Abstract
Introduction
Chronic pain is related to gastrointestinal (GI) functions because food components affect inflammation and pain through their action on the GI immune and/or neural system and because many analgesics interact with the gut to alter its structure and function. Immunoglobulin G4 (IgG4) are food-specific antibodies resulting from exposure of the gut immune system to nutrients. High IgG4 levels have been found to be associated with inflammation.
Methods
IgG4 were determined (both with the rapid test and enzyme-linked immunosorbent assay, ELISA) in men and women outpatients with chronic pain. All subjects were asked to exclude for 4 weeks all foods to which they had high blood levels of IgG4 antibodies. Pain and quality of life questionnaires were administered before (visit 1) and after (visit 2) the personalized exclusion diet period. Visual analogue scale (VAS), Italian Pain Questionnaire (QUID) and Margolis (MA) questionnaires were administered to determine pain intensity, pain features and pain extent, while Short Form Health Survey (SF-36) and Profile of Mood States (POMS) were used to test the quality of life and mood state. The nutritional status was evaluated in all subjects. Subject groups were women of reproductive age (pre-MW), women in menopause for at least 1 year (MW) and men.
Results
Fifty-four subjects with chronic pain (n = 12 neuropathic, n = 14 diffuse pain, n = 11 headache, n = 17 low back pain) completed the two visits and the 1-month exclusion diet. At visit 1, 47 (87%) subjects showed medium/high levels of IgG4 to at least one food. The foods showing the highest IgG4 values were eggs, dairy products, cereals and dried fruit. At visit 2, IgG4 levels were decreased, increased or unchanged. In all groups, the 4-week exclusion diet resulted in a significant reduction in all pain measures and an improvement of quality of life parameters. In particular, at visit 2, the VAS score determined in the morning decreased by more than 50%.
Conclusions
A food elimination diet based on IgG4 antibody levels may be effective in reducing pain and improving quality of life in patients with chronic pain.
Similar content being viewed by others
Chronic pain is a major clinical issue that interferes with daily functioning and quality of life. Strategies for chronic pain include symptomatic treatment with low benefits and significant side effects. |
High levels of immunoglobulin G (IgG), especially IgG4, may activate inflammatory processes. |
It is suggested that intestinal inflammation is involved in the onset and evolution of chronic pain diseases, and thus an exclusion diet could be beneficial in patients with chronic pain. |
More than five foods had to be excluded in 87% of subjects. |
The exclusion diet (food-specific IgG4 antibody-guided) is useful in subjects with chronic pain since visual analogue scale (VAS) decreased by 50%. |
As part of a whole-person approach to pain management, attention to dietary habits and the kinds of foods consumed could help avoid pain chronicization. |
Digital Features
This article is published with digital features, including an infographic, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.19646553.
Introduction
Chronic pain is described as a painful condition that lasts longer than 3 months [41]. It can be related to a body disease or, as more often occurs, be independent of any apparent pathological condition. In the last 30 years, impressive progress has been made in the understanding of the pathways, cells or molecules possibly involved in pain chronicization. Many candidates were found at both peripheral and central levels, most of them belonging to the inflammatory system. Inflammation is a condition originating from rupture of the equilibrium between inflammatory and anti-inflammatory activity [24]. This competition occurs every minute in our body and is a natural part of homeostatic processes. A chronic, low-grade inflammatory state [16] can be present in patients with food hypersensitivity and/or intolerance.
The gastrointestinal (GI) system includes several metres of gut tubes that are quite peculiar in each tract. They are able to transform any kind of food into basic elements utilized by cells for energy production or anabolic/metabolic processes, but also able to release substances interacting with the immune and nervous systems through neural and endocrine pathways [44]. On the other hand, the central nervous system (CNS) affects GI functions through both neural and endocrine actions. The autonomic nervous system (ANS) continuously modulates the physiological gut activities, regulating circulation, motility, secretion and reabsorption; in stressful conditions the ANS can impair GI functions via neural (sympathetic pathway) and endocrine (adrenaline) activation [31].
GI problems are common in patients with pain because the pain condition is stressful, i.e. able to affect GI functions, and because pain is commonly treated with drugs able to significantly affect the GI system. Pain treatment is largely based on nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, neuroleptics and antidepressants, all of which modify the GI state and functions [7]. Moreover, other drugs are often given to patients to treat the side effects of pain therapy (i.e. antacids).
Thus, in patients with pain the correct use of analgesics can easily induce problems in the gut [42]. Opioids in particular interact with the gut at different levels as a result of the strong presence of opioid receptors in the enteric nervous system (ENS) and in the epithelium [38]. Meng and his group have shown that morphine, in addition to constipation, can cause intestinal tight junction impairment and thus bacterial translocation from the gut lumen to the GI walls and blood [32]. NSAIDs like acetylsalicylic acid are well known to produce gastritis [21]
Foods have various components that may induce immune reactions, including the production of variable amounts of food-specific immunoglobulin (Ig), particularly type G4 (IgG4). IgG4 are described as Ig produced by the immune system to advise about the ingestion of ‘that food’ [6, 37]. IgG4 is an IgG subclass induced by type 2 cytokines such as interleukin (IL)-4 and IL-13 [1]. An excess of these specific food antigens activates inflammatory processes [17].
