Abstract
Introduction
Respiratory syncytial virus (RSV) leads to significant morbidity in newborn infants in the United Kingdom (UK). Nirsevimab, a long-acting monoclonal antibody, received approval from the European Medicines Agency and has been licensed by the Medicines and Healthcare products Regulatory Agency for preventing RSV lower respiratory tract disease (LRTD) in neonates and infants during their first RSV season. The objective of this study was to assess the potential impact of nirsevimab on RSV-associated LRTDs, related costs, and loss of quality-adjusted life years (QALYs) in infants experiencing their first RSV season.
Methods
The impact of administering nirsevimab across all infant populations compared to palivizumab in the high-risk palivizumab-eligible population was assessed via a static decision-analytic model specified for a UK birth cohort experiencing their first RSV season. The RSV-related health events of interest included primary care (PC), accident and emergency (A&E) visits, hospitalizations [including hospitalizations alone and those resulting in intensive care unit (ICU) admissions], recurrent wheezing in infants who were previously hospitalized, and all-cause LRTD hospitalizations.
Results
Under the current standard of practice (SoP), RSV was estimated to result in 329,425 RSV LRTDs annually, including 24,381 hospitalizations and ICU admissions, representing £117.8 million (2024 GBP) in costs. Comparatively, universal immunization of all infants with nirsevimab could avoid 198,886 RSV LRTDs, including 16,657 hospitalizations and ICU admissions, resulting in savings of £77.2 million in RSV treatment costs. Considering the impact on all-cause LRTD of a universal immunization strategy, nirsevimab could be valued between £243 and £274, assuming willingness-to-pay (WTP) thresholds of £20,000 and £30,000 per QALY saved, respectively.
Conclusions
This analysis demonstrated that the health and economic burden of RSV would be substantially reduced in all infants experiencing their first RSV season in the UK (including term, preterm, and palivizumab-eligible infants) as a result of a universal immunization strategy with nirsevimab.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Why carry out this study? |
Respiratory syncytial virus (RSV) is a leading cause of infant hospitalizations in the United Kingdom (UK) responsible for a substantial economic burden on the country’s resources. |
Current UK guidelines recommend prophylaxis against RSV within the high-risk population only. This leaves a large unmet need for protection against RSV for infants born full-term or late preterm. |
This study assessed the clinical and economic impact of all infant immunization with nirsevimab which was approved by the UK Medicines and Healthcare products Regulatory Agency in November 2022. |
What was learned from the study? |
Universal immunization with nirsevimab could substantially reduce the health and economic burden for UK infants during their first RSV season versus the current standard of practice. |
Implementation of this strategy would benefit all infants, including those at higher risk of serious disease and could play a significant role in reducing the strain of RSV LRTD on the NHS. |
Introduction
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract disease (LRTD) and hospitalizations among infants and young children [1, 2]. In 2015, worldwide RSV infection was associated with approximately 33.1 million hospital admissions and 5600 deaths among children under 5 years old [1]. In the United Kingdom (UK), an estimated 50–90% of pediatric bronchiolitis hospitalizations, and 5–40% of pediatric pneumonia hospitalizations, are caused by RSV [3]. Furthermore, RSV is expected to account for 83 deaths in children and adolescents each year [4].
RSV is a seasonal virus in the UK with peak circulation typically occurring between October and March and peak infections occurring in November and December. By 2 years of age, nearly all children are infected with RSV [2, 5, 6]. While most cases are mild, preterm birth and underlying medical conditions are associated with a higher risk of developing severe disease. Nonetheless, 85% of infants hospitalized due to RSV are otherwise healthy and born at full term [4, 7]. A previously published model predicted that 20,359 infants under 1 year and 33,561 children under 5 years are hospitalized in England during a typical RSV season [8]. During the seasonal peak of infections, hospital services are severely disrupted, placing significant burden on the National Health Service (NHS) [9].
Palivizumab is a monoclonal antibody approved by the United States Food and Drug Administration and the European Medicines Agency (EMA) for the prevention of serious RSV-associated LRTD requiring hospitalization in high-risk infants [10, 11]. Until September 2023, the UK Joint Committee for Vaccination and Immunization (JCVI) recommended using palivizumab in a smaller cohort of infants compared to the full EMA indication based on specific combinations of gestational age and the presence of chronic lung disease (CLD) or congenital heart disease (CHD) [10, 12]. These recommendations limit the use of palivizumab to < 1% of the UK infant population, rendering the majority unprotected.
Nirsevimab is a long-acting anti-RSV monoclonal antibody approved by the EMA [13] and the Medicines and Healthcare products Regulatory Agency [14]. The approval of nirsevimab was based on three pivotal clinical trials, in which a single dose of nirsevimab demonstrated high and consistent efficacy against RSV LRTD sustained through the entire RSV season.
In September 2023, the JCVI stated that a seasonal, seasonal-with-catch-up, or year-round passive (monoclonal antibody) RSV immunization program for newborns could be cost-effective. The JCVI advised that nirsevimab is suitable for a universal program to protect neonates and infants from RSV [15].
Previous modeling studies have examined the impact of prophylactic measures on RSV burden [16,17,18,19,20]. However, no studies have directly compared standard of practice (SoP) with nirsevimab in the UK, utilizing current clinical data [19, 20].
The objective of this study was to highlight the expected clinical and economic burden of nirsevimab versus palivizumab in RSV-associated LRTD prevention in UK infants, based on the latest clinical evidence for nirsevimab.
