Abstract
Introduction
As 5-HT1B receptor agonists, triptans produce vasoconstriction and have cardiovascular contraindications and precautions. Lasmiditan, a selective 5-HT1F receptor agonist, has a low affinity for 5-HT1B receptors, does not cause vasoconstriction, and is free of cardiovascular contraindications and precautions. The objective of this post hoc analysis was to evaluate the efficacy and safety of lasmiditan in patients with and without at least one triptan contraindication.
Methods
Patient subgroups, with and without triptan contraindications, were analyzed from pooled patient data from four randomized, double-blind, placebo-controlled clinical trials (SAMURAI, SPARTAN, CENTURION, and MONONOFU). Patients experiencing a single migraine attack of moderate or severe intensity were treated with lasmiditan 50 mg (SPARTAN and MONONOFU only), 100 mg, 200 mg, or placebo, and efficacy data were recorded in an electronic diary.
Results
Of 5704 patients, 207 (3.6%) patients had at least one contraindication to triptans. Overall subgroup analysis revealed that the effects of lasmiditan on pain freedom, pain relief, freedom from most bothersome symptom, disability freedom, and Patient Global Impression of Change at 2 h post-dose did not differ in patient groups with and without triptan contraindications. These outcomes generally showed a similar benefit pattern for lasmiditan in both subgroups, with all results being statistically significant in patients without contraindications, and pain relief being statistically significant in patients with contraindications. The safety and tolerability profiles of patients with triptan versus without triptan contraindications were similar, including dizziness in 18.3 to 22.8% and somnolence in 7.9 to 9.9% of patients at the highest dose of lasmiditan.
Conclusions
In pooled analyses from four trials, patients with and without triptan contraindications did not differ in their patterns of lasmiditan efficacy. Lasmiditan may be a treatment option in patients with contraindications to triptans.
Trial Registration Numbers
SAMURAI, NCT:02439320; SPARTAN, NCT:02605174; CENTURION, NCT:03670810; and MONONOFU, NCT:03962738.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Triptans are vasoconstrictors and have been associated with rare vascular adverse events, leading to contraindications for patients with cardiovascular conditions. |
Lasmiditan does not cause vasoconstriction and does not have these contraindications. |
The objective of this post hoc analysis was to evaluate the efficacy and safety of lasmiditan in patients with and without triptan contraindications. Pooled data were from randomized, double-blind, placebo-controlled trials SAMURAI, SPARTAN, MONONOFU, and CENTURION. |
The efficacy and safety of lasmiditan appeared to be independent of whether patients had (n = 207) or did not have (n = 5497) a contraindication to triptans, supporting that lasmiditan may be a therapeutic option for patients with contraindications to triptans. |
Introduction
Migraine is a chronic neurological disorder characterized by recurrent attacks of headache typically accompanied by additional symptoms such as nausea, vomiting, photophobia, or phonophobia [1]. As the leading cause of disability in those under 50 years old [2] and the second highest cause of disability worldwide, migraine imposes a significant global health burden [3]. Optimal migraine care depends on a careful, personalized analysis of individual needs. In the absence of contraindications, the triptan class of drugs has been a first-line approach for treating migraine acutely [4]. Triptans are serotonin 5-HT1B/1D/1F receptor agonists. These receptors are present predominantly on peripheral trigeminal sensory nerve endings, neurons in the trigeminal cervical complex, rostral brainstem, thalamus, and on blood vessels [5].
Based on the hypothesis that migraine attacks resulted from vasodilation [6], triptans were developed to selectively target cranial blood vessels and to produce vasoconstriction. This vasoconstrictive effect is mediated through 5-HT1B receptors [7, 8]. Preclinical and clinical studies have shown that triptans induce vasoconstriction in different intracranial and extracranial vessels, including the coronary arteries [7,8,9,10,11]. Consistent with this mechanism, triptans have been associated with rare vascular adverse events after dosing [12], leading to contraindications for patients with cardiovascular conditions [13]; these contraindications and precautions are present in the package insert for all triptans and are referred to as class labeling. Results from the 2009 American Migraine Prevalence and Prevention (AMPP) survey estimated that in the USA, there were roughly 2.6 million people with episodic migraine aged 22 years and older, living with one of more prior cardiovascular events, conditions, or procedures that were triptan contraindications; the prevalence of these contraindications increased with age [14]. Still more individuals have risk factor profiles that mandate caution in the use of triptans [15].
