Abstract
Introduction
This study aimed to evaluate the effectiveness of ensitrelvir, an oral antiviral, in reducing hospitalization risk in outpatients at high-risk for severe COVID-19 during the Omicron era.
Methods
This was a retrospective study using a large Japanese health insurance claims database. It included high-risk outpatients for severe symptoms who received their first COVID-19 diagnosis between November 2022 and July 2023. The study included outpatients aged ≥ 18 years. The primary endpoint was all-cause hospitalization during the 4-week period from the date of outpatient diagnosis and medication, comparing the ensitrelvir group (n = 5177) and the no antiviral treatment group (n = 162,133). The risk ratio and risk difference were evaluated after adjusting patient background distribution by the inverse probability of treatment weight (IPTW) method. Secondary endpoints were incidence of respiratory and heart rate monitoring, oxygen therapy, ventilator use, intensive care admission, and all-cause death.
Results
The risk ratio for all-cause hospitalization between the ensitrelvir group (n = 167,385) and the no antiviral treatment group (n = 167,310) after IPTW adjustment was 0.629 [95% confidence interval (CI) 0.420, 0.943]. The risk difference was − 0.291 [95% CI − 0.494, − 0.088]. The incidence of both respiratory and heart rate monitoring and oxygen therapy was lower in the ensitrelvir group. Ventilator use, intensive care admission, and all-cause death were difficult to assess because of the limited events.
Conclusions
The incidence of all-cause hospitalization was significantly lower in the ensitrelvir group than in the no antiviral treatment group, suggesting ensitrelvir is an effective treatment in patients at risk of severe COVID-19.
Plain Language Summary
COVID-19 still poses a risk for patients with serious health conditions and weakened immune systems, who are more likely to develop severe illness. Several studies have indicated that some oral antiviral medications might be effective in preventing severe disease. This study aimed to evaluate if ensitrelvir, an oral antiviral medication, can help prevent hospitalization in outpatients who are at risk of developing severe symptoms from the Omicron variant of the SARS-CoV-2 virus. The hospitalization rates of patients who received ensitrelvir was compared with those who did not receive any antiviral treatment, using medical records from a large health insurance database in Japan focused on outpatients who were at risk of severe symptoms and were diagnosed with COVID-19 between November 2022 and July 2023. Respiratory and heart rate monitoring, oxygen therapy, ventilator use, intensive care admission, and all-cause death were also evaluated. The study found that patients who received ensitrelvir had a lower risk of being hospitalized compared to those who did not receive any antiviral treatment. The ensitrelvir group also had lower rates of respiratory and heart rate monitoring and oxygen therapy. However, it was challenging to assess the effects on ventilator use, intensive care admission, and all-cause death due to the small number of events in the population under evaluation. Based on these findings, ensitrelvir appears to be an effective treatment for reducing the risk of hospitalization in patients at risk of severe COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The Omicron variant of COVID-19 was less severe than previous variants, but hospitalizations were still more frequent compared to seasonal influenza. Therefore, anti-SARS-CoV-2 drugs were considered important for reducing severe disease, particularly in high-risk patients. |
We evaluated the effectiveness of ensitrelvir in reducing the risk of hospitalization from SARS-CoV-2 infection in outpatients at risk of developing severe symptoms during the Omicron era. |
What was learned from the study? |
This retrospective study using a large Japanese health insurance claims database showed that the risk of all-cause hospitalization was significantly lower in the ensitrelvir group than in the no antiviral treatment group. |
Our results suggested that ensitrelvir is an effective treatment in patients at risk of severe COVID-19. |
Introduction
SARS-CoV-2 infection remains a threat for patients with a severe comorbidity burden and immunocompromised patients who are at high risk of progression to severe disease [1]. Several studies have suggested that some oral antiviral drugs may reduce the risk of severe disease. For example, nirmatrelvir achieved a relative reduction of approximately 89% in COVID-19-related hospitalizations and all-cause deaths in the pre-Delta, pre-Omicron variant period of the pandemic [2]. Molnupiravir had lower efficacy, with a relative reduction of approximately 48% [3]. Those clinical trials were conducted early in the COVID-19 pandemic, when most people had not been vaccinated against COVID-19 and were at risk of progression to severe disease. Since the arrival of variants of concern, especially the Omicron variant, mixed results have been reported on the effectiveness of antiviral drugs in reducing the severity of COVID-19 among vaccinated patients. Three real-world studies reported that patients treated with nirmatrelvir had a lower risk of hospitalization (adjusted hazard ratio 0.27–0.54) [4,5,6]. However, a prospective randomized controlled trial in the Omicron period found that molnupiravir did not reduce hospitalization or death among high-risk patients with a history of multiple vaccinations [7]. Another retrospective study showed that molnupiravir was associated with preventing deterioration after hospitalization (odds ratio 0.448 [8], hazard ratio 0.76 [9]).
