Abstract
Introduction
Inflammation has been suggested to be involved in the pathogenesis of osteoarthritis and pain. We sought to explore the associations between inflammatory serum markers and magnetic resonance imaging-defined long-term structural change and pain trajectory.
Methods
A total of 169 randomly selected participants (mean age 63 years; 47% female) from a prospective cohort study were included in this study. Circulating levels of interleukin 6 (IL-6), tumour necrosis factor alpha (TNF-α) and high-sensitivity C-reactive protein (CRP) were measured at baseline. A knee MRI scan was performed to measure cartilage volume (CV) and bone marrow lesions (BMLs) at baseline and at 10.7 years. Knee pain at four visits was measured by the WOMAC pain questionnaire, and pain trajectories were identified using group-based trajectory modelling. Linear, log-binomial and multi-nominal logistic regression were used for the analyses.
Results
IL-6 was associated with lateral but not medial tibial CV loss (β = − 0.25% per annum, per standard deviation [SD] log pg/ml; P < 0.05) in the multivariate analysis. IL-6 was also associated with a ‘Moderate pain’ trajectory (relative risk ratio 1.93 per SD log pg/ml; 95% confidence interval 1.02–3.65) relative to the ‘Minimal pain’ trajectory group. There was no significant association of TNF-α and CRP with CV loss and pain trajectory groups with the exception of a beneficial relationship between CRP and medial tibial CV loss (β = 0.20% per annum, per SD log mg/l). No association between inflammatory markers and change in BML size was observed.
Conclusions
IL-6 was independently associated with compartment-specific CV loss and worse pain trajectory, but the other markers studied were not, suggesting that components of inflammation are implicated in the pathogenesis of cartilage loss and developing a worse pain course.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Inflammation has been suggested to be involved in the pathogenesis of osteoarthritis and pain. |
Osteoarthritis and pain are heterogenous, so inflammation may be a driving mechanism in developing the heterogeneity of osteoarthritis and pain. |
The aim of this study was to investigate whether circulating levels of inflammatory markers are associated with knee pain trajectories/subgroups that were previously identified and structural change visible on magnetic resonance imaging, over a long follow-up period. |
What was learned from the study? |
Elevated levels of interleukin 6 increased the risk of developing worse pain trajectory and more lateral tibial cartilage volume loss. |
The findings suggest that components of inflammation are implicated in developing a worse pain course and the pathogenesis of cartilage loss. |
Introduction
Osteoarthritis (OA) is a painful and entire-joint structural condition that involves cartilage, subchondral bone and synovium [1]. Unfortunately, no treatment is currently available that targets both disease progression and the pain that is the most defining symptom of OA. Current OA treatments are palliative and primarily focus on pain relief, but the effect of commonly prescribed medications is modest with a substantial risk of adverse effects [2].
Although the aetiology of OA is not fully understood, inflammation is increasingly considered to be a crucial driver of OA pathology and pain [3, 4]. There is evidence suggesting that inflammatory factors such as proinflammatory cytokines are key mediators in the pathophysiology of OA [3, 5]. Tumour necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β), produced by activated chondrocytes, synoviocytes and mononuclear cells [6], are considered to be the prominent mediators among proinflammatory cytokines in OA [3, 7], able to induce the inflammatory cascade independently or together with other cytokines [3], such as by stimulating IL-6 production [8]. C-reactive protein (CRP), a systemic marker of inflammation, is regulated by proinflammatory cytokines (e.g. IL-6). Epidemiological studies, including our own research, have shown that local/circulating levels of these proinflammatory cytokines (e.g. TNF-α, IL-6) and CRP are linked to X-ray/magnetic resonance imaging (MRI)-defined knee OA progression and pain change in the short term [9,10,11,12,13,14,15,16].