Methods
Model Overview
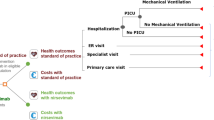
A static decision-analytic model that tracks the UK infant cohort (by month of birth) during their first RSV season was developed in Microsoft Excel®. The model considers possible RSV-related health events and costs in all infants under 1 year of age. All infants in the model were considered susceptible to RSV LRTD, though risk evolved with age, depending on the RSV circulation density and relative risk of health events based on gestational age. A full model description has previously been published [21]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Infants enter the model in monthly birth cohorts by subpopulation and are tracked across a specified time horizon, based on the number of RSV seasons or years. The efficacy, coverage rate, and duration of protection associated with each prophylaxis are combined with the associated risk reductions of RSV-related health events during the protection window and compared across two scenarios. The model considers the UK RSV season to span from October to February, as defined in the Green Book [2], with a peak in infections in December based on data collected between 2012 and 2016 (Figure S1) [2].
This study compared the expected impact of nirsevimab versus previous SoP (i.e., palivizumab) from the NHS payer perspective for a typical RSV season observed prior to the COVID-19 pandemic. Disease burden was calculated as the number and associated costs of hospitalizations [including intensive care unit (ICU) admissions], accident and emergency (A&E) visits, primary care (PC) visits, non-medically attended (MA) RSV events, all-cause LRTD hospitalizations, recurrent wheezing as a complication associated with inpatient RSV events, and the number of deaths among infants with RSV. Costs are reported in 2024 GBP. The time horizon was the first RSV season for all health events, except for recurrent wheezing and premature deaths, which had 3-year and lifetime horizons, respectively. Quality-adjusted life years (QALY) were estimated to calculate the economically justifiable price of each comparator given the UK willingness-to-pay threshold.
Target Population and Immunization Strategies
The cohort is stratified by RSV-related LRTD risk: (1) late preterm and term infants [born at or after 35 weeks gestational age (wGA)]; (2) preterm infants (born between 29 wGA and < 35 wGA, not eligible for palivizumab); and (3) palivizumab-eligible infants (born < 29 wGA or those with CLD or CHD, based on JCVI recommendations) [12], aligned with the nirsevimab clinical trials [22,23,24].
The model compared two immunization strategies: Strategy 1 follows the previous SoP for each subpopulation, consisting of a “catch-up” or seasonal-based approach in the palivizumab-eligible population, in which infants born before the RSV season received up to five monthly palivizumab administrations throughout the RSV season. Infants born during the season received monthly administration from birth until the end of the RSV season. Preterm and term infants not eligible for palivizumab received no prophylaxis, in line with previous JCVI guidelines [12]. In Strategy 2, immunization with nirsevimab among term infants born before the RSV season coincided with the UK National Immunization Program (NIP). For infants born before the RSV season, a single dose of nirsevimab was administered during their regular NIP immunization appointment (at weeks 8, 12, or 16 after birth) closest to but no later than the beginning of the RSV season. Infants born during the RSV season received administration at birth. For preterm and palivizumab-eligible infants, nirsevimab was administered following a “catch-up” strategy similar to the SoP described in Strategy 1: infants born before the RSV season received one dose of nirsevimab in October, and at birth for those born during the RSV season. The duration of protection for nirsevimab was 5 months, based on the European Union and UK marketing authorization [13, 25], with a conservative assumption of no residual efficacy beyond that. Both prophylaxes assumed an immediate and full onset of protection after dosing. A model overview is presented in Figure S2.
Model Inputs
An evidence-based decision analysis was performed to systematically combine UK-specific empirical data concerning epidemiology, prophylaxis efficacy, and number of health events, based on unit costs from national databases, with published literature to inform the model. Model parameters and their associated base-case estimates are listed in Table 1.
Annual births in the UK were sourced from cumulative birth data for England [26], Wales [26], Scotland [27], and Northern Ireland [28]. The demographic inputs regarding the proportion of births per month and the proportion of infants born before 35 wGA were obtained from the UK Office for National Statistics, based on 2019 population characteristics in order to avoid any confounding impacts associated with the COVID-19 pandemic [29, 30]. The proportion of annual births eligible for prophylaxis with palivizumab was informed by IQVIA prescribing cost data (data on file) The seasonality and incidence of RSV were obtained from Public Health England 2017 data of monthly reported laboratory-confirmed respiratory infections that were categorized as RSV in England and Wales [31].
The efficacy of nirsevimab against RSV-related health events, in term and preterm infants not eligible for palivizumab, was based on Phase IIb, Phase III (MELODY) and Phase IIIb (HARMONIE) trials [22, 23, 32]. In the global randomized placebo-controlled MELODY trial, nirsevimab significantly reduced RSV-associated LRTD compared to placebo, with a 74.5% reduction in MA RSV-associated LRTD [95% confidence interval (CI) 49.6–87.1; P < 0.001] among late preterm and term infants not eligible for palivizumab [23]. Similarly, in a population of healthy infants born preterm from the pivotal Phase IIb trial, a single dose of nirsevimab resulted in fewer MA RSV LRTDs and hospitalizations versus placebo throughout the RSV season [22]. Additionally, in both trials, there was no statistically significant difference in the incidence of serious adverse events between infants receiving nirsevimab versus placebo [22, 23]. The open-label HARMONIE trial, conducted in France, Germany, and the UK, compared nirsevimab with standard of care in preventing RSV-related hospitalizations in infants under 12 months not eligible for palivizumab. Nirsevimab significantly reduced RSV-associated LRTD hospitalizations by 83.2% overall (n = 8058; 95% CI 67.8–92.0; P < 0.001) and 83.4% in the UK (n = 4092; 95% CI 34.3–97.6; P = 0.003). Nirsevimab also demonstrated a significant reduction in all-cause LRTD hospitalizations by 58.0% (95% CI 39.7, 71.2; P < 0.0001) [32]. Finally, in the randomized, double-blind MEDLEY trial (Phase II/III) the safety profiles of nirsevimab and palivizumab were similar in infants eligible for palivizumab [24].