Lasmiditan is a selective 5-HT1F receptor agonist [16, 17] approved in the USA for the acute treatment of migraine, with or without aura, in adults. The efficacy and safety profile of lasmiditan in the treatment of acute migraine has been reported in phase 3 trials [18,19,20,21,22]. Like triptans, lasmiditan also works on the trigeminal system but has a low affinity for 5-HT1B receptors and does not cause vasoconstriction [16]. Lasmiditan aborts migraine attacks by decreasing neural transmission and inhibiting the release of neurotransmitters involved in migraine pathophysiology, including calcitonin gene-related peptide (CGRP) [23]. In experiments using sumatriptan as a positive control, lasmiditan did not cause vasoconstriction in human proximal or distal coronary, internal mammary, or middle meningeal arteries ex vivo, or in dog coronary or carotid arteries in vivo [24]. Additionally, the presence of cardiovascular risk factors has not been associated with differences in the efficacy or safety profile of lasmiditan based on two studies reported previously [25].
Because lasmiditan is free of the 5-HT1B agonism thought to mediate vasoconstriction associated with triptans, the drug was developed with the hope that it would be free of cardiovascular contraindications. Nonetheless, both lasmiditan and triptans are serotonergic agonists that have sufficient pharmacologic overlap to make shared adverse events possible. In addition, lasmiditan may be preferentially used in those with triptan contraindications. Therefore, the efficacy and tolerability of lasmiditan in people with triptan contraindications is especially relevant to clinical practice. Though prior work focused on those with cardiovascular risk factors [25], that group is much larger and may be less vulnerable to adverse events than those with firm contraindications.
The objective of this post hoc analysis was to evaluate the efficacy and safety of lasmiditan in patients with and without at least one triptan contraindication, and to assess whether the presence of cardiovascular contraindications could interfere with lasmiditan treatment outcomes, given its distinct mechanism of action.
Methods
Statement of Ethics Compliance
All studies were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guideline, and local regulatory requirements. The study protocols were approved by an independent ethics committee or institutional review board at each study site. All participants provided written consent before the start of the study. All studies were registered at ClinicalTrials.gov (SAMURAI, NCT:02439320; SPARTAN, NCT:02605174; CENTURION, NCT:03670810; and MONONOFU, NCT:03962738).
Study Design
This post hoc analysis examined pooled data from four randomized, double-blind, placebo-controlled trials (SAMURAI, SPARTAN, CENTURION, and MONONOFU). All trials had a common design. Participants were asked to treat a single migraine attack (SAMURAI, SPARTAN, and MONONOFU) or four migraine attacks (CENTURION; first attack data only included). Participants were randomized 1:1:1 to placebo, lasmiditan 100 mg, or lasmiditan 200 mg (SAMURAI and CENTURION first attack), 1:1:1:1 to placebo, lasmiditan 50 mg, lasmiditan 100 mg, or lasmiditan 200 mg (SPARTAN), and 7:3:7:6 to placebo, lasmiditan 50 mg, lasmiditan 100 mg, or lasmiditan 200 mg (MONONOFU). Full details of each trial’s study design can be found in the primary report for each trial [18, 19, 22, 26].
Trial Population and Definition of Cardiovascular Contraindications to TRIPTANS
Eligibility criteria were similar across all studies with the exception that SAMURAI excluded patients with known coronary artery disease, clinically significant arrhythmia, and uncontrolled hypertension [18]. Participants were aged 18 years or older, with a diagnosis of migraine with or without aura fulfilling the criteria of the International Classification of Headache Disorders (ICHD). Criteria were from ICHD-2 for SAMURAI, SPARTAN, and MONONOFU [1], and ICHD-3 for CENTURION [27]. Participants were required to have a history of 3 to 8 migraine attacks per month, a history of disabling migraine for at least 1 year, and a Migraine Disability Assessment (MIDAS) score of ≥ 11.