High levels of IgG4 were reported to underlie the so-called IgG4-related disease (IgG4-RD), a heterogeneous, subacute and usually silent autoimmune disease involving many organs [2]; nonetheless, high IgG4 in serum is not necessarily indicative of an IgG4-RD [34].
For GI disorder (i.e. irritable bowel syndrome, IBS), several studies have tried to modulate pain through diet [11, 26], whereas fewer attempts have been made for chronic painful syndromes apparently unrelated to the GI system [8, 15, 27].
The aim of the present study was to determine IgG4 levels in women and men with chronic pain. IgG4 determination is used as a marker for the foods to be excluded, i.e. a marker of foods able to induce an immune reaction. In the present study we started using the rapid NutriSmart test®. This test is commonly used when subjects need to lose weight, and the test is done only at the beginning, to see which foods should be excluded. The IgG4 determination by the enzyme-linked immunosorbent assay (ELISA) was considered later in the study and was carried out before and after the exclusion diet to verify possible changes in IgG4 levels. We decided to use this test in patients with chronic pain, all asked to follow a 1-month personalized food-specific elimination diet based on IgG4 levels. The marked pain reduction observed strongly encourages the regular application of this non-pharmacological approach to patients with chronic pain as part of a whole-person approach to pain management [10].
Methods
Male and female subjects with non-oncological chronic pain were recruited by means of online social media and local advertising. The experimental protocol adhered to the principles of the Declaration of Helsinki of 1964 and its later amendments; the experimental procedure was approved by the University of Siena Local Ethics Committee (CAREUS, 7/2020 of 15/09/2020). Once included in the study, each subject provided informed consent for the analysis and publication of the data, and then met with the experimental team twice (visit 1 and visit 2). Visit 1 was considered as basal/control while at visit 2 all determinations were repeated to highlight possible changes.
Experimental Procedure
During visit 1, subjects underwent the following general experimental procedures to evaluate:
-
Demographic data
-
Food-specific IgG4 levels with one of the following tools:
-
NutriSMART® rapid test
-
Analytical determination through the ELISA method
-
-
Pain intensity and features with the following tests:
-
Visual analogue scale, VAS
-
Margolis test, MA
-
Present Pain Intensity scale, PPI
-
Italian Pain Questionnaire, QUID
-
-
Quality of life state with the following questionnaires:
-
Ad hoc questionnaire on smoking habits, sleep problems, menstrual cycle alterations, GI problems, birth control pill intake, allergies/intolerances, presence of other clinical problems
-
Profile of Mood States, POMS
-
Short Form-36, SF-36
-
-
Nutritional state with anthropometric measures and bioelectrical impedance analysis (BIA)
The data were analyzed and IgG4 levels were determined. On the basis of each food’s IGg4 level, each patient received a personalized list of foods to be excluded for 4 weeks.
Visit 2 took place after 4 weeks. All subjects repeated the general experimental procedure as at visit 1. Questionnaires were self-administered with support from an expert clinician. Questionnaires were scored by a researcher blinded to the testing phase (visit 1 or visit 2).
Methods and Tools
Determination of Specific IgG4 Levels: NutriSMART® or ELISA Methods
For each subject, a few drops of blood were collected (capillary sampling) and used in the NutriSMART® rapid test (DST, Schwerin, Germany) or left to dry in an ad hoc absorbent paper for ELISA determination.
The NutriSMART® test was immediately processed following the manufacturer’s instructions. It consists of 40 wells containing food-specific antigens referring to 57 common foods. At the end of the process a three-level score was obtained: mild (reported as 1 or +), medium (reported as 2 or ++) or high (reported as 3 or +++).
The analytical determinations were carried out by the ELISA method. Briefly, the serum samples were diluted and added to reaction wells coated with one of 80 food-specific allergens. Less than 1 µL of serum per allergen was required. The plate was then sealed and incubated at room temperature for 1 h. Sample-specific IgG4 antibodies bound to antigens in the wells during incubation. Unbound components of the serum were washed and patted dry after the sample incubation. Anti-human IgG4 antibodies coupled to horseradish peroxidase (HRP) were added to the wells and they bound to IgG4 antibodies from the sample, standards or controls. After another incubation at room temperature for 30 min, washing and patting dry, 100 µL of a substrate mixture consisting of equal proportions of 3,3′,5,5′-tetramethylbenzidine (TMB) and hydrogen peroxide was added. The reaction was stopped after 10 min by adding a stop solution. A yellow dye was formed and the respective intensity was related to the proportional amount of bound antibodies. The absorbance was measured at 450 nm. The concentrations of food-specific IgG4 (U/mL; 1 U = 1.47 ng) were calculated using the standard curves of 80 types of food-specific IgG4 provided by the manufacturer. The detection limit was 0.01 U/mL.
The results were then reported to each subject who was asked to completely avoid for 4 weeks any foods with a score of 2 or 3 (NutriSMART® test) or foods showing IgG4 levels higher than 3.5 U/mL (ELISA test) independently of the number of foods to be excluded.
Pain Measures
The visual analogue scale (VAS) is a unidimensional measure of pain. It consists of a 10-cm-long horizontal line (0—no pain, 10—maximum pain experienced). Patients were asked to indicate the average VAS experienced in the last week at three times of the day (morning, VASm; afternoon, VASa; night, VASn) [22].