Similar to previous economic evaluations of nirsevimab [33], we applied the RSV LRTD data from the MELODY and Phase IIb trials in the corresponding subpopulation for the prevention of outpatient events (i.e., A&E and PC visits). RSV-related hospitalization and all-cause LRTD hospitalization data from HARMONIE were used for the preterm and term infant subpopulations not eligible for palivizumab. Finally, as pharmacokinetic data show a similar degree of efficacy extrapolated to infants born extremely preterm, with CLD/CHD, we have assumed the non-inferiority of nirsevimab versus palivizumab for this subpopulation (given the absence of efficacy data for this subgroup in MEDLEY) [24]. The efficacy for palivizumab was informed by a network meta-analysis of three randomized controlled trials assessing the prevention of hospitalization associated with palivizumab versus placebo (assumed equal across inpatient and outpatient settings) [34]. As the anticipated administration schedule for nirsevimab was based on the NIP, its uptake was assumed to be similar to the primary series vaccinations in infants under 5 years old in the UK COVER program (i.e., 91%) [35]. As palivizumab requires monthly administrations during the RSV season (for a maximum of 5 months), the coverage rate of palivizumab within SoP was informed to reflect the sales in UK annually (i.e., 70%) [Data on File].
Scenario and Sensitivity Analysis
Alternative scenarios were tested based on implementation strategies, considering immunization at birth for all infants (“year-round” scenario) and immunization for infants born during the season only (“in-season” scenario). An extended duration of protection was applied in the “year-round” scenario, assuming sustained protection over 5 months, and a linear decay of efficacy from month 6 through month 12. Although the trajectory for the decline is yet to be determined, there is a building evidence base suggesting protection may extend beyond 150 days [23, 36]. We also tested the assumptions proposed by Hodgson et al. [20] (Table S1). The different immunization strategies are presented in Figure S3. To further test uncertainty in the model results, we conducted a deterministic sensitivity analysis (DSA) by varying key model parameters to test their impact on healthcare costs. The upper and lower bounds for each tested parameter are presented in Table 1. It is important to note that variability in the tested parameters were based on an assumed deviation of 20%, except for treatment efficacy which was extracted from the respective clinical trial results. The assumed deviation of 20% is expected to provide a reasonable estimate of the variability that could be observed in a real-world scenario.
Results
Results are reported for Strategy 1 (SoP) and Strategy 2 (NIP), as well as prevented events, costs avoided and QALY saved with nirsevimab (Table 2). Outcomes are also reported by subpopulation in Table S2 (events), Table S3 (costs), and Table S4 (QALYs). Distribution of health events by month of birth is reported in Table S5 (all events) and Table S6 (RSV-related hospitalizations).
Disease Burden under the Current SoP
Under the current SoP, the model estimated a total of 23,234 hospitalizations (excluding ICU admissions) and 1147 ICU admissions. Of those hospitalized, there were an estimated 18,286 cases of recurrent wheezing over the first 3 years. RSV infections led to a total of 64,575 A&E visits, 159,371 PC visits and 62,793 non-MA RSV events. The estimated number of all-cause LRTD hospitalizations (excluding RSV-related hospitalizations) was 45,940 (Table 2).
The RSV-related cost for all subpopulations was £117.8 million. The largest share is attributable to hospitalizations alone (58% of the total costs), while ICU costs account for approximately 9% of the total costs. The cost of all-cause LRTD hospitalizations (excluding RSV hospitalizations) was £134.2 million. The largest contributor of QALY loss was PC visits, which represented 28% of the 3,557 total QALY loss.
Term infants accounted for 92% and 83% of total hospitalizations (excluding ICU admissions) and ICU admissions, respectively, whereas palivizumab-eligible infants accounted for only 2% and 3.5%, respectively.
The analysis of the RSV-related health event distribution by month of birth showed that 68% was attributable to infants born between March to September (Table S5, Figure S4). The distribution of RSV hospitalizations by month of birth, shows that infants born out of season account for 58%, and infants born in-season account for 42% (Table S6 and Fig. 1).
Distribution of RSV-related hospitalization per month of birth over the first RSV season and avoided events with nirsevimab under the NIP immunization strategy. Infants born from March through September, considered born before the start of their first RSV season (OoS); infants born from October through February, considered born during the RSV season (WiS). mo month old, OoS out of season, RSV respiratory syncytial virus, SoP standard of practice, WiS within season
Disease Burden under All-Infant Immunization with Nirsevimab
Immunization with nirsevimab was estimated to avoid 162,376 RSV MA LRTDs and 36,511 RSV non-MA LRTDs. Moreover, nirsevimab reduced RSV hospital and ICU admissions by 68% (16,657) and avoided substantial numbers of A&E and PC visits (Table 2). Additionally, 9736 all-cause LRTD hospitalizations (excluding RSV hospitalizations) were avoided.
The use of nirsevimab was estimated to reduce total costs associated with RSV MA LARTDs by £77.2 million (66%). Reduced hospital admissions (including ICU admission) accounted for the largest portion of avoided costs (£53.7 million, 68% reduction). Reduced all-cause LRTD events saved an additional £28.4 million. The replacement of palivizumab by nirsevimab in the eligible population resulted in £11 million in savings. Nirsevimab was estimated to conserve 2023 QALYs compared to SoP. As most RSV LRTDs occurred among term infants, 93% of events avoided by an all-infant immunization strategy with nirsevimab were in this group.
The modeled impact of universal immunization with nirsevimab on infants born during versus before the RSV season was significant, regardless of the age at the start of the RSV season (Table S4, Fig. 1). An estimated 64% of the total avoided health burden (134,329 RSV LRTD and all-cause LRTD hospitalizations) was attributable to infants born before the RSV season, while infants born in-season accounted for the remaining 36% (74,293 RSV LRTDs).