For this analysis, participants from the four trials were pooled and then separated into two distinct patient subpopulations: patients with at least one triptan contraindication and patients with no triptan contraindications. The definition of triptan contraindications was based on a previous report [5]. In this research, a team of headache specialists, cardiologists, and health economics and outcomes researchers identified triptan contraindications by reviewing labels and then identified current procedural terminology (CPT) diagnostic codes corresponding to these contraindications in some or all of the triptan labels. Because medical conditions are coded in Medical Dictionary for Regulatory Activities (MedDRA) terms in clinical trials, each of the CPT diagnostic codes was converted to MedDRA codes to generate a comprehensive list of triptan contraindications (see supplementary material, including number of patients with each contraindication in the safety population). Medical histories were then reviewed to identify study participants who did and did not have at least one of the triptan contraindications.
Outcomes/Endpoints/Assessments
Patient-reported efficacy outcomes were recorded by the patient in an electronic diary at baseline and at 0.5, 1, 1.5, 2, 3, 4, 24, and 48 h after treatment or at baseline, 0.5, 1, 2, 4, 6, 24, and 48 h after treatment (CENTURION only). For the efficacy analysis, only the time points up to 2 h post treatment were evaluated because rescue medications were permitted after the assessment at 2 h. At the designated time points, patients recorded their level of headache pain as “none”, “mild”, “moderate”, or “severe.” Headache pain freedom is defined as a reduction in headache severity from mild, moderate, or severe at baseline to none at the indicated assessment time. A subject is not counted as being pain-free at a specific time point if she or he used rescue or recurrence medication at or before the specific time point. Sustained pain-free is defined as experiencing headache pain-free at 2 h after dosing and at the indicated assessment time, having not used any medications after the first dose. Patients also indicated the presence or absence of migraine-associated symptoms, including nausea, vomiting, photophobia, or phonophobia. At baseline, patients identified their most bothersome symptom (MBS) from choices, including nausea, phonophobia, or photophobia. At each time point, patients indicated their level of migraine-related disability by responding to the following question: “How much is your migraine interfering with your normal activities?” with response options “none”, “mild interference”, “marked interference”, or “need complete bed rest.” At 2 h post treatment, patients indicated their global impression of change (PGIC) in response to the following question: “How do you feel after taking study medication?” with seven response options ranging from ”very much better” to ”very much worse.”
Statistical Analyses
The patient demographics and baseline migraine attack characteristics were summarized using descriptive statistics for patients with and without triptan contraindications. All efficacy analyses were conducted in the efficacy population defined as all randomized patients who used at least 1 dose of study drug to treat an attack of at least mild pain severity and had any non-missing post-dose pain severity assessment at or before 2 h after dose. Mantel–Haenszel odds ratio with 95% confidence intervals and general association p values at 2 h post treatment, stratified by study, were calculated for two distinct patient subpopulations: “Triptan contraindication” versus “Triptan no contraindication.” Additionally, p values for treatment-by-triptan contraindicated status interaction were calculated based on a logistic regression model with treatment-by-triptan contraindicated status interaction term and study, treatment group, and triptan contraindicated status as covariates.
The safety population (all patients who were randomized and received at least one dose of study medication) was used for all safety analyses. Treatment-emergent adverse events were defined as new or worsening adverse events within 48 h of dosing.
Due to the post hoc nature of these analyses, the analyses were not corrected for multiplicity. Subgroup tests for interaction were considered statistically significant based on a p value of 0.10; otherwise, p values less than 0.05 were used to define statistical significance.
Results
Patient Demographics and Baseline Characteristics
A total of 5704 patients in the efficacy population, pooled from the SAMURAI, SPARTAN, MONONOFU, and CENTURION trials, were included in this analysis. Of the 5704 patients, 207 (3.6%) patients had at least one contraindication to triptans. The patient demographics and baseline disease characteristics for the efficacy population are summarized in Table 1. In comparison to patients free of triptan contraindications, those with a triptan contraindication were older, more likely to be White, more likely to have a family history of coronary artery disease, more likely to take a migraine preventive agent, and more likely to have a history of migraine with aura (Table 1).