Ensitrelvir fumaric acid (known as ensitrelvir) is an oral SARS-CoV-2 3CL protease inhibitor developed by Shionogi & Co. Ltd. for the treatment of infection caused by SARS-CoV-2. It showed potent antiviral efficacy against the Delta and Omicron variants in clinical studies [10,11,12]. In the recently reported phase 3 study, SCORPIO-SR, it also demonstrated a significantly shorter time to resolution of COVID-19 symptoms compared with a placebo in patients aged 12–69 years with mild to moderate COVID-19 [10]. Based on these data, and its documented efficacy in an earlier phase 2b study [11], ensitrelvir received emergency approval for use in Japan in November 2022, and standard approval in March 2024 as an oral antiviral against SARS-CoV-2 regardless of the presence of risk factors. The phase 2b and phase 3 SCORPIO-SR studies were conducted when the Omicron variant was prevalent and more than 80% of the target population were vaccinated. Those studies were therefore designed to assess the endpoint of symptom resolution in non-hospitalized patients with mild to moderate disease (independent of vaccination status and risk factors), rather than hospitalization and death in patients at risk of developing severe symptoms. Several case reports also showed the effectiveness of ensitrelvir treatment in the real world [13,14,15,16]. Yamato et al. reported on the use of ensitrelvir in 32 hospitalized patients (average age 73.5 years old), all of whom survived to Day 28 [17]. However, evidence of its ability to reduce severe disease in high-risk patients is limited.
This paper reports a retrospective analysis using a large Japanese health insurance claims database to evaluate the association between ensitrelvir treatment and hospitalization and other severe events, compared with no antiviral treatment in high-risk patients with COVID-19.
Methods
Data Sources
We used anonymized data from the JMDC Claims Database (JMDC Inc., Tokyo, Japan), a claims database with a traceable, cumulative dataset of medical (inpatient and outpatient) Diagnosis Procedure Combinations, and dispensing claims for approximately 17 million people. Individuals could be followed-up within the database even if they used multiple medical institutions of different types (e.g., clinics and hospitals), provided they had insurance.
During the data period, ensitrelvir was used from both Japanese government procurement and general distribution. Government-procured ensitrelvir, not being subject to insurance claims, was recorded differently in claims. Nevertheless, by request to JMDC Inc., data for both types were extracted.
Study Design and Population
This retrospective database cohort study compared the incidence of severe outcomes between patients treated with ensitrelvir (ensitrelvir group) and those who did not receive any anti-SARS-CoV-2 drugs (no antiviral treatment group).
The study included all outpatients aged 18 years or older who had their first diagnosis of COVID-19 (ICD-10 code: U071, U072) on or after 22 November, 2022, when ensitrelvir obtained emergency regulatory approval from the Ministry of Health, Labour and Welfare (MHLW) in Japan, and before 31 July, 2023, and who could be tracked from 6 months before the date of diagnosis (Day 1) to 1 month following that date. Patients with at least one risk factor for severe disease (Table 1) based on COVID-19 guidelines from MHLW [18,19,20] were defined as high-risk patients and were set as the primary analysis target population of this study. They were divided into the ensitrelvir group and no antiviral treatment group based on their use of antiviral drugs on the date of diagnosis. A detailed definition of high-risk patients based on the Japanese COVID-19 treatment guidelines is included in the Supplementary Methods.
This study was conducted according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects. It used anonymized information from an existing database, and informed consent was therefore not required. This study was registered in UMIN Clinical Trials Registry (study ID: UMIN000053217).
Study Outcomes
The study outcome measures were all-cause hospitalization (starting on Days 2–28) as a primary endpoint, and as secondary endpoints, respiratory and heart rate monitoring (starting on Days 2–28), oxygen therapy (starting on Days 2–28), ventilator use (starting on Days 2–28), intensive care unit (ICU) admission (starting on Days 2–28), and all-cause death (within the following month). All-cause death was considered the outcome if there was 1 month of insurance withdrawal due to death or 1 month with a diagnosis outcome of death, and if that month matched the month of Day 1 or the following month.