For this reason, targeting inflammation has been the focus of OA clinical trials; however, these trials mostly have not shown positive effects [2, 17, 18]. This scarcity of effects may be partially due to OA being not one disease, but rather composed of distinct subgroups with different mechanisms underlying each subgroup, such that inflammation may be only present in one subset of OA patients [19,20,21]. Hence, assessment of inflammatory markers may allow for the identification of subgroups of OA patients who are at a higher risk of disease progression. However, there are currently very few biomarkers that can predict structural progression, particularly in the long term [22]. We recently identified distinct knee OA pain trajectories/subgroups and found worse pain trajectories to be associated with the presence of metabolic syndrome [23, 24], suggesting that the underlying mechanisms of different pain trajectories may be different. Based on this presumption, inflammation may also play a different role in the distinct pain subgroups, and thus may be used to classify pain subgroups to apply a tailored treatment. Currently, research on the long-term relationship between inflammatory markers and MRI-defined structural changes is scarce, and no study has yet examined whether inflammation increases the risk of developing different pain subgroups. Therefore, the aim of this study was to investigate whether circulating levels of IL-6, TNF-α and CRP are associated with MRI-defined structural change over a long follow-up period and the knee pain trajectories we identified.
Methods
Participants
The Tasmanian Older Adult Cohort Study (TASOAC) is a prospective and population-based cohort study of 1099 participants in Southern Tasmania, Australia (population 229,000) who were randomly selected from the electoral roll between 2002 and 2004, with an equal number of men and women. The electoral roll included eligible electors if they were aged ≥ 18 years, an Australian citizen and an elector entitled to vote at House of Representatives election or qualified to become such an elector. Institutionalised older adults were excluded. Participants were aged between 50 and 80 years at the time of recruitment and were followed up at 2.6, 5.1 and 10.7 years post-recruitment; a total of 875, 768 and 563 participants participated in the follow-up at these time points, respectively. The flow chart of the TASOAC study is shown in Electronic Supplementary Material (ESM) Fig. S1. The participants provided detailed information via questionnaires and interview, allowed blood sample collection and underwent clinical assessments. The current study was a sub-study of the TASOAC in which 193 participants were randomly selected for inflammatory marker testing at baseline. The TASOAC received ethical approval from the Southern Tasmanian Health and Medical Human Research Ethics Committee. All participants provided written informed consent to participate in the TASOAC. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Outcome Measurements
Knee Structures Visible on MRI
Participants underwent MRI examinations of their right knee at baseline and at 10.7 years of follow-up using a 1.5–T whole-body MR unit (Picker International Inc., Cleveland, USA) equipped with a commercial transmit–receive extremity coil, as previously described [25]. The imaging sequences consisted of: (1) a T1-weighted fat saturation three-dimensional (3D) gradient-recalled acquisition in the steady-state; and (2) a T2-weighted fat saturation 2D fast spin-echo.
Knee Cartilage Volume
Knee cartilage volume (CV) on T1-weighted MR images was measured by two trained and blinded observers using the image-manipulation softward Osiris (University of Geneva, Geneva, Switzerland) as previously described [26]. Disarticulation contours around the cartilage boundaries were manually drawn on a section-by-section basis to isolate the medial and lateral tibial CV from the total volume, these were then resampled using bilinear and cubic interpolation for the final 3D rendering. The coefficient of variation for medial and lateral tibial CV measures was 2.1% and 2.2%, respectively [26]. The following formula was used to calculate the rate of CV change per annum: percentage change per annum = 100 × ([follow-up CV − baseline CV]/baseline CV)/time between two scans in years.
Bone Marrow Lesion
Bone marrow lesion (BML) was defined as areas of increased signal adjacent to the subcortical bone on T2-weighted MR images and was scored by a trained observer using OsiriX software. The maximum area (in mm2) of the lesion in the subregion of medial and lateral tibial sites was scored. Intraobserver repeatability was established with an intraclass correlation coefficient (ICC) of 0.98 (95% confidence interval 0.96–0.99). We defined the BML size increase if any change in BML size from baseline to the follow-up examination at 10.7 years was greater than the least significant criterion (52 mm2) that took into account the measurement error and the correlation between the two BML measurements [27].
Knee Pain
Knee pain was evaluated at baseline and at the three follow-ups using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain questionnaire. This pain questionnaire comprises five questions, with participants asked to state their perceived pain while walking on a flat surface, going up or down stairs, sleeping at night, sitting or lying and standing. Each question was scored on a 10-point scale (0–9) with a higher score indicating greater pain intensity [28], and a total WOMAC pain score was calculated by summing the scores for the five questions (0–45).