The Margolis test (MA) is a drawing rating system that evaluates percentages of pain distribution in the body. This test is composed of 45 anatomical areas each with a corresponding percentage value (0–100) of body surface in order to compute a total weighted score. Patients were asked to indicate the areas where pain was experienced in the last week at three times of the day (morning, MAm; afternoon, MAa; night, MAn) [28].
Italian Pain Questionnaire (QUID) is the validated Italian version of the McGill Pain Questionnaire [13]. It provides a subjective measurement of quality and intensity of the pain experienced in the last week. It is a semantic interval scale composed of 42 pain descriptors divided into four main classes: sensory (S), affective (A), emotional (E) and miscellaneous (M). The patient has to choose only the exact words that match their current feelings from the descriptor list of the QUID. From the value acquired for each dimension (S, A, E, M) a Pain Rating Index rank value (PRIr) is obtained, and from the sum of the rank value a Pain Rating Index rank-Total (PRIr-T) is acquired. Moreover, it includes the present pain intensity (PPI), a 6-point scale where the subject has to report the pain intensity experienced on the testing day. It consists of a number–word combination chosen by the respondent, from no pain to the worst pain experienced [none, 0; mild, 1; discomforting, 2; distressing, 3; horrible, 4; excruciating, 5].
Quality of Life Questionnaires
The Profile of Mood States (POMS) is widely used to assess transient, distinct mood states and mood changes [30]. POMS consists of 58 words or brief phrases examining eight different dimensions of mood swings over the last week. It comprises six subscales: Tension-Anxiety (T-A), Depression-Dejection (D-D), Anger-Hostility (A-H), Vigour-Activity (V-A), Fatigue-Inertia (F-I) and Confusion-Bewilderment (C-B). Participants are asked to assess their mood state on a 5-point scale, ranging from “not at all” (0) to “a little” (1), “moderately” (2), “quite a bit” (3) and “extremely” (4). In each subscale, values higher (T-A, D-D, A-H, F-I, C-B) or lower (V-A) than 55 were considered significantly altered with respect to the normal population.
Short Form-36 (SF-36) is one of the most used instruments evaluating perceived health status [3]. It consists of 36 items grouped into two components and divided into eight scales: the first four scales—physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH)—are included in the Physical Component Summary (PCS); the other four—vitality (V), social functioning (SF), role emotional (RE), mental health (MH)—are included in the Mental Component Summary (MCS). Each one of the eight scales is assigned a score from 0 to 100, where a higher score means better health in that area.
Nutritional Status and Body Composition
The following anthropometric measures were collected: weight (with an electronic scale), height (with a stadiometer), waist circumference (WC), hip circumference (HC). Weight and height data were used to calculate body mass index (BMI) using the Quetelet equation [body mass/height2 (kg/m2)].
Bioelectrical impedance analysis (BIA) was used to estimate body composition with a bioimpedentiometer (Akern Srl, Florence, Italy). Impedance/resistance (Rz) and reactance (Ohm) values were examined using specific software (BodyGram 1.31) to acquire phase angle (PhA), total body water (TBW), fat free mass (FFM), fat mass (FM), body cell mass (BCM), body cell mass index (BCMI) and extracellular water (ECW).
Statistical Analysis
After a data normality check (Kolmogorov–Smirnov test), data were analyzed with analysis of variance (ANOVA) carried out with the factors group (three levels: pre-MW, premenopausal women; MW, menopausal women; men), test (two levels: visit 1 and visit 2) and other factors detailed in the “Results” section. Post hoc analysis was carried out by Fisher’s least significant difference (LSD) test when necessary. Correlations were carried out with Pearson correlation coefficient. A p < 0.05 was considered significant. All analyses were performed with Statistica® (StatSoft Inc, Tulsa, USA) software. Data are reported as the mean and standard error of the mean (SEM).
Results
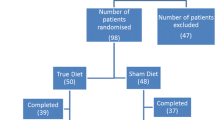
Of the 60 subjects who agreed to participate in the study (46 women and 14 men), 54 were included (43 women and 11 men). Menopause is a physiological condition occurring when menses stop. When the ovary decreases and stops estrogen production, the woman’s body starts to change and many functions are different from the reproductive period. Women were divided into two groups. Women were considered in menopause when menses had not been present for more than 1 year. Thus subjects were divided into three groups depending on sex and menopausal status (premenopausal women, pre-MW; menopausal women, MW; men). Demographic and clinical information are detailed in Table 1.
During their lives, 19 women had experienced sleep problems, 12 menstrual cycle alterations and 13 had taken birth control pills; among the men, 3 had experienced sleep problems. Gastrointestinal problems were reported by 8 women and 2 men. Allergies and other clinical problems were reported by 19 women and 8 men. Two subjects reported that they had suffered a cancer condition, but the pain felt at present was not related to the cancer itself.
All subjects signed the informed consent form and agreed to exclude the foods from their diet for the 4-week period; all described high compliance regarding food exclusion with minimal and insignificant exceptions (not reported).
Food-specific IgG4 levels were determined in all subjects with one of the two methods; the rapid test (NutriSMART®, able to test 57 foods) was used at the beginning because of its ease of use and immediate response. Then it was possible to test analytically, by an ELISA method, 80 foods. In those subjects it was possible to repeat the test at the end of the exclusion diet. The second IgG4 determination was not necessary for the study, but it was carried out only to test possible changes in IgG4 levels. Detailed results per subject are reported in Table 2 where foods are grouped into 10 classes (fish, meat, cereals, vegetables, legumes, dairy products, eggs, fruit, dried fruit, various).