Efficacy Outcomes
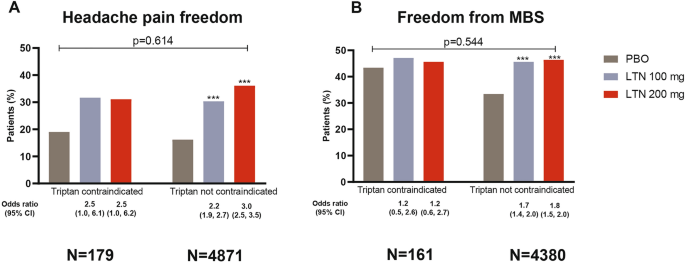
The subgroup analysis revealed that the effects of lasmiditan on the study co-primary endpoints of headache pain freedom at 2 h post-dose (2hPF) and freedom from MBS at 2 h post-dose (2hMBS) were not statistically different in patients with and without triptan contraindications (interaction p values > 0.1; Fig. 1). For example, for the 100-mg dose, 2hPF rates were 31.7% in the group with triptan contraindications and 30.3% in the group without contraindications, and odds ratios relative to placebo had overlapping confidence intervals (Fig. 1A). For the 100-mg dose, 2hMBS freedom rates were 47.1% in the group with triptan contraindications and 45.6% in the group without contraindications, and odds ratios relative to placebo had overlapping confidence intervals (Fig. 1B). Figure 2 shows that the magnitude of the treatment effect for 2-h headache pain relief, PGIC, and migraine-associated disability were similar, the confidence intervals for the odds ratios relative to placebo overlapped, and again the interaction p values were > 0.1. These outcomes generally showed a similar pattern of benefit for lasmiditan in both subgroups, with all results being statistically significant in the larger subgroup of patients without contraindications, and the results for pain relief being statistically significant in the smaller subgroup of patients with contraindications (Figs. 1 and 2).
Headache pain freedom (A) and freedom from MBS (B) in patients with and without a contraindication to triptans, 2 h following treatment with placebo, lasmiditan 100 or 200 mg. Comparisons of lasmiditan effect in the group of patients with triptan contraindications versus those without were not significant for any treatment group for either pain freedom or freedom from MBS (all interaction p values > 0.1). Odds ratio compared to patients who received placebo in the same subgroup. ***p < 0.001 vs. placebo. CI confidence interval, LTN lasmiditan, MBS most bothersome symptom, N number of patients, PBO placebo
Headache pain relief (A), Patient Global Impression of Change (B), and patient-reported freedom from migraine-related disability (C) in patients with and without a contraindication to triptans, 2 h following treatment with placebo, lasmiditan 100 or 200 mg. Comparisons of lasmiditan effect in the group of patients with triptan contraindications versus those without were not significant for any treatment group for either headache pain relief (percentage of patients with a reduction in pain severity from “moderate” or “severe” at baseline to “mild” or “none” at 2 h), PGIC (percentage of patients with responses “very much better” or “much better”), or freedom from migraine-related disability (percentage of patients with response option “none”) (all interaction p values > 0.1). Odds ratio compared to patients who received placebo in the same subgroup. *p < 0.05, ***p < 0.001 vs. placebo. CI confidence interval, LTN lasmiditan, N number of patients, PBO placebo, PGIC Patient Global Impression of Change
The proportion of patients achieving headache pain freedom over the 2 h post treatment is shown in Table 2. This time course was similar in patients with and without triptan contraindications.
Safety Outcomes
Proportions of patients with treatment-emergent adverse events (TEAEs) were generally similar in the triptan contraindicated and triptan not contraindicated population. There were no deaths in either group, and the proportions of patients experiencing adverse events (AEs) or serious AEs (SAEs) were similar between the two groups. There were no cardiovascular events in either subgroup suggesting vasoconstriction. The proportion of patients with triptan contraindications reporting SAEs was 0% (0/67) in the placebo group and 1.5% (2/138) in the lasmiditan group. The reported SAEs (menorrhagia, somatic symptom disorder) were not treatment-emergent (new or worsening within 48 h after dosing) and were not considered related to study drug. TEAEs with at least 2% frequency are reported in Table 3 and were similar in the subgroups, with dizziness in 18.3–22.8% and somnolence in 7.9–9.9% of patients at the highest lasmiditan dose.