Exposure Measurements
Serum levels of IL-6, TNF-α and CRP were measured at baseline, as previously described [11, 13]. The minimum levels of detection of the assay for IL-6, TNF-α and CRP were 2.0 pg/ml, 4.0 pg/ml and 0.01 mg/l, respectively. The coefficients of variation for IL-6, TNF-α and CRP were 8, 6 and 4.8%, respectively.
Covariate Measurements
Participants’ age and sex were collected via a questionnaire. Height was measured using a stadiometer, and weight was measured using a single pair of electronic scales (Delta Model 707; Seca GmbH & Co., Hamburg, Germany), following which body mass index (BMI) was calculated (kg/m2). Pedometers (models Omron HJ-003 & HJ-102; Omron Healthcare, Kyoto, Japan) were used to measure participants’ physical activity, expressed as steps per day, as previously described [25]. Participants were asked to state ‘how much have they been bothered by emotional problems during the past 4 weeks, such as feeling anxious, depressed or irritable?’. Response options included ‘not at all’, ‘very little’, ‘moderately’, ‘quite a lot’ and ‘extremely’. If participants gave a response of ‘very little’ or worse, they were considered to be experiencing emotional problems. Participants were asked to report any common conditions diagnosed by a doctor, including diabetes, heart attack, hypertension, thrombosis, asthma, bronchitis/emphysema, hyperthyroidism, hypothyroidism, and rheumatoid arthritis. The total number of comorbidities was calculated and ranged from zero to nine. Radiograph assessment was completed at baseline and has been previously described in detail [29]. Briefly, knee radiographs with a standing anteroposterior semiflexed view of the right knee were obtained and scored using the Altman atlas for osteophytes and joint space narrowing (JSN) on a 4-point scale (0–3) [30]. The ICCs were 0.99 for osteophytes and 0.98 for JSN. The presence of radiographic knee OA (ROA) was defined as any score of ≥ 1 for osteophytes or JSN.
Statistical Analysis
The distribution of the values of inflammatory markers was checked using the Shapiro–Wilk test. The levels of all markers were natural log-transformed for normalisation. Any outlier was excluded if the concentration was either greater than the mean + 3× the standard deviation (SD) or less than the mean + 3× the SD. Two outliers were removed from our analyses. Means ± SD and medians (interquartile range) and percentages were used to describe continuous and categorical variables, respectively. The t-test, Mann–Whitney U-test and Chi-square test were used to compare differences in participants’ characteristics between participants included and not included in this study where appropriate. Linear and log-binomial regression were used to evaluate the association of each marker with CV loss (percentage change) and BML size increase before and after adjustment for covariates, including age, sex, physical activity, comorbidities and ROA, respectively.
As previously described, we identified three knee pain trajectories using group-based trajectory modelling [23]. Multi-nominal logistic regression was then used to assess the associations between markers and pain trajectory groups before and after controlling for covariates, including age, sex, physical activity, comorbidities, emotional problems and ROA. TNF-α was additionally adjusted for IL-6 to test whether IL-6 was associated with CV loss and pain trajectories, independent of TNF-α. We standardised IL-6, TNF-α and CRP values by dividing each one by the corresponding SD to make the results comparable across different markers. It has been reported that IL-6, TNF-α and CRP can be produced in adipose tissue [31,32,33]; therefore, BMI may be part of the cytokine–structural change–pain causal pathway, and was not adjusted in the models. The data analyses were performed using Stata software (V.16) (StataCorp, College Station, TX, USA). A p value of < 0.05 was considered to be statistically significant.