Economically Justifiable Price and Number Needed to Immunize (NNI)
The economically justifiable price of nirsevimab (including administration costs) was estimated at £243 and £274 following an incremental cost-effectiveness ratio threshold of £20,000/QALY saved and £30,000/QALY saved, respectively. The estimated NNI to prevent one RSV LRTD in any setting was 4 in the overall infant population, and 39 to prevent one RSV LRTD hospitalization.
Scenario and Sensitivity Analysis
Avoided RSV events under the base-case strategy (NIP), the year-round strategy, and the in-season-only implementation are similar for term infants born during the season (Fig. 2). In term infants born before the start of the RSV, year-round immunization prevents more than 100,000 cases. Results for in-season-only implementation highlight the missed opportunity of protecting these infants. The impact on high-risk infants (preterm and palivizumab-eligible) and the prevention of all-cause LRTD hospitalizations follow the same pattern, but on a smaller scale (Table 3).
Avoided events based on implementation strategies. NIP immunization strategy refers to an all-infant immunization with a catch-up for infants born before the RSV season fitting to routine visits already scheduled; Year-round immunization strategy refers to immunization at birth for preterm and term infants; In-Season immunization strategy refers to immunization of preterm and term infants born during the season only, from October to March. All-cause LRDT hospitalizations exclude RSV-related hospitalization, accounted in events for term, preterm, and high-risk infants. High-risk infants refer to palivizumab-eligible infants; Prevented events sum the four first categories. exc. excluding LRTD lower respiratory tract disease, NIP National Immunization Program, RSV respiratory syncytial disease
The results of the DSA are presented as a tornado diagram in Fig. 3. The analysis showed that parameters related to the term infant population had the largest impact on model results. Specifically, the risk of RSV by age, coverage rate for nirsevimab, and RSV treatment costs were the most significant drivers of healthcare costs. These results align with observations from the scenario and base-case analysis, which highlight a significant burden and unmet need for infants in this subgroup.
Deterministic sensitivity analysis results. It is important to note that variability in the tested parameters were based on an assumed deviation of 20%, except for treatment efficacy which was extracted from the respective clinical trial results. The assumed deviation of 20% is expected to provide a reasonable estimate of the variability that could be observed in a real-world scenario. DSA deterministic sensitivity analysis, RSV respiratory syncytial disease
Discussion
This study adds to the recent literature on the characterization of RSV burden, with the most up-to-date clinical data. It estimates the impact of universal infant immunization with nirsevimab on RSV-related health outcomes, all-cause LRTD hospitalizations, expenditures and QALY loss, based on a previously published static decision-analytic model adapted for the UK setting [19, 20]. The model predicts that infants currently ineligible for palivizumab (term and preterm) account for 98% of the hospitalization burden, despite having a lower risk of severe events compared to those infants eligible to receive palivizumab. These results demonstrate that immunization of otherwise healthy infants born at or close to term (approximately 96% of cohort) can avoid the majority of poor health outcomes and expenditures associated with RSV, despite premature infants with CHD or CLD being at greater risk of serious outcomes.
The results show that immunizing all infants with nirsevimab could reduce RSV-related LRTDs by 60% and associated costs by 66%. The estimated reduction in all-cause LRTD hospitalizations (excluding RSV events) was 21%; this additional benefit may be key in relieving pressure on the health system during winter. Immunization of infants with nirsevimab born before the RSV season can avoid 134,329 total RSV LRTDs (64%). For infants born during the RSV season, immunization with nirsevimab is estimated to avoid 74,293 RSV LRTDs (36%). This distribution of RSV LRTDs occurring in infants born during the RSV season versus those born before the RSV season aligns with previous estimates [8, 37, 38]. Infants born before the RSV season make up the majority of the annual birth cohort (i.e., 7 months of the year; 60%) [29, 30], explaining the higher absolute number of health events and costs versus those born during the season. Similarly, as the majority of the infant population (96%) is born full-term, most health events occur among the term Infant population, born both during and before of the RSV season. This highlights the need to immunize all infants to reduce the overall RSV burden, including those born full-term and those born before the RSV season. The sensitivity analysis lends additional credibility to these results which show that parameters related to term infants have the strongest impact on the model results.
RSV seasonality informs a critical component of any immunization strategy. A minimum 5-month duration of protection of nirsevimab suggests that the optimal immunization timing for infants born before the RSV season is October, thus providing protection throughout the RSV season. This reinforces health equity by offering optimal coverage for infants born before the season, while also protecting the youngest and most vulnerable infants born during the season. However, immunizing a substantial portion of the infant population in October (approximately 62% of the UK’s annual infant birth cohort) could be logistically difficult and reduce real-world coverage. Therefore, the strategy evaluated in our study aligns immunization with nirsevimab in the term infant population with the UK NIP. The difference between the October catch-up and the NIP strategy is marginal in terms of reduction in hospitalizations and direct costs for the entire infant population, considering most infants are born at term (Table S7).
By integrating nirsevimab prophylaxis within the UK NIP, the health system burden of immunization for infants born pre-season is spread out across July, August, and September, instead of concentrating all immunizations in October, thereby maximizing coverage, particularly in infants less vulnerable to severe RSV-related health events. If the 150-day nirsevimab duration of protection is strictly assumed, then integrating nirsevimab within the UK NIP could leave a small portion of older infants with limited protection toward the end of the season when RSV is still in circulation. However, these infants will be less vulnerable to severe RSV-related MA LRTDs as they are older in age; plus, there is evidence that some infants may experience residual protection beyond 150 days.