Statistical Analyses
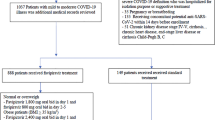
The analysis population included all patients who met the eligibility criteria and did not meet the exclusion criteria (Fig. 1). The comparison groups were the ensitrelvir-treated group (patients who received ensitrelvir on Day 1) and the no antiviral treatment group (patients who did not receive any anti-SARS-CoV-2 virus drugs on Day 1). Comparisons were made between the standardized ensitrelvir group and the standardized no antiviral treatment group. We calculated the risk ratios (RRs) and risk differences (RDs) by dividing the incidence of severe outcomes in the group without antiviral treatment by the incidence in the ensitrelvir group (RR) or subtracting it (RD). The 95% confidence intervals (CIs) for both RRs and RDs were determined after standardizing each group in the comparison pair. Statistical significance was determined when the 95% CIs for RR and RD did not include 1 and 0, respectively (two-sided significance level, 0.05).
In order to standardize the baseline demographic and clinical characteristics of patients between the ensitrelvir group and the no antiviral treatment group, we employed the propensity score and applied the inverse probability of treatment weighting (IPTW) method. The propensity score was calculated through a logistic regression analysis, with the ensitrelvir group set as the response variable (assigned a value of 1 for the ensitrelvir group, and 0 otherwise), and the patient baseline characteristics (covariates) serving as the explanatory variables. The propensity score, which represents the predicted probability (P) of being in the ensitrelvir group for each patient based on the logistic regression analysis, was used to assign weights (i.e., 1/P for the ensitrelvir group and 1/(1−P) for the no antiviral treatment group).
The covariates used in calculating the propensity score were selected based on their relevance to the severity of COVID-19 [18,19,20]. Patient age was categorized as 18–49, 50–64, and ≥ 65 years. Comorbidities were defined using ICD-10 codes, requiring a corresponding medical record within 6 months prior to the month of Day 1, or, if the record was from the month of Day 1, it had to be documented before Day 1. The covariates included age, gender, and the presence or absence of the following conditions (diagnosis was determined by the administration of relevant medication within 6 months before Day 1, excluding Day 1): malignant tumor, chronic respiratory disease, diabetes, chronic kidney disease, hypertensive disease, dyslipidaemia, cardiovascular disease, cerebrovascular disease, morbid obesity, immunosuppressive state, and AIDS/HIV (the lists of ICD-10 codes are provided in the Supplementary Methods). Most of these covariates were found to be associated with all-cause hospitalization by logistic regression performed as post hoc analyses (Table S2).
Standardized mean differences (SMDs) between groups were calculated for each baseline characteristic after adjusting for IPTW. The adjusted Kaplan–Meier estimator with IPTW was used to plot the cumulative incidence of all-cause hospitalization from Day 1 to Day 28. Missing data were not imputed, and no adjustment was made for multiple tests. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 4.1.0.
Similar methods have been presented previously [21].
Results
Patients
From November 2022 to July 2023, during the B.1.1.529 (Omicron) variant dominant period, 167,310 eligible patients were identified in the JMDC claims database. Of those patients, 5177 (3.1%) were treated with ensitrelvir and 162,133 (96.9%) received no antiviral treatment (Fig. 1).
In the high-risk population, characteristics of patients were generally similar between those who received ensitrelvir and no antiviral treatment (Table 2). After IPTW adjustment, the SMDs between the ensitrelvir and no antiviral treatment groups were < 0.1 for all characteristics, indicating that the baseline characteristics were well-balanced after adjustment (Table 3).
Study Outcomes
After IPTW adjustment, the incidence of all-cause hospitalization in the high-risk population was significantly lower in the ensitrelvir group than in the no antiviral treatment group (0.494% vs. 0.785%; RR: 0.629, 95% CI 0.420 to 0.943; RD: − 0.291, 95% CI − 0.494 to − 0.088) (Fig. 2). The number needed to treat to prevent one all-cause hospitalization was 344. Similar results were observed in an analysis of the overall population, including those not at risk of severe disease ( Fig. S1). As a pos hoc analysis, the incidence of all-cause hospitalization for aged ≥ 65 was compared between the ensitrelvir group and the no antiviral treatment group using Fisher’s exact test, and the percentages were 0% (0/380) in the ensitrelvir group and 1.298% (199/15,336) in the no antiviral treatment group, with the p value of 0.0166.
