Foundation of China (Grant No. 81900064 and No. 82170079). The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Pei Zhang, Wei Long, and Le Xu. The first draft of the manuscript was written by Le Xu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Le Xu, Pei Zhang, Wei Long, Rurong Wang, Xuehan Li declare that they have no conflict of interest.
Compliance with Ethics Guidelines
This study is a single-center randomized superiority trial. This randomized controlled trial (RCT) complies with the ethical standards of the Helsinki Declaration. First, the Ethics Committee of Chengdu Shang Jin Nan Fu hospital provided ethical approval (No. 2019042504) for the study protocol and then the research protocol prospectively was registered in the Chinese Clinical Trial Registry (ChiCTR2000038425). All participants provided informed consent to participate in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Xu, L., Zhang, P., Long, W. et al. Comparison of Patient-Controlled Caudal Epidural Analgesia and Patient-Controlled Intravenous Analgesia After Perianal Surgery: A Randomized Controlled Trial. Pain Ther 11, 1025–1035 (2022). https://doi.org/10.1007/s40122-022-00411-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00411-y