In 23/54 subjects (pre-MW = 6, MW = 11, men = 6), IgG4 were determined by the NutriSMART® rapid test only at visit 1, as part of a pilot study. Score values for each food ranged from 1 to 3; values equal to or higher than 2 were considered higher than normal and the subjects were asked to exclude those foods. Table 2 reports, for each subject in which the analysis was carried out with this method, the highest score for each food class and the list of foods to be excluded.
In 31/54 subjects (pre-MW = 14, MW = 12, men = 5), IgG4 levels were quantified by ELISA twice (visit 1 and visit 2). The IgG4 values ranged from 0.08 to 1259.7 U/mL; values higher than 3.50 U/mL were considered higher than normal and the subjects were asked to exclude those foods. Table 2 reports, for each subject, the total IgG4 amount per food class and the list of foods to be excluded.
On the basis of the IgG4 levels determined at visit 1, each subject received a list of foods to be excluded, suggestions to replace foods and a booklet to note all changes occurring during the 4-week period.
Foods with IgG4 higher than normal (i.e. values higher than 3.50 U/mL or values of 2 or higher) were present in all subjects; in particular, 63% of patients showed more than five foods to be excluded. Table 3 reports the list of all excluded foods and the percentages of patients that excluded that food. Several foods had to be excluded in a very high percentage of subjects (at least 15%).
To determine the foods’ IgG4 levels, the total sum was calculated for all subjects; Fig. 1 shows the IgG4 levels determined for each of the 80 foods at visit 1 in all subjects tested with the ELISA method. Spelt and wheat showed the highest IgG4 levels in the cereals class, while casein and cow milk were highest among dairy products and hazelnut and almond were highest in the dried fruit class. Eggs, both yolk and white (but particularly egg white), also showed very high levels.
Pain Measures
All subjects had suffered chronic pain longer than 1 year, and in most of them more than one pain condition could be determined; however, on the basis of clinical symptoms and previous diagnosis, each subject was included in one of four different pain conditions (i.e. those prevalent as intensity of symptoms and diagnosis): diffuse pain (DP), headache (HA), low back pain (LBP) and neuropathic pain (NP). As reported in Table 4, DP was present in 14 subjects, LBP in 17 subjects, NP in 12 subjects and HA in 11 subjects. In women, chronic pain syndromes were equally represented with a small prevalence of HA (7/20) in pre-MW and LBP (7/23) in MW, while in men the majority reported LBP (7/11) (Table 4). Figure 2 reports the IgG4 levels determined in the four pain syndromes independently of group; NP and LBP subjects showed the highest levels.
Visual Analogue Scale (VAS)
VAS was carried out at three times of the day (VASm, VASa, VASn). As reported in Supplementary Table 1 and Fig. 3, at visit 1 several subjects reported VAS scores higher than 5 (pre-MW: 16/20; MW: 20/23; men: 8/11). In pre-MW, pain levels appeared to be lower in the morning and increased in the night, whereas in MW and men the VAS score was higher in the morning and decreased during the day (Fig. 3). After 4 weeks of exclusion diet, VAS decreased in all groups and in all determinations. The decrease was greater in pre-MW than in the other two groups. In particular, ANOVA was applied to the VAS data with the factors group (three levels: pre-MW, MW and men), test (two levels: visit 1 and visit 2) and VAS (three levels: VASm, VASa, VASn repeated). A significant effect was found for test [F1,89 = 8.27, p < 0.01], as VAS was lower at visit 2 than visit 1. Moreover, there was a significant group × VAS interaction [F4,178 = 3.73, p < 0.01]: in the pre-MW group, VASm was lower than VASa (p < 0.05); in the MW group, VASm was higher than VASa (p < 0.05) and VASn (p < 0.001); VASm was higher in MW than in pre-MW (p < 0.01). No significance was found for the factor group.
Visual analogue scale (VAS) scores determined in pre-MW (premenopausal women, n = 17), MW (menopausal women, n = 18) and men (n = 7), during visit 1 (T1) and visit 2 (T2) at three times of the day (morning, afternoon, night). *p < 0.05 VAS morning vs VAS afternoon in pre-MW, #p < 0.05 VAS morning vs VAS afternoon in MW, ###p < 0.001 VAS morning vs VAS night in MW, §§p < 0.01 VAS morning in MW vs VAS morning in pre-MW
Margolis Test (MA)
The Margolis (MA) test was carried out at three times of the day (MAm, MAa, MAn). As reported in Table 5, pain areas ranged from 5% to 13% of the subjects’ bodies, similarly in the three groups. ANOVA applied to MA values with the factors group, test and Margolis (three levels: MAm, MAa, MAn) revealed a significant effect of the factor Margolis [F2,158 = 5.24, p < 0.01] since MAm was higher than MAa (p < 0.01) and MAn (p < 0.05), independently of group and test. the significant group × Margolis interaction [F4,158 = 2.93], p < 0.05) was due to MW having higher values in the morning than in the afternoon (p < 0.01). Men showed higher levels of MAm than MAa (p < 0.01) and MAn (p < 0.05).