The incidences of respiratory and heart rate monitoring (0.143% vs. 0.269%) and oxygen therapy (0.095% vs. 0.149%) were also lower in the ensitrelvir group than in those receiving no antiviral treatment (Fig. 2), with an RR for respiratory and heart rate monitoring of 0.531 (95% CI 0.261–1.081; RD: − 0.126, 95% CI − 0.230 to − 0.022) and an RR for oxygen therapy of 0.635 (95% CI 0.258–1.563; RD: − 0.055, 95% CI − 0.141 to 0.032). Other events (ventilator use, ICU admission and all-cause death) were difficult to evaluate because of the limited number of unadjusted incidences, only 0–2 cases.
Figure 3 shows the cumulative incidence adjusted by IPTW of all-cause hospitalization for the high-risk population, comparing those treated with ensitrelvir and those who did not receive antiviral treatment up to Day 28. Ensitrelvir suppressed the incidence of all-cause hospitalization, and there was no overlap of the 95% CIs of each group from Day 2 to Day 12. There were very few cases of administration of an antiviral after Day 2 in the no antiviral treatment group (1.468%), and the impact seemed minimal on the comparison results for severe events.
Discussion
In this retrospective database cohort study of patients with COVID-19 at risk of developing severe symptoms, we found that ensitrelvir was associated with a significantly lower risk of hospitalization. The Omicron variant was less severe than previous variants of COVID-19 [22], and the percentage of the population who had been vaccinated and/or previously had COVID-19 had increased. However, severe events including hospitalization were still more frequent than with seasonal influenza [21], and a supplemental analysis revealed that the majority of high-risk factors were found to be associated with the likelihood of hospitalization. Anti-SARS-CoV-2 drugs were therefore considered important to reduce severe disease, especially in high-risk patients. Several studies have shown the effect of ritonavir-boosted nirmatrelvir and molnupiravir on the risk of hospitalization in patients infected with the Omicron variant who were at high risk of developing severe COVID-19 symptoms [4,5,6, 8, 9]. However, this is the first study to show the effect of ensitrelvir in reducing the level of severe disease in high-risk patients using real-world data.
The higher prevalence of COVID-19 vaccination and the described decreasing rate of severe SARS-CoV-2 infections during the Omicron variant predominance make difficult to assess the risk reduction of severe disease in prospective randomized control studies. When studying influenza, symptom alleviation and viral reduction were used as the endpoints in clinical studies of oseltamivir and other antivirals [23], and the risk reduction of severe events was assessed only after sufficient real-world data had been accumulated [24, 25]. This led to a growing consensus within influenza research that early antiviral treatment suppresses viral growth, which in turn suppresses severe events. Similarly, the viral load of SARS-CoV-2 may be associated with the severity of both COVID-19 and the development of severe symptoms [26]. Early antiviral treatment leading to early viral growth suppression may result in fewer severe outcomes. Ensitrelvir showed similar or better antiviral activity across variants of concern than other anti-SARS-CoV-2 drugs in a nonclinical setting [27], and it was expected to show similar efficacy in reduction of severe disease as ritonavir-boosted nirmatrelvir and molnupiravir in clinical studies.
In this study, the incidence of all-cause hospitalization was significantly lower in the ensitrelvir group than in the no antiviral treatment group. Ensitrelvir in particular seemed to suppress the incidence of all-cause hospitalization in the early phase (< Day 7), which could support the assumed correlation between viral load and severity of disease [28] (Fig. 3). Under Japanese insurance claim rules, it is stipulated that the respiratory and heart rate monitoring is considered when there is constant monitoring for patients with severe cardiac or respiratory disfunction or those at risk of it [21, 29], which could reflect emergency department visit.
The risk of respiratory and heart rate monitoring during hospitalization was also significantly lower in the ensitrelvir group than the no antiviral treatment group. Due to the limited number of other secondary events (oxygen therapy, ventilator use, ICU admission, and all-cause death), the statistical significance of their lower number in the ensitrelvir group was not established. However, those secondary endpoints supported the finding of the primary endpoint, and those endpoints with small numbers of events need further investigations.