Results
A total of 193 participants were randomly selected from the entire cohort of TASOAC for inflammatory marker testing at baseline; of these participants, data on all inflammatory markers pertinent to this study were available for 182 of them. Of the 182 participants, 82 and seven were excluded from analyses of knee structural change due to missing MRI data and missing covariate measurements, respectively, and 13 participants were excluded from the pain trajectory analyses due to missing covariate measurements (Fig. 1). Thus, the analyses of the current study included 93 participants in the analyses on MRI-defined structural change and 169 participants in those for pain trajectory. Of the 169 participants, the mean (± SD) age was 63.2 ± 7.2 years and mean BMI was 27.4 ± 4.4 kg/m2; 47% were women; and 56% had ROA (Table 1). There were no significant differences in participants’ characteristics between participants included and not included in this study (ESM Table S1). Over 10.7 years of follow-up, CV decreased and BML size increased, while pain level appeared to be stable (ESM Table S2).
Tables 2 and 3 show the associations of IL-6, TNF-α and CRP with CV loss and BML size increase in the medial/lateral tibial compartment over 10.7 years of follow-up. In the univariate analyses, IL-6, TNF-α and CRP were not found to be associated with medial or lateral tibial CV loss, except that IL-6 was associated with more lateral tibial CV loss than the other two infammatory markers (Table 2). The significant association between IL-6 and lateral tibial CV loss persisted after adjustment for age, sex, physical activity, comorbidities and ROA. Further adjustment for TNF-α for IL-6 also did not change the association (data not shown). Surprisingly, after controlling for covariates, CRP became significant with medial tibial CV loss with per SD log-transformed CRP increase associated with less medial tibial CV loss. No inflammatory marker was found to associate with medial or lateral BML size (Table 3).
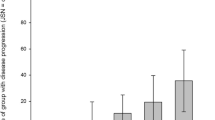
Of 169 participants, 54% (n = 92), 35% (n = 59) and 11% (n = 18) fell into ‘Minimal pain’, ‘Mild pain’ and ‘Moderate pain’ trajectory group, respectively. There was a trend toward an increase in the concentration of IL-6 as pain trajectory groups worsened (Fig. 2). Table 4 presents the association between inflammatory markers with pain trajectory groups. Per SD log-transformed increase in IL-6 was associated with the ‘Moderate pain’ trajectory as compared to the ‘Minimal pain’ trajectory before and after adjusting for covariates. There were no statistically significant associations between TNF-α and CRP and pain trajectory groups. When BMI was also additionally adjusted in the analyses, the results of the associations of IL-6 with lateral tibial CV loss and ‘Moderate pain’ trajectory did not largely alter (ESM Tables S3, S4).
Discussion
This study describes the associations between inflammatory serum markers and MRI-defined knee structural change and pain trajectory over the long term. We found that higher levels of IL-6 were associated with lateral (but not medial) tibial CV loss and with an increased risk of belonging to a worse pain trajectory group. Neither TNF-α nor CRP was found to associate with knee structural change on MR scans and pain trajectory except for an unexpected positive relationship between CRP and medial tibial CV loss. These findings support the role of inflammation in the pathogenesis of both OA structural change and pain, but also reflect that inflammation is a driving mechanism for developing a worse pain course only in a subset of the patient population with pain. To our knowledge, this study is the first to examine the role of inflammatory markers in different pain subgroups.
That local/circulating IL-6 is implicated in the pathophysiology of OA is supported by a large body of evidence [3, 18], but few studies so far have explored its relationship with MRI-defined structural change. Livshits et al. [10] reported that increased circulating levels of IL-6 were associated with the incidence of ROA in a 15-year follow-up study. Pelletier et al. [12] showed that baseline serum levels of IL-6 were significantly associated with CV loss at the medial compartment over 2 years in patients with knee OA. Similarly, an earlier study on our cohort also found that baseline serum levels of IL-6 were predictive of both medial and lateral tibial CV loss over 2.9 years of follow-up. Consistent with these studies, the present study found more lateral tibial CV loss in association with elevated levels of IL-6 independent of covariates, even TNF-α, thereby providing further support for a detrimental role of elevated levels of IL-6 in cartilage metabolism. However, different associations at the medial/lateral compartment between studies likely reflect a greater contribution of mechanical loading to the medial compartment. As such, the loss of CV at the medial compartment was higher and occurred earlier than that at the lateral compartment; consequently, there was not too much CV to lose even with a longer observation period. In contrast, the present study did not find an association between IL-6 and BML size increase. This result is contrary to that of a 2-year follow-up study which found that higher levels of baseline IL-6 were related to a greater risk of BML score increase in 192 patients with knee OA [15]. Other than a discrepancy in participants’ characteristics, another possible explanation for this inconsistency might be that BML often fluctuates in a short period [34].