Discussion
The results of this post hoc analysis show that the effects of lasmiditan in terms of efficacy and safety appeared to be independent of whether patients had or did not have a contraindication to triptans. Because lasmiditan is not a vasoconstrictor, these results support that lasmiditan may be a therapeutic option for patients with contraindications to triptans.
The four clinical trials included in the current analysis shared a common design. No upper age limit was applied, and patients were not excluded on the basis of having cardiovascular risk factors or disease, with the exception that SAMURAI excluded patients with known coronary artery disease, clinically significant arrhythmia, and uncontrolled hypertension [18].
As expected, patients with triptan contraindications were older, more likely to have a family history of coronary artery disease, use medications for migraine prevention, and have a history of migraine with aura. Efficacy data were similar among patients with and without triptan contraindications, as defined by all interaction p values being greater than 0.37. For pain freedom, pain relief, and PGIC at 2 h, and the time course of pain freedom, the results were similar visually and in terms of odds ratios for patients with and without contraindications. For MBS at 2 h, responses for patients treated with lasmiditan and those having contraindications versus not having contraindications were similar, but the placebo response was higher in triptan contraindicated patients, leading to odds ratios of 1.2 for patients treated with lasmiditan and having a contraindication compared to 1.7–1.8 for patients not having a contraindication. For migraine-related disability, the odds ratios for lasmiditan versus placebo were 1.2–1.8 in the contraindicated group compared with 1.7–1.9 in the not contraindicated group. Statistical testing of lasmiditan versus placebo within the subset of patients with contraindications to triptans showed statistical significance only for pain relief; these statistical tests not showing statistical significance should be considered within the context of the limited sample size and power in the subset of triptan contraindicated patients.
Safety data revealed that the proportions of patients with TEAEs were generally similar in the triptan contraindicated and triptan not contraindicated population. There were no deaths in either group, and the proportions of patients experiencing AEs or SAEs were similar between the two groups. TEAEs also displayed a similar profile across the subgroups, including dizziness in 18.3–22.8% and somnolence in 7.9–9.9% of patients at the highest lasmiditan dose. There were no cardiovascular events in either subgroup suggesting vasoconstriction.
Lasmiditan is a highly selective 5-HT1F receptor agonist distinct from triptans in chemical structure and in pharmacology and was developed to have a neurological mechanism of action [16]. Importantly, lasmiditan lacks significant activity at the vasoconstrictive 5-HT1B receptor and so is not vasoconstrictive. Patients who have contraindications to triptans might be candidates for lasmiditan therapy, and the results from the current analysis suggest that these patients may benefit from lasmiditan therapy.
A strength of this analysis is that results from four similarly conducted clinical trials with similar inclusion and exclusion criteria were pooled to maximize the available patients with triptan contraindications.
There are limitations to consider when interpreting the results of this report. The first limitation is that all analyses were completed post-hoc and that the studies were not powered to detect treatment-by-triptan contraindicated status interaction. The relatively small sample size of patients with triptan contraindications limits the power to detect significant changes within this subset and to detect differences in effects between patients having versus not having triptan contraindications (subset effects). Prior efforts to enroll patients with cardiovascular disease into migraine studies have proven challenging. For example, a study of telcagepant in patients with stable coronary artery disease and migraine enrolled 165 of the planned 400 patients and was stopped for futility due to enrollment difficulties (28).
Conclusions
In conclusion, the efficacy and safety findings for lasmiditan appeared to be independent of whether patients had or did not have contraindications to triptans. Lasmiditan may be a therapeutic option in patients unable to take triptans because of contraindications.
References
IHS. ICHD-II Classification: parts 1–3: primary, secondary and other. Cephalalgia. 2004;24(1_suppl):23–136.
Steiner TJ, Stovner LJ, Vos T, Jensen R, Katsarava Z. Migraine is first cause of disability in under 50s: will health politicians now take notice? J Headache Pain. 2018;19(1):17.
Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390(10100):1211–59.
Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3–20.