Present Pain Intensity (PPI)
At visit 1 all groups had PPI values ranging from 1.5 to 2.5, i.e. from “mild” (1) to “strong” (3) intensity (Fig. 4). The exclusion diet significantly decreased (improved) this parameter, as shown by the significance of the factor test [F1,86 = 5.41, p = 0.02] owing to the higher levels at visit 1 than at visit 2.
Italian Pain Questionnaire (QUID)
The study of pain features by means of the QUID questionnaire revealed a high significance among groups due to the higher levels (worst) in pre-MW than in MW and men; indeed the values were higher in all the classes and the Pain Rating Index rank-Total (PRIr-T) values were mostly double in pre-MW than in the other groups. In particular, as reported in Table 6, ANOVA with the factors group, test and QUID (four levels: QUIDs, sensorial; QUIDa, affective; QUIDe, emotional; QUIDm, miscellaneous, repeated) revealed a significant effect of group [F2,87 = 10.71, p < 0.001] due to the higher values in pre-MW than in the others (both p < 0.001), independently of test and QUID. The factor QUID was significant [F3,261 = 44.12, p < 0.001] since QUIDs was higher than the other dimensions (all p < 0.001). No differences were found for the factor test. Similarly, ANOVA applied to PRIr-T (Table 6 and Supplementary Table 1) revealed a significant effect of group [F2,85 = 12.72, p < 0.001] due to pre-MW having higher values than the others (both p < 0.001).
Determination of Specific IgG4 Levels
As reported in Table 2, to know the total amount of IgG4 per subject and their changes between visits, the IgG4 values obtained by ELISA for the 80 foods were summed and ANOVA was applied to the total amount with the factors group (three levels; pre-MW, MW and men) and test (two levels: visit 1 and visit 2). Neither group [F2,56 = 1.11, n.s.] nor test [F1,56 = 0.41, n.s.] showed significance, indicating that all groups had the same IgG4 levels and there was no change in IgG4 from visit 1 to visit 2.
To study possible subject group differences and variations between the two visits in the different food classes, ANOVA was applied to the IgG4 values with the factors group, test and food (10 levels repeated: fish, meat, cereals, vegetables, legumes, dairy products, eggs, fruit, dried fruit, various). As shown in Fig. 5, ANOVA revealed the significance of food [F9,504 = 11.24, p < 0.001] due to IgG4 levels for cereals, dairy products, eggs and dried fruit being higher than the others. Group and test did not show significance, suggesting no influence of sex, age or time since it appears that after 1 month of food-specific exclusion the IgG4 levels were not modified.
IgG4 levels. a IgG4 total for all groups; b pre-MW (premenopausal women); c MW (menopausal women) and d men during visit 1 (T1) and visit 2 (T2). *p < 0.05 cereals vs all the other foods; dairy products vs all the other foods except cereals; eggs vs all the other foods except cereals, dairy products and dried fruit; dried fruit vs fish, meat, fruit and various
Quality of Life Questionnaires
Profile of Mood States (POMS)
The POMS data are reported in Table 7 and Supplementary Table 2. ANOVA with the factors group, test and POMS (six levels: T-A, Tension-Anxiety; D-D, Depression-Dejection; A-H, Anger-Hostility; V-A, Vigour-Activity; F-I, Fatigue-Inertia; C-B, Confusion-Bewilderment) revealed a significant effect of group [F2,67 = 5.92, p < 0.01] due to pre-MW showing higher (worst) values than the other groups (MW, p < 0.001; men p < 0.05); moreover there was a significant effect of POMS [F5,335 = 9.86, p < 0.001] since POMS V-A was lower than all the other scales (all p < 0.001) and POMS F-I was higher than all the others (all p < 0.01) except POMS A-H. The significant group × POMS interaction [F10,335 = 2.91, p < 0.001] was due to pre-MW having higher levels than men and MW in all classes except POMS V-A.
Short Form-36 (SF-36)
The Short Form-36 results are reported in Table 8 and Supplementary Table 3. ANOVA was applied with the factors group, test and SF-36/PCS (for the Physical Component Summary, four levels: PF, RP, BP, GH) or SF-36/MCS (for the Mental Component Summary, four levels: V, SF, RE, MH). There was a significant effect of SF-36/PCS [F3,261 = 25.8, p < 0.001] since PF was higher than the other PCS classes (all p < 0.05), independently of group and test; moreover, the significance of SF-36/MCS [F3,271 = 6.58, p < 0.001] was due to V being lower than the other MCS classes (all p < 0.05), independently of group and test.
Nutritional Status and Body Composition
As reported in Table 9 and Supplementary Table 4, at visit 1 BMI scores higher than 25, i.e. in the overweight classes, were present in 10/19 pre-MW, 13/22 MW and 7/10 men; these frequencies did not change significantly at visit 2. The BMI data were subjected to ANOVA with the factors group and test. No significant effects were found. There were no differences in waist circumference (WC) or hip circumference (HC) among groups and tests.
BMI was negatively correlated with SF-36/PF (visit 1/visit 2, n = 89, r = − 0.4, p < 0.001), indicating that higher PF (physical functioning) was related to lower BMI.
Bioelectrical impedance analysis (BIA) results are reported in Supplementary Table 4. ANOVA with the factors group and test revealed a significant effect of group on total body water (TBW) and free fat mass (FFM) [F2,86 = 42.08, p < 0.001; F2,86 = 40.90, p < 0.001 respectively] since men had higher values than pre-MW and MW (all p < 0.001). There were no significant differences in body cell mass (BCM), body cell mass index (BCMI), extracellular water (ECW), phase angle (PhA) or fat mass (FM).