IL-6 has been found to serve as pronociceptive mediators which may be directly implicated in pain generation by acting on nociceptive neurons [35, 36]. Several clinical studies also have shown that IL-6 in synovial fluid/serum was correlated with pain scores [37, 38], but only few studies have investigated the relationship of IL-6 with pain change [13, 39]. Higher synovial fluid levels of IL-6 at the time of surgery were found to predict less pain improvement at 2 years following total knee arthroplasty in 28 patients with OA [39]. Likewise, we previously reported that higher baseline serum levels of IL-6 were predictive of greater change in knee pain while standing over a 5-year follow-up [13]. In line with these studies, the findings of this study that a higher concentration of IL-6 was associated with a more severe pain trajectory not only confirm the IL-6–pain link, but also extend previous studies into worsening pain from the group level using traditional longitudinal analysis to the individual level that can differentiate distinct pain subgroups. Our recent studies together with previous studies have demonstrated that pain in OA is heterogenous and consists of different pain subgroups [23, 24, 40,41,42,43]. The present study found that higher levels of IL-6 increased the risk of “Moderate pain”—but not “Mild pain”—relative to “Minimal pain”, suggesting that inflammation is only present and participates in pain generation and maintenance in a subset of the OA population. This finding may partly explain why IL-6 inhibitors (i.e. tocilizumab) did not improve pain more than placebo in patients with hand OA after 12 weeks of treatment [44], while there is a possibility that there may not be a sufficient drug penetration in hand OA where there is less inflammation. Although it is speculative, an observed relationship between IL-6 and “Moderate pain” may indicate pain sensitisation induced by IL-6, since pain level in the “Moderate pain” group was highest and stable over time (data not shown). Supporting this speculation, studies have found that IL‐6‐induced sensitisation cannot be reversed with the administration of an IL‐6‐neutralising compound, suggesting that IL-6-induced sensitisation is persistent once it is established [45, 46].
TNF-α is also considered to be another important proinflammatory cytokine that is involved in cartilage destruction [3] and activation of nociceptors [47]. However, the current study was unable to show significant associations of TNF-α with structural change and pain trajectories. This is broadly in accordance the results reported in earlier studies, which showed no link between TNF-α and incident ROA over 15 years [10], and is indirectly supported by our recent clinical trial of TNF inhibitor treatment in hand OA patients, showing no pain improvement as compared to the placebo group [48]. On the contrary, Botha-Scheepers et al. [9] reported that TNF-α was associated with radiological progression of knee OA over 2 years. Our earlier studies from this cohort also showed that elevated levels of TNF-α at baseline were associated with a greater change in knee pain while standing [13], but not in CV loss [11], over up to 5 years of follow-up. Given that TNF-α can initiate and propagate inflammation [3] and that studies with a shorter follow-up period were more likely to observe a significant association, it may be that TNF-α is only involved in the early stage of structural change and pain generation. The current study also did not find an association between CRP and MRI-defined structural change and pain trajectories, except for an unexcepted positive relationship between CRP and medial tibial CV loss. This seems to be supported by studies that showed no association between CRP level and OA progression [16] and CV loss [11]. Further, the results from previous studies between CRP level and pain at the group level are conflicting [13, 14], with the inconsistencies possibly suggesting that CRP is not an optimal marker in monitoring structural changes or pain evolution. The finding that the CRP level was associated with more medial tibial CV was unexpected and may be due to the non-specificity of CRP as an indicator of inflammation other than infection or tissue damage [49].