Dodick DW, Shewale AS, Lipton RB, Baum SJ, Marcus SC, Silberstein SD, et al. Migraine patients with cardiovascular disease and contraindications: an analysis of real-world claims data. J Prim Care Community Health. 2020;11:2150132720963680.
Humphrey PP, Feniuk W, Perren MJ, Beresford IJ, Skingle M, Whalley ET. Serotonin and migraine. Ann N Y Acad Sci. 1990;600:587–98.
Martin GR. Inhibition of the trigemino-vascular system with 5-HT1D agonist drugs: selectively targeting additional sites of action. Eur Neurol. 1996;36(Suppl 2):13–8.
Parsons AA, Raval P, Smith S, Tilford N, King FD, Kaumann AJ, et al. Effects of the novel high-affinity 5-HT(1B/1D)-receptor ligand frovatriptan in human isolated basilar and coronary arteries. J Cardiovasc Pharmacol. 1998;32(2):220–4.
Longmore J, Razzaque Z, Hargreaves R, Schofield W, Pickard J, Boulanger C. Rizatriptan selectively contracts human middle meningeal over coronary artery: comparison with sumatriptan. Cephalagia. 1997;17(suppl 19):388–9.
Gupta P, Scatchard J, Shepperson N, Wallis R, Wythes M. In vitro pharmacology of eletriptan (UK-116044), a potent partial agonist at the “5HT 1D-like” receptor in the dog saphenous vein. Cephalalgia. 1996;16:386.
MacIntyre PD, Bhargava B, Hogg KJ, Gemmill JD, Hillis WS. Effect of subcutaneous sumatriptan, a selective 5HT1 agonist, on the systemic, pulmonary, and coronary circulation. Circulation. 1993;87(2):401–5.
Dodick D, Lipton RB, Martin V, Papademetriou V, Rosamond W, MaassenVanDenBrink A, et al. Consensus statement: cardiovascular safety profile of triptans (5-HT agonists) in the acute treatment of migraine. Headache. 2004;44(5):414–25.
Negro A, Koverech A, Martelletti P. Serotonin receptor agonists in the acute treatment of migraine: a review on their therapeutic potential. J Pain Res. 2018;11:515–26.
Buse DC, Reed ML, Fanning KM, Kurth T, Lipton RB. Cardiovascular events, conditions, and procedures among people with episodic migraine in the US population: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2017;57(1):31–44.
Lipton RB, Reed ML, Kurth T, Fanning KM, Buse DC. Framingham-based cardiovascular risk estimates among people with episodic migraine in the US population: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2017;57(10):1507–21.
Nelson DL, Phebus LA, Johnson KW, Wainscott DB, Cohen ML, Calligaro DO, et al. Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia. 2010;30(10):1159–69.
Rubio-Beltran E, Labastida-Ramirez A, Villalon CM, MaassenVanDenBrink A. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88–97.
Kuca B, Silberstein SD, Wietecha L, Berg PH, Dozier G, Lipton RB, et al. Lasmiditan is an effective acute treatment for migraine. Neurology. 2018;91:e2222–32.
Goadsby PJ, Wietecha LA, Dennehy EB, Kuca B, Case MG, Aurora SK, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142(7):1894–904.
Brandes JL, Klise S, Krege JH, Case M, Khanna R, Vasudeva R, et al. Interim results of a prospective, randomized, open-label, Phase 3 study of the long-term safety and efficacy of lasmiditan for acute treatment of migraine (the GLADIATOR study). Cephalalgia. 2019;39(11):1343–57.
Brandes JL, Klise S, Krege JH, Case M, Khanna R, Vasudeva R, et al. Long-term safety and efficacy of lasmiditan for acute treatment of migraine: final results of the GLADIATOR study. Cephalalgia Rep. 2020;3:2515816320958176.
Ashina M, Reuter U, Smith T, Krikke-Workel J, Klise SR, Bragg S, et al. Randomized, controlled trial of lasmiditan over four migraine attacks: findings from the CENTURION study. Cephalalgia. 2021;41(3):294–304.
Clemow DB, Johnson KW, Hochstetler HM, Ossipov MH, Hake AM, Blumenfeld AM. Lasmiditan mechanism of action—review of a selective 5-HT1F agonist. J Headache Pain. 2020;21(1):71.