Another option considered by the JCVI involves immunizing all infants at birth [15]. Existing and expected data suggesting prolonged protection with nirsevimab[23] could render this strategy pragmatic. Sufficient evidence to properly model this duration and waning of protection is not fully available at this time as a strong correlation between protection with respect to serum concentration has not yet been established. A scenario in which all infants receive administration with nirsevimab at birth was tested under the assumption of a linear decay in protection between months 6 and 12. The outcomes of this strategy were similar to the NIP strategy. A final scenario tested was the immunization of the infants born during the season only. This strategy would result in most infants experiencing their first RSV season unprotected, with only a third of health events avoided compared to all-infant protection.
The primary strength of our model is its granularity compared to existing studies in infant RSV [17, 20, 39]. In addition to considering differentiated risks of RSV-related health events by infant subpopulation, the use of hospitalization rates by wGA (from [8]) allows for stratification of subpopulations by monthly age. Our model can therefore evaluate the potential impact of nirsevimab independently for infants within each subpopulation, in addition to those born during versus before the RSV season. Disaggregating the population granularly allows multiple strategies to be evaluated and trade-offs assessed, weighing up pragmatism versus outcomes to determine the most impactful intervention and inform the decision-making process. Another strength of our model is that the analysis is based on the most up-to-date data for the risk of hospitalization due to RSV among infants in the UK, collected shortly before the COVID-19 pandemic and so not confounded by the influence of social distancing on circulating respiratory viruses [40, 41].
The results of our evaluation can be compared with recent studies assessing the impact of immunization on RSV LRTDs [16, 17, 20, 42]. The most recent Hodgson study uses a dynamic transmission model, leveraging an adapted version of the susceptible-exposed-symptomatic-recovered model structure which includes an asymptomatic state as well as the potential effects of maternal-protective antibodies [20]. The Hodgson study estimates fewer avoided RSV LRTDs associated with nirsevimab prophylaxis compared to the results of this analysis. While differences in model structures between the Hodgson and current study can explain some of the differences in results, the primary driver of the discrepancy is related to several other dimensions. First, Hodgson et al. assumed sterilizing immunity induced by nirsevimab, causing an increase of susceptible infants over the second RSV season, and therefore an increase in the number of cases in the 1–4 years age group. This assumption on the mechanism of action of nirsevimab is incorrect, as RSV exposure in nirsevimab-immunized infants is accompanied by subclinical manifestations of disease, indicating that sterilizing immunity is not induced by nirsevimab [43]. This is backed by new data showing no increase in severity in the second season of nirsevimab-immunized infants [44]. The Hodgson model also underestimated key parameters like RSV hospitalization costs, where it is assumed that RSV-related hospital costs are the same across all age groups from 0 to 15 years, and that this is equivalent to the cost of hospitalized acute bronchiolitis in all individuals aged < 18 years. Given RSV disease severity is known to be greatest in infants aged under 1 year and decreases with age, Hodgson’s approach likely underestimates the cost of hospitalization in infants aged under 1 year. Furthermore, QALY loss inputs used in the Hodgson 2024 model are outdated and not specific to the target age group for immunization. Our model utilizes more recent, relevant and comprehensive QALY loss data from Mao et al. [45], a prospective observational cohort study conducted in the UK, Spain, Finland, and the Netherlands, which estimates QALY losses in infants aged under 1 year with a confirmed RSV case during the 2017–18, 2018–19 or 2019–20 season. Hodgson’s model also failed to account for the proven benefits of nirsevimab in reducing all-cause LRTD hospitalizations [22, 23, 32], and the direct impact on infants of replacing palivizumab with nirsevimab [24]. Finally, substantial evidence indicates nirsevimab will have greater real-world impact than maternal vaccination, through the protection of all infants, regardless of gestational age, with sustained efficacy over the RSV season and timely administration to allow protection throughout the RSV season. Early real-world evidence shows robust and consistent outcomes with a pooled effectiveness in preventing RSV LRTDs of approximately 84.4% (95% CI 76.8–90.0) [46]. Using Hodgson’s assumptions, our model results in 4178 fewer hospitalizations avoided, £69 million fewer direct costs avoided, and 1678 fewer QALY saved versus SoP, suggesting significant underestimation of the impact of nirsevimab in reducing RSV-related burden compared to the results presented here.
Furthermore, a formal model comparison was conducted to ensure the cross-validity of our model in accordance with guidelines for multi-model comparisons executed by the Respiratory Syncytial Virus Consortium in Europe (RESCEU) [47]. This study aimed to compare the outcomes of different available, model-based, analytical approaches designed to estimate the cost-effectiveness of RSV prevention in infancy and pregnancy, using a standardized set of input parameters across three static models—one produced by researchers at Antwerp University, the Novavax model, and the Sanofi-generated model presented here. Our model produced identical results to those generated with the same inputs in the Antwerp University model, for the overall infant population and per age group, supporting the validity of the results presented here.
Limitations
Although a key strength of our model centers around the level of granularity available in modeling infant birth cohorts, one key limitation in this analysis is the lack of some granular source data. In these situations, assumptions were applied to fit the available data. For example, the risk of A&E visits was assumed to be consistent across all subpopulations. Estimates for hospitalizations are also unavailable by month of age; therefore, the monthly trend for the overall infant population from Reeves and colleagues [8] is assumed to be applicable to all subpopulations.
Similarly, data on the route into admission of infants who are hospitalized is largely unavailable, particularly with respect to infants who are first admitted to A&E. As the only available source to inform A&E visits presents incidence rates only for infants who do not go on to be hospitalized [48], this analysis treats these health events as mutually exclusive and therefore cannot capture any impact the potential reduction in A&E visits has on the number of hospitalizations for this population of infants. For the same reason, the model also does not include NHS contacts which do not lead to hospitalization.