The long observation period is a strength of this study; however, there are several limitations to be acknowledged. First, serum inflammatory markers were measured at baseline, so we were unable to evaluate structural change on the MR scans and pain subgroups in relation to the variation of these markers. Second, serum levels of markers reflect systemic inflammation in the body, not just in the joint; thus, they are likely affected by other conditions. Although we have considered the impact of potential comorbidities on inflammatory markers, we cannot rule out a possibility that acute inflammation/infection was present in some participants at the time of blood collection. Also, our study did not collect data on treatments as a result of inflammation/infection, so we may have underestimated the associations of IL-6 with CV loss and pain trajectories. In addition, we selected IL-6, TNF-α and CRP for biomarker testing when the TASOAC was initiated. With an in-depth understanding of inflammation in OA and pain, further investigations on other inflammatory markers involved in MRI-defined structural change and pain heterogeneity are warranted. Third, some participants, particularly those with severe pain, may have been receiving pharmacological and/or non-pharmacological treatment; however, whether pain management has influenced results cannot be assessed due to unavailability of the data.
Conclusions
IL-6, but not other markers, was independently associated with compartment-specific CV loss and a worse pain trajectory, suggesting that components of inflammation may be implicated in the pathogenesis of cartilage loss and developing worse pain course.
References
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–707. https://doi.org/10.1002/art.34453.
Conaghan PG, Cook AD, Hamilton JA, Tak PP. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat Rev Rheumatol. 2019;15(6):355–63. https://doi.org/10.1038/s41584-019-0221-y.
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. https://doi.org/10.1038/nrrheum.2010.196.
Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16–21. https://doi.org/10.1016/j.joca.2012.11.012.
Chow YY, Chin KY. The role of inflammation in the pathogenesis of osteoarthritis. Mediat Inflamm. 2020;2020:8293921. https://doi.org/10.1155/2020/8293921.
Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6(11):625–35. https://doi.org/10.1038/nrrheum.2010.159.
van den Bosch MHJ, van Lent P, van der Kraan PM. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthritis Cartilage. 2020;28(5):532–43. https://doi.org/10.1016/j.joca.2020.01.016.
Guerne PA, Carson DA, Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990;144(2):499–505.
Botha-Scheepers S, Watt I, Slagboom E, et al. Innate production of tumour necrosis factor alpha and interleukin 10 is associated with radiological progression of knee osteoarthritis. Ann Rheum Dis. 2008;67(8):1165–9. https://doi.org/10.1136/ard.2007.084657.
Livshits G, Zhai G, Hart DJ, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: the Chingford Study. Arthritis Rheum. 2009;60(7):2037–45. https://doi.org/10.1002/art.24598.
Stannus O, Jones G, Cicuttini F, et al. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18(11):1441–7. https://doi.org/10.1016/j.joca.2010.08.016.
Pelletier JP, Raynauld JP, Caron J, et al. Decrease in serum level of matrix metalloproteinases is predictive of the disease-modifying effect of osteoarthritis drugs assessed by quantitative MRI in patients with knee osteoarthritis. Ann Rheum Dis. 2010;69(12):2095–101. https://doi.org/10.1136/ard.2009.122002.
Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72(4):535–40. https://doi.org/10.1136/annrheumdis-2011-201047.
Zhu Z, Jin X, Wang B, et al. Cross-sectional and longitudinal associations between serum levels of high-sensitivity C-reactive protein, knee bone marrow lesions, and knee pain in patients with knee osteoarthritis. Arthritis Care Res (Hoboken). 2016;68(10):1471–7. https://doi.org/10.1002/acr.22834.
Zhu Z, Otahal P, Wang B, et al. Cross-sectional and longitudinal associations between serum inflammatory cytokines and knee bone marrow lesions in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2017;25(4):499–505. https://doi.org/10.1016/j.joca.2016.10.024.
Jin X, Beguerie JR, Zhang W, et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(4):703–10. https://doi.org/10.1136/annrheumdis-2013-204494.
Philp AM, Davis ET, Jones SW. Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatology (Oxford). 2017;56(6):869–81. https://doi.org/10.1093/rheumatology/kew278.
Wiegertjes R, van de Loo FAJ, Blaney Davidson EN. A roadmap to target interleukin-6 in osteoarthritis. Rheumatology (Oxford). 2020;59(10):2681–94. https://doi.org/10.1093/rheumatology/keaa248.