Rubio-Beltrán E, Labastida-Ramírez A, Haanes KA, van den Bogaerdt A, Bogers AJJC, Zanelli E, et al. Characterization of binding, functional activity, and contractile responses of the selective 5-HT1F receptor agonist lasmiditan. Br J Pharmacol. 2019;176(24):4681–95.
Shapiro RE, Hochstetler HM, Dennehy EB, Khanna R, Doty EG, Berg PH, et al. Lasmiditan for acute treatment of migraine in patients with cardiovascular risk factors: post-hoc analysis of pooled results from 2 randomized, double-blind, placebo-controlled, phase 3 trials. J Headache Pain. 2019;20(1):90.
Sakai F, Takeshima T, Homma G, Tanji Y, Katagiri H, Komori M. Phase 2 randomized placebo-controlled study of lasmiditan for the acute treatment of migraine in Japanese patients. Headache. 2021;61(5):755–65.
(IHS) HCCotIHS. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
Ho TW, Ho AP, Chaitman BR, Johnson C, Mathew NT, Kost J, et al. Randomized, controlled study of telcagepant in patients with migraine and coronary artery disease. Headache. 2012;52(2):224–35.
Acknowledgements
We would like to acknowledge the site staff and investigators and to thank all the patients and their families and caregivers who participated in this trial.
Funding
This study was funded by Eli Lilly and Company. All publication costs including the journal’s Rapid Service Fee were also funded by Eli Lilly and Company.
Medical Writing Assistance
Deirdre Hoban, PhD, as part of her role as an employee of Eli Lilly and Company, provided writing support and manuscript preparation. Editorial support was provided Lauren Linsangan, Anthony Marca, and Patrick Tenedero of Certara Synchrogenix, who were contracted by Eli Lilly and Company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conception: John H. Krege, Richard B. Lipton. Design of the work: John H. Krege, Sinéad M. Ryan, Maurice Vincent. Acquisition of data: Mika Komori. Analysis of data: Simin K. Baygani. Interpretation of data: John H. Krege, Richard B. Lipton, Mika Komori, Maurice Vincent. Drafting of the work: Sinéad M. Ryan. Critical revision of the work for important intellectual content: John H. Krege, Richard B. Lipton, Simin K. Baygani, Mika Komori, Maurice Vincent. All authors provided input and gave final approval for the work to be published.
Disclosures
John H. Krege, Simin K. Baygani, Mika Komori and Maurice Vincent are full-time employees and minor shareholders of Eli Lilly and Company. Sinéad M. Ryan is a full-time employee of Eli Lilly and Company. Maurice Vincent has received funds from Eli Lilly and Company for attending meetings and or/travel as part of his work in the company. Richard B. Lipton has received research funding from NIH/NIA 2PO1 AG003949 (Einstein Aging Study), the S&L Marx Foundation, Czap Foundation, the NIH, the FDA, the Migraine Research Foundation and the National Headache Foundation. Richard B. Lipton has served/serves as consultant, advisory board member, and received honoraria from or research support from: AbbVie (Allergan), American Academy of Neurology, American Headache Society, Amgen, Biohaven, Biovision, Boston, Dr. Reddy’s (Promius), Electrocore, Eli Lilly and Company, eNeura, Equinox, GlaxoSmithKline, Grifols, Lundbeck (Alder), Merck, Pernix, Pfizer, Teva, Vector and Vedanta. Richard B. Lipton has stock or stock options in Biohaven and CtrlM Health.
Compliance with Ethics Guidelines
All studies were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guideline, and local regulatory requirements. The study protocols were approved by an independent ethics committee or institutional review board at each study site. All patients provided written consent before the start of the study. All studies were registered at ClinicalTrials.gov (SAMURAI, NCT:02439320; SPARTAN, NCT:02605174; CENTURION, NCT:03670810; and MONONOFU, NCT:03962738).
Data Availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Krege, J.H., Lipton, R.B., Baygani, S.K. et al. Lasmiditan for Patients with Migraine and Contraindications to Triptans: A Post Hoc Analysis. Pain Ther 11, 701–712 (2022). https://doi.org/10.1007/s40122-022-00388-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00388-8