Assumptions for wheezing similar to those of a recent cost-effectiveness analysis on RSV prevention in the EU [49] are applied in our base case; an estimated avoided 12,493 cases, £3.5 million in direct costs and 476 QALYs saved were associated with wheezing over 3 years as a result of universal nirsevimab immunization. However, a key assumption in the model is the indirect impact of nirsevimab on the incidence of wheezing as a result of prevented hospitalizations. While prevention efficacy against RSV disease is proven, prevention efficacy against sequelae was not assessed in clinical trials and remains a point of conjecture.
Finally, the model adopts a static structure from a payer perspective, meaning potential indirect effects on the transmission of RSV are not captured. While no evidence shows an impact of nirsevimab on infection susceptibility, the potential effects of RSV antibodies on viral shedding, and therefore transmission, should be explored in order to further characterize the indirect effects of nirsevimab [50]. This analysis does not consider the lifetime lost earnings due to an infant RSV death or any other societal impact. This narrow, direct NHS-focused perspective may not fully capture the overall benefits of immunization; the impact of introducing nirsevimab from a societal perspective should be considered by decision-makers in particular.
Conclusion
An all-infant immunization strategy with nirsevimab could considerably alleviate the health and economic implications of RSV infection for infants entering their first RSV season in the UK, compared to existing SoP. This reduction is driven by term infants, who account for most of the RSV LRTD burden; however, this immunization strategy would benefit all infants, including those considered eligible for palivizumab and preterm infants, who suffer disproportionately from higher rates of serious disease. The implementation of such a strategy could play a significant role in reducing the strain of RSV MA LRTD on the NHS during winter months when hospital capacity is most stretched.
Data Availability
All data generated or analyzed during this study are included in this published article or as supplementary information files.
References
Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–58.
UK Health Security Agency. Respiratory syncytial virus: the green book. 2015 [cited 2024 Feb 19]. pp. 434–5. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/458469/Green_Book_Chapter_27a_v2_0W.PDF.
Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–28.
Oxford Vaccine Group. Respiratory syncitial virus. 2019. https://vk.ovg.ox.ac.uk/rsv
Lively JY, Curns AT, Weinberg GA, Edwards KM, Staat MA, Prill MM, et al. Respiratory syncytial virus-associated outpatient visits among children younger than 24 months. J Pediatr Infect Dis Soc. 2019;8:284–6.
Glezen W, Taber L, Frank A, Kasel J. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;6:543–6.
Murray J, Bottle A, Sharland M, Modi N, Aylin P, Majeed A, et al. Risk factors for hospital admission with RSV bronchiolitis in England: a population-based birth cohort study. PLoS ONE. 2014;9: e89186.
Reeves RM, Hardelid P, Panagiotopoulos N, Minaji M, Warburton F, Pebody R. Burden of hospital admissions caused by respiratory syncytial virus (RSV) in infants in England: a data linkage modelling study. J Infect. 2019;78:468–75.
Fusco F, Hocking L, Stockwell S, Bonsu M, Marjanovic S, Morris S, et al. The burden of respiratory syncytial virus: understanding impacts on the NHS, society and economy. Rand Health Q. 2022;1:2.
AstraZeneca AB; Södertälje Sweden. SYNAGIS (INN-palivizumab) summary of product characteristics. [cited 2024 Feb 19]. https://www.ema.europa.eu/en/documents/product-information/synagis-epar-product-information_en.pdf
MedImmune LLC; Gaithersburg MD. SYNAGIS (palivizumab) prescribing information. 2014 [cited 2024 Feb 19]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/103770s5185lbl.pdf
JCVI. Joint Committee on Vaccination and Immunisation Statement on immunisation for Respiratory Syncytial Virus. 2010. https://www.nitag-resource.org/sites/default/files/ddf283f66b77b34a6821e8d6f000947b74f6b68b_1.pdf
AstraZeneca AB; Södertälje Sweden. BEYFORTUS (INN-nirsevimab) summary of product characteristics. [cited 2024 Feb 19]. https://www.ema.europa.eu/en/documents/product-information/beyfortus-epar-product-information_en.pdf.
MHRA grants approval of Beyfortus® (nirsevimab) for prevention of RSV disease in infants. businesswire. 2022.
JCVI. Respiratory syncytial virus (RSV) immunisation programme for infants and older adults: JCVI full statement, 11 September 2023. 2023 [cited 2024 Feb 19]. https://www.gov.uk/government/publications/rsv-immunisation-programme-jcvi-advice-7-june-2023/respiratory-syncytial-virus-rsv-immunisation-programme-for-infants-and-older-adults-jcvi-full-statement-11-september-2023
Cromer D, van Hoek AJ, Newall AT, Pollard AJ, Jit M. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: a modelling and cost-effectiveness analysis for England. Lancet Public Health. 2017;2:e367–74.
Getaneh AM, Li X, Mao Z, Johannesen CK, Barbieri E, van Summeren J, et al. Cost-effectiveness of monoclonal antibody and maternal immunization against respiratory syncytial virus (RSV) in infants: evaluation for six European countries. Vaccine. 2023. https://www.sciencedirect.com/science/article/pii/S0264410X23000920
Li X, Willem L, Antillon M, Bilcke J, Jit M, Beutels P. Health and economic burden of respiratory syncytial virus (RSV) disease and the cost-effectiveness of potential interventions against RSV among children under 5 years in 72 Gavi-eligible countries. BMC Med. 2020;18:82.
Hodgson D, Koltai M, Krauer F, Flasche S, Jit M, Atkins KE. Optimal Respiratory Syncytial Virus intervention programmes using Nirsevimab in England and Wales. Vaccine. 2022;40:7151–7.
Hodgson D, Wilkins N, van Leeuwen E, Watson CH, Crofts J, Flasche S, et al. Protecting infants against RSV disease: an impact and cost-effectiveness comparison of long-acting monoclonal antibodies and maternal vaccination. Lancet Region Health Eur. 2024;38:100829.