Dell’Isola A, Allan R, Smith SL, Marreiros SS, Steultjens M. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord. 2016;17(1):425. https://doi.org/10.1186/s12891-016-1286-2.
Deveza LA, Melo L, Yamato TP, Mills K, Ravi V, Hunter DJ. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthritis Cartilage. 2017;25(12):1926–41. https://doi.org/10.1016/j.joca.2017.08.009.
Deveza LA, Loeser RF. Is osteoarthritis one disease or a collection of many? Rheumatology (Oxford). 2018;57(Suppl_4):iv34–42. https://doi.org/10.1093/rheumatology/kex417.
Saberi Hosnijeh F, Bierma-Zeinstra SM, Bay-Jensen AC. Osteoarthritis year in review 2018: biomarkers (biochemical markers). Osteoarthritis Cartilage. 2019;27(3):412–23. https://doi.org/10.1016/j.joca.2018.12.002.
Pan F, Tian J, Aitken D, Cicuttini F, Jones G. Predictors of pain severity trajectory in older adults: a 10.7-year follow-up study. Osteoarthritis Cartilage. 2018;26(12):1619–26. https://doi.org/10.1016/j.joca.2018.08.002.
Pan F, Tian J, Cicuttini F, Jones G. Metabolic syndrome and trajectory of knee pain in older adults. Osteoarthritis Cartilage. 2020;28(1):45–52. https://doi.org/10.1016/j.joca.2019.05.030.
Dore DA, Winzenberg TM, Ding C, et al. The association between objectively measured physical activity and knee structural change using MRI. Ann Rheum Dis. 2013;72(7):1170–5. https://doi.org/10.1136/annrheumdis-2012-201691.
Jones G, Glisson M, Hynes K, Cicuttini F. Sex and site differences in cartilage development: a possible explanation for variations in knee osteoarthritis in later life. Arthritis Rheum. 2000;43(11):2543–9. https://doi.org/10.1002/1529-0131(200011)43:11%3c2543::AID-ANR23%3e3.0.CO;2-K.
Nguyen TV, Eisman JA. Assessment of significant change in BMD: a new approach. J Bone Miner Res. 2000;15(2):369–72. https://doi.org/10.1359/jbmr.2000.15.2.369.
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40.
Jones G, Ding C, Scott F, Glisson M, Cicuttini F. Early radiographic osteoarthritis is associated with substantial changes in cartilage volume and tibial bone surface area in both males and females. Osteoarthritis Cartilage. 2004;12(2):169–74. https://doi.org/10.1016/j.joca.2003.08.010.
Altman RD, Hochberg M, Murphy WA Jr, Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(Suppl A):3–70.
Hoene M, Weigert C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obes Rev. 2008;9(1):20–9. https://doi.org/10.1111/j.1467-789X.2007.00410.x.
Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582(1):117–31. https://doi.org/10.1016/j.febslet.2007.11.051.
Anty R, Bekri S, Luciani N, et al. The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, Type 2 diabetes, and NASH. Am J Gastroenterol. 2006;101(8):1824–33. https://doi.org/10.1111/j.1572-0241.2006.00724.x.
Felson DT, Parkes MJ, Marjanovic EJ, et al. Bone marrow lesions in knee osteoarthritis change in 6–12 weeks. Osteoarthritis Cartilage. 2012;20(12):1514–8. https://doi.org/10.1016/j.joca.2012.08.020.
Ebbinghaus M, von Segond BG, Massier J, et al. Interleukin-6-dependent influence of nociceptive sensory neurons on antigen-induced arthritis. Arthritis Res Ther. 2015;17:334. https://doi.org/10.1186/s13075-015-0858-0.
Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: the cytokine connection. Cytokine. 2014;70(2):185–93. https://doi.org/10.1016/j.cyto.2014.06.019.
Giordano R, Petersen KK, Andersen HH, Simonsen O, Arendt-Nielsen L. Serum inflammatory markers in patients with knee osteoarthritis: a proteomic approach. Clin J Pain. 2020;36(4):229–37. https://doi.org/10.1097/AJP.0000000000000804.