Discussion
The main result of the present study is the significant improvement in pain conditions in the chronic pain sufferers merely from excluding from their diet the foods related to their high blood levels of IgG4.
Chronic pain is a major challenge for patients. It interferes with all personal, familial and social activities. Thus patients ask for drugs chosen among the many painkillers available [12]. With such treatment it is possible that the pain is reduced or cancelled; however, in some cases the therapy may not work and the patient returns to the physician, who will change the drug, increase the dose or add another drug. In these patients the drug-induced side effects are added to the causes responsible for pain. In our sample of patients with pain, it was clear that often the use of analgesics was continued for several months or years independently of their efficacy; it appears that many patients continued to take drugs not to treat pain (often still present) but only for the fear of pain, often stronger than the pain itself.
Drugs and other conditions (bacterial and viral infections, stressful events) can affect gastrointestinal (GI) physiology, altering gut permeability and causing certain foods to become immunostimulant and pro-inflammatory [5, 14, 29, 36, 43]. The inflamed GI structures can be accompanied by strong disruption of the tight junctions present among enterocytes and the development of leaky gut [19]. Low grade inflammation is the common result in these conditions, with patients reporting gastritis, reflux, colitis, diarrhea, constipation or simply low back pain [16]. Cytokines and/or toxins can then circulate and directly and/or indirectly affect the CNS, pain pathways in particular. This condition can be ascertained through IgG4 determination [25], commonly used to identify possible food-related hypersensitivity [20]. IgG4 levels are considered also in our experience a good marker for foods. We consider IgG4 a good indication of those foods that can play a role in increasing inflammation and pain. Foods have thousands of components and each subject is used to eating differently from the others for many reasons. Each food can act as ‘good’ or ‘not good’ depending on many factors; in practice it would take years to verify the possible ‘beneficial’ effects of exclusion testing one food per time. Moreover, IgG4 resulted in several ‘high’ also for foods considered good, i.e. the apple. In the present experiment the apple was found to be positive in many patients.
In the present study a large-scale determination was carried out to identify, among 80 foods, those to which the patient with pain had developed high IgG4 levels. The subjects with pain were not chosen on the basis of the pathology but merely because of the presence of chronic pain. Indeed, as the study considered pain not directly related to the gut, the aim was to assess the possibility to improve the pain condition independently of the pain diagnosis. Unrelated to age and sex, IgG4 were found at medium/high levels in 87% of subjects and were higher in patients with neuropathic pain and low back pain than in the others. Four of the food groups showed very high IgG4 levels: dairy products, eggs, cereals and dried fruit, in agreement with several studies related to allergic reactions [9, 35, 45]. Indeed, these foods are well known to exert a strong stimulating action on the immune system and in most countries it is mandatory to report their presence on food labels in order to advise allergy sufferers. The patients with pain were asked to exclude these foods and/or other ones with high IgG4 levels. To the best of our knowledge this is the first time that this kind of exclusion diet procedure was applied to patients with pain with the primary aim to improve their painful conditions.
An exclusion diet consists in avoiding specific foods for a certain period of time. We asked the patients to avoid for 4 weeks foods against which they had high levels of IgG4. At the end of this period, pain measures were decreased in all subjects: VAS, as an index of pain intensity, was reduced by more than 50% and the Present Pain Intensity, i.e. the pain experienced by the subject at the moment of the interview, was also significantly decreased. Questionnaire results showed an improvement in mood and quality of life scales. Hence it appears that just asking patients with pain to avoid these foods could improve their quality of life without increasing their drug intake.
VAS is a well-known method used worldwide to determine pain intensity and to evaluate drug efficacy; a decrease of 50% is considered adequate to establish the efficacy of an analgesic [40]. The VAS decrease was present at all three determinations (morning, afternoon and night), carried out to better evaluate the features of the chronic pain condition as it is well known that some kinds of pain are more intense during the morning (low back pain) and others in the afternoon/night [23]. In the premenopausal women (pre-MW), VAS tended to increase from morning to evening, while the opposite was true in the menopausal women (MW) and men. Since the pain syndromes were similarly represented in all groups, this different trend could be related to the work activity, with younger women being more active during the day than older ones. In pre-MW this diurnal variation seems to have been lost, with the significant VAS reduction observed particularly in the morning.
A weight increase could be an indirect cause of chronic pain and be related to GI disorders. Weight is known to affect pain, firstly as a result of the clear mechanical interaction at spinal and joint levels and secondly because obesity is often accompanied by an inflammatory state [18]; moreover, being overweight often induces patients to limit food intake with an altered consumption of nutrients. In the present study most subjects showed a body mass index (BMI) higher than 25, i.e. in the overweight classes, and interestingly the BMI was higher in those in which IgG4 tended to increase after a 1-month exclusion diet. Thus, although also in these subjects VAS scores decreased in the same period, the tendency towards an increase shown by IgG4 levels strongly suggests the presence in these patients’ diet of other foods able to stimulate immunity or the presence of a particularly sensitive GI immune system.