Kieffer A, Beuvelet M, Sardesai A, Musci R, Milev S, Roiz J, et al. Expected impact of universal immunization with nirsevimab against RSV-related outcomes and costs among all US infants in their first RSV season: a static model. J Infect Dis. 2022;226:S282–92. https://doi.org/10.1093/infdis/jiac216.
Griffin MP, Yuan Y, Takas T, Domachowske JB, Madhi SA, Manzoni P, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383:415–25. https://doi.org/10.1056/NEJMoa1913556.
Hammitt LL, Dagan R, Yuan Y, Cots MB, Bosheva M, Madhi SA, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;10:140–1.
Simoes EAF, Madhi SA, Muller WJ, Atanasova V, Bosheva M, Cabañas F, et al. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: a pooled analysis of randomised controlled trials. Lancet Child Adolesc. Health. 2023. https://www.thelancet.com/journals/lanchi/article/PIIS2352-4642(22)00321-2/fulltext
Businesswire. MHRA grants approval of Beyfortus® (nirsevimab) for prevention of RSV disease in infants. 2022. https://www.businesswire.com/news/home/20221109005601/en/MHRA-Grants-Approval-of-Beyfortus%C2%AE%E2%96%BC-nirsevimab-for-Prevention-of-RSV-Disease-in-Infants
Office for National Statistics. Births in England and Wales: summary tables. Secondary Births in England and Wales: summary tables. [cited 2024 Feb 19]. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/datasets/birthsummarytables.
National Records of Scotland. Vital events—births. Secondary vital events—births. [cited 2024 Feb 19]. https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/vital-events/births
Northern Ireland Statistics and Research Agency. Registrar general annual report 2021 births. Secondary registrar general annual report 2021 births. [cited 2024 Feb 19]. https://www.nisra.gov.uk/publications/registrar-general-annual-report-2021-births
Office for National Statistics. Table 2: live births: month of occurrence, 1938 to 2019. Secondary table 2: live births: month of occurrence, 1938 to 2019 . 2019. [cited 2024 Feb 19]. https://www.ons.gov.uk/file?uri=%2fpeoplepopulationandcommunity%2fbirthsdeathsandmarriages%2flivebirths%2fdatasets%2fbirthcharacteristicsinenglandandwales%2f2019/birthcharacteristics201912112020134444.xls
Office for National Statistics. Table 8: births by gestational age at birth and ethnicity of live births, 2019. Secondary table 8: births by gestational age at birth and ethnicity of live births, 2019 2019.. [cited 2024 Feb 19]. https://www.ons.gov.uk/file?uri=%2fpeoplepopulationandcommunity%2fbirthsdeathsandmarriages%2flivebirths%2fdatasets%2fbirthcharacteristicsinenglandandwales%2f2019/birthcharacteristics201912112020134444.xls
Public Health England. Respiratory infections: laboratory reports 2017. Secondary Respiratory infections: laboratory reports 2017 2018. https://www.gov.uk/government/publications/respiratory-infections-laboratory-reports-2017
Drysdale SB, Cathie K, Flamein F, Knuf M, Collins AM, Hill HC, et al. Nirsevimab for prevention of hospitalizations due to RSV in infants. N Engl J Med. 2023;389:2425–35.
Hutton DW. Economic analysis of nirsevimab in pediatric populations. 2023 [cited 2023 Aug 3]. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-23/RSV-Pediatric-02-Hutton-508.pdf
Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Vrca VB, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev. 2013. https://doi.org/10.1002/14651858.CD006602.pub4.
UK Health Security Agency. Quarterly vaccination coverage statistics for children aged up to 5 years in the UK (COVER programme): July to September 2021. Secondary Quarterly vaccination coverage statistics for children aged up to 5 years in the UK (COVER programme): July to September 2021 2021. [cited 2024 Feb 19]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1065452/hpr2021_COVER.pdf
Sanofi, AstraZeneca. Nirsevimab for the prevention of RSV in all infants. 2022 [cited 2024 Apr 2]. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/02-RSV-Mat-Ped-Felter-508.pdf
Hall CB, Blumkin AK, Staat MA, Schultz AF, Poehling KA, Szilagyi PG, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:10.
Demont C, Petrica N, Bardoulat I, Duret S, Watier L, Chosidow A, et al. Economic and disease burden of RSV-associated hospitalizations in young children in France, from 2010 through 2018. BMC Infect Dis. 2021;21:730. https://doi.org/10.1186/s12879-021-06399-8.
Rainisch G, Adhikari B, Meltzer MI, Langley G. Estimating the impact of multiple immunization products on medically-attended respiratory syncytial virus (RSV) infections in infants. Vaccine. 2020;38:251–7.
Narayan O, Bentley A, Mowbray K, Hermansson M, Pivonka D, Kemadjou EN, et al. Updated cost-effectiveness analysis of palivizumab (synagis) for the prophylaxis of respiratory sync. J Med Econ. 2020. https://doi.org/10.1080/13696998.2020.1836923.
Hospitalisation Rates Associated with Respiratory Syncytial Virus (RSV) Infection in Children ≤24 Months in England, 2015–2019. In: Poster presented at the 42nd annual meeting of the European Society for Paediatric Infectious Diseases (ESPID), Copenhagen, Denmark, May 20–24, 2024.
Hodgson D, Pebody R, Panovska-Griffiths J, Baguelin M, Atkins KE. Evaluating the next generation of RSV intervention strategies: a mathematical modelling study and cost-effectiveness analysis. BMC Med. 2020;18:348. https://doi.org/10.1186/s12916-020-01802-8.
Wilkins D, Yuan Y, Chang Y, Aksyuk AA, Núñez BS, Wählby-Hamrén U, et al. Durability of neutralizing RSV antibodies following nirsevimab administration and elicitation of the natural immune response to RSV infection in infants. Nat Med. 2023;29:1172–9.