Cuellar JM, Scuderi GJ, Cuellar VG, Golish SR, Yeomans DC. Diagnostic utility of cytokine biomarkers in the evaluation of acute knee pain. J Bone Jt Surg Am. 2009;91(10):2313–20. https://doi.org/10.2106/JBJS.H.00835.
Gandhi R, Santone D, Takahashi M, Dessouki O, Mahomed NN. Inflammatory predictors of ongoing pain 2 years following knee replacement surgery. Knee. 2013;20(5):316–8. https://doi.org/10.1016/j.knee.2012.10.015.
Collins JE, Katz JN, Dervan EE, Losina E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2014;22(5):622–30. https://doi.org/10.1016/j.joca.2014.03.009.
Nicholls E, Thomas E, van der Windt DA, Croft PR, Peat G. Pain trajectory groups in persons with, or at high risk of, knee osteoarthritis: findings from the Knee Clinical Assessment Study and the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2014;22(12):2041–50. https://doi.org/10.1016/j.joca.2014.09.026.
Dai Z, Lu N, Niu J, Felson DT, Zhang Y. Dietary fiber intake in relation to knee pain trajectory. Arthritis Care Res (Hoboken). 2017;69(9):1331–9. https://doi.org/10.1002/acr.23158.
Wesseling J, Bastick AN, ten Wolde S, et al. Identifying trajectories of pain severity in early symptomatic knee osteoarthritis: a 5-year followup of the Cohort Hip and Cohort Knee (CHECK) Study. J Rheumatol. 2015;42(8):1470–7. https://doi.org/10.3899/jrheum.141036.
Richette P, Latourte A, Sellam J, et al. Efficacy of tocilizumab in patients with hand osteoarthritis: double blind, randomised, placebo-controlled, multicentre trial. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-218547.
Schaible HG, von Banchet GS, Boettger MK, et al. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci. 2010;1193:60–9. https://doi.org/10.1111/j.1749-6632.2009.05301.x.
Boettger MK, Leuchtweis J, Kummel D, Gajda M, Brauer R, Schaible HG. Differential effects of locally and systemically administered soluble glycoprotein 130 on pain and inflammation in experimental arthritis. Arthritis Res Ther. 2010;12(4):R140. https://doi.org/10.1186/ar3079.
Cook AD, Christensen AD, Tewari D, McMahon SB, Hamilton JA. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 2018;39(3):240–55. https://doi.org/10.1016/j.it.2017.12.003.
Aitken D, Laslett LL, Pan F, et al. A randomised double-blind placebo-controlled crossover trial of Humira (adalimumab) for erosive hand osteoarthritis: the HUMOR trial. Osteoarthritis Cartilage. 2018;26(7):880–7. https://doi.org/10.1016/j.joca.2018.02.899.
Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–12. https://doi.org/10.1172/JCI18921.
Acknowledgements
Funding
This study was supported by the National Health and Medical Research Council (NHMRC) project grant of Australia (302204). FP is funded by the NHMRC Early Career Fellowship; JT is funded by National Heart Foundation Fellowship; FC is funded by NHMRC Leadership Fellowship; GJ is funded by NHMRC Practitioner Fellowship. No funding or sponsorship was received for publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
FP participated in the conceptualisation, contributed to the data analysis and interpretation of results and drafted the manuscript; JT contributed to the data analysis, interpretation of results and revising the manuscript; FC contributed to the TASOAC study design, data collection and interpretation of results; GJ contributed to the TASOAC study design, data collection, interpretation of results and revising the manuscript. All authors have read and approved the final version of the manuscript.
Disclosures
Feng Pan, Jing Tian, Flavia Cicuttini, Graeme Jones have nothing to disclose.
Compliance with ethics guidelines
This study was approved by the Southern Tasmanian Health and Medical Human Research Ethics Committee (Ref. no: H0006488), and written informed consent of all participants was obtained. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pan, F., Tian, J., Cicuttini, F. et al. Prospective Association Between Inflammatory Markers and Knee Cartilage Volume Loss and Pain Trajectory. Pain Ther 11, 107–119 (2022). https://doi.org/10.1007/s40122-021-00341-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-021-00341-1