IgG4 determination is not a common test in clinical practice, even though rapid tests are commercially available. In the present study we determined the IgG4 levels related to 80 foods. The list includes most of the common foods present in the diet of an adult living in a Western country. The 80 foods were grouped into 10 main classes (fish, meat, cereals, vegetables, legumes, dairy products, eggs, fruit, dried fruit, various). We measured IgG4 before and after 4 weeks of an exclusion diet. Some values were identical in the same subject at the first and second determinations. Before the second determination we verified that the patient had taken care to follow the exclusion diet. Therefore, we can only hypothesize that in some cases the IgG4 antibodies remain in the blood longer than 30 days after consumption of the related food has stopped. Further studies will be carried out to verify the persistence of these IgG in the blood.
Chronic pain, of any origin, may have an inflammatory component [39] and IgG4 production is related to inflammation [1, 17]. Thus the presence of IgG4 in the blood could represent a good marker to establish if a subject is consuming foods able to stimulate their increase. It is possible that food-specific IgG hypersensitivity renders patients more reactive to a low-grade inflammatory process which would not necessarily cause symptoms per se but can amplify other pathologies [33]. This would explain why excluding foods to which patients have IgG antibodies might be particularly beneficial in pain despite the fact that these antibodies may also be present in the general population [4]. Interestingly the highest IgG4 values were shown by patients with neuropathic pain and low back pain.
Limitations
The study does have an important limitation that needs highlighting, i.e. the lack of a control group. However, since determinations were carried out twice in each patient, we consider the first values as a reference point. Moreover, despite the lack of the control group, the number of tests used and the consistency of the different outcomes make the results unlikely to be a consequence of placebo effects. Future studies should ideally include additional controls, for example a control group asked to avoid (non-IgG4 related) foods chosen at random.
Conclusion
This food-related approach to the treatment of chronic pain deserves to be adopted before patients are given pain therapy involving analgesics. Many people in the general population experience GI problems and it is not always easy to find a solution to them. As part of a whole-person approach to pain management, greater attention by clinicians to dietary habits and the kinds of foods consumed could help avoid pain and pain chronicization.
Infographic

References
Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39(4):469–77.
Al-Khalili OM, Erickson AR. IgG-4 related disease: an introduction. Mo Med. 2018;115(3):253–6.
Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998;51(11):1025–36.
Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomized controlled trial. Gut. 2004;53(10):1459–64. https://doi.org/10.1136/gut.2003.037697.
Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105-120.
Bernardi D, Borghesan F, Faggian D, et al. Time to reconsider the clinical value of immunoglobulin G4 to foods? Clin Chem Lab Med. 2008;46(5):687–90. https://doi.org/10.1515/cclm.2008.131.
Berrocoso E, Sánchez-Blázquez P, Garzón J, Mico JA. Opiates as antidepressants. Curr Pharm Des. 2009;15(14):1612–22.
Bjørklund G, Aaseth J, Doşa MD, et al. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition. 2019;66:153–65.
Bordoni A, Danesi F, Dardevet D, et al. Dairy products and inflammation: a review of the clinical evidence. Crit Rev Food Sci Nutr. 2017;57(12):2497–525.
Brain K, Burrows TL, Bruggink L, et al. Diet and chronic non-cancer pain: the state of the art and future directions. J Clin Med. 2021;10:5203. https://doi.org/10.3390/jcm10215203.
Casado-Bedmar M, Keita ÅV. Potential neuro-immune therapeutic targets in irritable bowel syndrome. Therap Adv Gastroenterol. 2020;13:1756284820910630.
Cuesta SA, Meneses L. The role of organic small molecules in pain management. Molecules. 2021;26(13):4029.
De Benedittis G, Massel R, Nobili R, Pieri A. The Italian pain questionnaire. Pain. 1988;33(1):53–62.
Docherty MJ, Jones RC 3rd, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol. 2011;7(9):592–601.
Dragan S, Şerban MC, Damian G, Buleu F, Valcovici M, Christodorescu R. Dietary patterns and interventions to alleviate chronic pain. Nutrients. 2020;12(9):2510.
El-Hakim Y, Bake S, Mani KK, Sohrabji F. Impact of intestinal disorders on central and peripheral nervous system diseases. Neurobiol Dis. 2022;165: 105627.
Finkelman FD, Khodoun MV, Strait R. Human IgE-independent systemic anaphylaxis. J Allergy Clin Immunol. 2016;137(6):1674–80.
Forsythe LK, Wallace JM, Livingstone MB. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21(2):117–33.
Fukui H. Increased intestinal permeability and decreased barrier function: does it really influence the risk of inflammation? Inflamm Intest Dis. 2016;1(3):135–45.
Gocki J, Bartuzi Z. Role of immunoglobulin G antibodies in diagnosis of food allergy. Postepy Dermatol Alergol. 2016;33(4):253–6.
Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept. 2009;155(1–3):11–7.
Huskisson EC, Jones J, Scott PJ. Application of visual-analogue scales to the measurement of functional capacity. Rheumatol Rehabil. 1976;15(3):185–7.
Jankowski KS. Morning types are less sensitive to pain than evening types all day long. Eur J Pain. 2013;17(7):1068–73.
Jiang BC, Liu T, Gao YJ. Chemokines in chronic pain: cellular and molecular mechanisms and therapeutic potential. Pharmacol Ther. 2020;212: 107581.
Kasashima S, Kawashima A, Kasashima F, Endo M, Matsumoto Y, Kawakami K. Inflammatory features, including symptoms, increased serum interleukin-6, and C-reactive protein, in IgG4-related vascular diseases. Heart Vessels. 2018;33(12):1471–81.