Dagan R, Hammitt LL, Nuñez BS, Cots MB, Bosheva M, Madhi SA, et al. Infants receiving a single dose of nirsevimab to prevent RSV do not have evidence of enhanced disease in their second RSV season. J Pediatr Infect Dis Soc. 2024;13:144–7. https://doi.org/10.1093/jpids/piad113.
Mao Z, Li X, Dacosta-Urbieta A, Billard M-N, Wildenbeest J, Korsten K, et al. Economic burden and health-related quality-of-life among infants with respiratory syncytial virus infection: a multi-country prospective cohort study in Europe. Vaccine. 2023. https://doi.org/10.1016/j.vaccine.2023.03.024.
López-Lacort M, Muñoz-Quiles C, Mira-Iglesias A, López-Labrador FX, Mengual-Chuliá B, Fernández-García C, et al. Early estimates of nirsevimab immunoprophylaxis effectiveness against hospital admission for respiratory syncytial virus lower respiratory tract infections in infants, Spain, October 2023 to January 2024. Eurosurveillance. 2024;29:2400046.
Li X, Hodgson D, Flaig J, Kieffer A, Herring WL, Beyhaghi H, et al. Cost-effectiveness of respiratory syncytial virus preventive interventions in children: a model comparison study. Value in Health. 2022. https://www.sciencedirect.com/science/article/pii/S1098301522047465
Ajayi-Obe EK, Coen PG, Handa R, Hawrami K, Aitken C, McIntosh EDG, et al. Influenza A and respiratory syncytial virus hospital burden in young children in East London. Epidemiol Infect. 2008;136:1046–58.
Li X, Bilcke J, Fernández LV, Bont L, Willem L, Wisløff T, et al. Cost-effectiveness of respiratory syncytial virus disease prevention strategies: maternal vaccine versus seasonal or year-round monoclonal antibody program in Norwegian children. J Infect Dis. 2022;226:jiac064.
Voirin N, Virlogeux V, Demont C, Kieffer A. Potential impact of nirsevimab on RSV transmission and medically attended lower respiratory tract illness caused by RSV: a disease transmission model. Infect Dis Ther. 2021. https://doi.org/10.1007/s40121-021-00566-9.
Sanofi. BEYFORTUS prescribing information. 2023. https://products.sanofi.us/beyfortus/beyfortus.pdf
Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–40.
Study TIm-RSTIm-R. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–7.
Thwaites R, Buchan S, Fullarton J, Morris C, Grubb E, Rodgers-Gray B, et al. Clinical burden of severe respiratory syncytial virus infection during the first 2 years of life in children born between 2000 and 2011 in Scotland. Eur J Pediatr. 2020;179:791–9. https://doi.org/10.1007/s00431-019-03564-9.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2004.
Wildenbeest JG, Billard M-N, Zuurbier RP, Korsten K, Langedijk AC, van de Ven PM, et al. The burden of respiratory syncytial virus in healthy term-born infants in Europe: a prospective birth cohort study. Lancet Respir Med. 2022. https://www.sciencedirect.com/science/article/pii/S2213260022004143
Office for National Statistics. Child and infant mortality in England and Wales: 2019. Secondary Child and infant mortality in England and Wales: 2019. [cited 2024 Feb 19]. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/childhoodinfantandperinatalmortalityinenglandandwales/2019
Taylor S, Taylor RJ, Lustig RL, Schuck-Paim C, Haguinet F, Webb DJ, et al. Modelling estimates of the burden of respiratory syncytial virus infection in children in the UK. BMJ Open. 2016;6: e009337.
Office for National Statistics (ONS). CPIH INDEX 06: health 2015=100. 19 June 2014. [cited 2024 Jun 25]. https://www.ons.gov.uk/economy/inflationandpriceindices/timeseries/l528/mm23
National Health Service England. 2021/22 National Cost Collection Data Publication. https://www.england.nhs.uk/publication/2021-22-national-cost-collection-data-publication/
Curtis L, Burns A. Unit costs of health and social care 2020. Canterbury: Personal Social Services Research Unit, University of Kent; 2020.
Personal Social Services Research Unit. Unit costs of health and social care 2023 manual. https://kar.kent.ac.uk/105685/.
Funding
This work was funded by AstraZeneca and Sanofi. The journal’s Rapid Service Fee was funded by AstraZeneca and Sanofi.
Author information
Authors and Affiliations
Contributions
Alexia Kieffer, Matthieu Beuvelet, Aditya Sardesai and Robert Musci were responsible for the conceptualization of the study. Richard Hudson, Mersha Chetty, Gerald Moncayo supervised the study. Alexia Kieffer, Matthieu Beuvelet, Aditya Sardesai, and Robert Musci were responsible for methodology and visualization. Robert Musci wrote the original draft, and Richard Hudson, Gerald Moncayo, Aditya Sardesai, Alexia Kieffer, Matthieu Beuvelet, and Mersha Chetty edited and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Alexia Kieffer, Matthieu Beuvelet, Geraldo Moncayo, Mersha Chetty, and Richard Hudson are employees of Sanofi and may hold shares and/or stock options in the company. Aditya Sardesai and Robert Musci are salaried employees of Evidera, Inc. and are not allowed to accept renumeration from any clients for their services.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior Publication: A full model description has previously been published for the US setting (Kieffer et al. 2022, J Infect Dis 226; https://doi.org/10.1093/infdis/jiac216).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kieffer, A., Beuvelet, M., Moncayo, G. et al. Disease Burden Associated with All Infants in Their First RSV Season in the UK: A Static Model of Universal Immunization with Nirsevimab Against RSV-Related Outcomes. Infect Dis Ther (2024). https://doi.org/10.1007/s40121-024-01037-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40121-024-01037-7