Kim MY, Choi SW. Dietary modulation of gut microbiota for the relief of irritable bowel syndrome. Nutr Res Pract. 2021;15(4):411–30.
Lee HS, Lee KJ. Alterations of food-specific serum IgG4 titers to common food antigens in patients with irritable bowel syndrome. J Neurogastroenterol Motil. 2017;23(4):578–84.
Margolis RB, Tait RC, Krause SJ. A rating system for use with patient pain drawings. Pain. 1986;24(1):57–65.
Martel MO, Finan PH, Dolman AJ, et al. Self-reports of medication side effects and pain-related activity interference in patients with chronic pain: a longitudinal cohort study. Pain. 2015;156(6):1092–100.
McNair DM, Lorr M, Droppelman LF. EDITS manual for the profile of mood states. San Diego: Educational and Industrial Testing Service; 1971. p. 27.
Megha R, Farooq U, Lopez PP. Stress-induced gastritis. 2021 Aug 31. Treasure Island (FL): StatPearls; 2022 (PMID: 29763101).
Meng J, Yu H, Ma J, et al. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS ONE. 2013;8(1): e54040.
Minihane AM, Vinoy S, Russell WR, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114(7):999–1012.
Nambiar S, Oliver TI. IgG4 related disease. Treasure Island (FL): StatPearls; 2021 (PMID: 29763000).
Reig-Otero Y, Mañes J, Manyes L. Amylase-trypsin inhibitors in wheat and other cereals as potential activators of the effects of nonceliac gluten sensitivity. J Med Food. 2018;21(3):207–14.
Rueda-Ruzafa L, Cruz F, Cardona D, et al. Opioid system influences gut-brain axis: dysbiosis and related alterations. Pharmacol Res. 2020;159: 104928.
Speciani AF, Piuri G. Measuring biomarkers for an innovative personal food profile. J Am Coll Nutr. 2015;34(Suppl 1):34–8.
Sternini C. Receptors and transmission in the brain-gut axis: potential for novel therapies. III. Mu-opioid receptors in the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2001;281(1):G8–15.
Tal MA. Role for inflammation in chronic pain. Curr Rev Pain. 1999;3(6):440–6.
Thong I, Jensen M, Miró J, Tan G. The validity of pain intensity measures: what do the NRS, VAS, VRS, and FPS-R measure? Scand J Pain. 2018;18(1):99–107.
Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–7.
Tsigos C, Kyrou I, Kassi E, et al. Stress: endocrine physiology and pathophysiology. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. https://www.ncbi.nlm.nih.gov/books/NBK278995/. [Updated 2020 Oct 17]
Wang F, Roy S. Gut homeostasis, microbial dysbiosis, and opioids. Toxicol Pathol. 2017;45(1):150–6.
Wärnberg J, Gomez-Martinez S, Romeo J, Díaz LE, Marcos A. Nutrition, inflammation, and cognitive function. Ann N Y Acad Sci. 2009;1153:164–75.
Yu Z, Yin Y, Zhao W, Chen F, Liu J. Application and bioactive properties of proteins and peptides derived from hen eggs: opportunities and challenges. J Sci Food Agric. 2014;94(14):2839–45.
Acknowledgements
The authors thank the participants of the study, the University of Siena for funding this research, the Project srl (Modena, Italy) and DST Diagnostische Systeme & Technologien GmbH (Schwerin, Germany) for supporting the IgG4 determination.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Editorial Assistance
The authors thank Dr Peter Christie, a freelance bioscience editor, for the English language revision and the Association “Biomedicina e Nutrizione” for payment of his fee.
Author Contributions
IC: concept and design, data analysis, literature review, results interpretation, manuscript drafting and revision; EF: results interpretation, manuscript revision; AG: results interpretation, manuscript revision; LM: data analysis, results interpretation; SP: literature review, manuscript revision; IC: data analysis, literature review, results interpretation, manuscript drafting and revision; AMA: concept and design, data analysis, literature review, results interpretation, manuscript drafting and revision. All authors have read and agreed to the published version of the manuscript.
Prior Presentation
Casini I.; Fatighenti E.; Giannantoni A.; Massai L.; Pieretti S. and Aloisi A.M. Nutrition and chronic pain in women. 44th AISD National Congress. Online, 23–25 September 2021.
Disclosures
Ilenia Casini, Elena Fatighenti, Lauretta Massai, Stefano Pieretti, Ilaria Ceccarelli and Anna Maria Aloisi declare no conflicts of interest with respect to the research, authorship, and/or publication of this article. Antonella Giannantoni has received funding from Astellas and Ipsen.
Compliance with Ethics Guidelines
The experimental protocol adhered to the principles of the Declaration of Helsinki of 1964 and its later amendments; the experimental procedure was approved by the University of Siena Local Ethics Committee (CAREUS, 7/2020 of 15/09/2020). Once included in the study, each subject provided informed consent for the analysis and publication of the data, then met with the experimental team twice (visit 1 and visit 2).
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Casini, I., Fatighenti, E., Giannantoni, A. et al. Food-Specific IgG4 Antibody-Guided Exclusion Diet Improves Conditions of Patients with Chronic Pain. Pain Ther 11, 873–906 (2022). https://doi.org/10.1007/s40122-022-00391-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00391-z