In a clinical setting, physicians decide whether to prescribe antiviral agents based on the severity of COVID-19, its associated symptoms, days since onset of symptoms, and vaccination status. These data were not included in the study database. This study lacked for adjustment by baseline symptom severity at diagnosis, days from symptom onset to diagnosis, and vaccination status. The COVID-19 medical treatment guidelines in Japan [18,19,20] recommend ensitrelvir for patients with severe COVID-19 symptoms such as fever, sore throat, and cough. This may have meant that COVID-19 was more likely to progress in patients undergoing ensitrelvir treatment. Even with this potential selection bias, ensitrelvir reduced the relative risk of hospitalization by 37.1%.
Patients aged 65 years or older accounted for 2.4% in this study population (Table 2), while patients with aged 70 or older in Japan from January to September 2022, the Omicron dominant period, were about 7% [30]. Benefit packages essentially remain the same regardless of health insurance schemes in Japan. All individuals in the JMDC database have access to the comprehensive equality health coverage; therefore, there are no expected impacts on the socioeconomic factors in the patient population seeking healthcare.
Ensitrelvir reduced the risk of hospitalization in the study population. Ongoing studies such as STRIVE (Strategies and Treatment for Respiratory Infections & Viral Emergencies: Shionogi Protease Inhibitor) [31] and other retrospective studies using more extensive datasets may provide additional evidence.
Limitations
Several limitations arose from using the JMDC health insurance claims database. Firstly, age is considered the most relevant risk factor for severe disease [32], but the proportion of older people (aged ≥ 65 years) in the database was low (approximately 4% [33]), because retired individuals are not members of employer-based health insurance associations. Although only 2.4% of patients over the age of 65 were included in this study, ad hoc analysis demonstrated a risk reduction of hospitalization among those patients who received ensitrelvir. It can be presumed that our findings would remain consistent even if this database was to reflect aging demographics in Japan. Secondary, the database does not include baseline information of patients, particularly regarding the time from diagnosis or the onset of COVID-19 to the start of treatment, the severity of the COVID-19, or vaccination status. In particular, although the vaccination levels in Japan from January to September 2022, the Omicron-dominant period, was estimated to be approximately 80% across all ages and approximately 95% in those aged ≥ 65 years [34], whether and when a patient has been vaccinated may lead to potential bias in their willingness to seek early medical examination or to receive antiviral drugs. Therefore, it was not possible to match the patient background at baseline in either group, and this should be a direction for future research. Thirdly, the primary endpoint was all-cause hospitalization, which may have included hospitalization for reasons other than COVID-19. As the claim data serve for the purpose of medical expense invoicing, the disease names used are those designated for insurance purposes, and the accuracy of the diagnosis remains unknown. Therefore, all-cause hospitalization without subjective interpretations may be a reasonable endpoint. Fourthly, the lack of information on patient compliance and treatment adherence, a common limitation of claims data, may affect the real-world outcome of treatment, but this limitation could affect the ensitrelvir group only by attenuating its effectiveness, thus not altering the conclusion regarding ensitrelvir efficacy. This was a retrospective cohort study, and there may therefore have been other unmeasured confounding factors. Hence, it would be worthwhile to conduct further research aiming to reflect the demographics in Japan, along with comprehensive baseline information, to enhance the scientific validity of study findings.
Conclusions
This real-world database study found that the incidence of hospitalization from all causes was significantly lower in the ensitrelvir group than in those receiving no antiviral treatment. This suggests that ensitrelvir may be an effective treatment option for patients at risk of severe COVID-19.
Data Availability
The data that support the findings of this study are available from JMDC Inc. Restrictions apply on the availability of these data, which were used under license for this study. Data are available from the authors with the permission of JMDC Inc.
References
Manchanda V, Mitra S, Rafique I, et al. Is Omicron really mild?—comparative analysis of comorbidities and disease outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants. Indian J Med Microbiol. 2023;45: 100391.
Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–408.
Jayk Bernal A, da Silva MMG, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–20.
Arbel R, Wolff Sagy Y, Hoshen M, et al. Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge. N Engl J Med. 2022;387(9):790–8.
Shah MM, Joyce B, Plumb ID, et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19–United States, April–September 2022. MMWR Morb Mortal Wkly Rep. 2022;71(48):1531–7.
Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2023;76(3):e342–9.
Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 2023;401(10373):281–93.
Suzuki Y, Shibata Y, Minemura H, et al. Real-world clinical outcomes of treatment with molnupiravir for patients with mild-to-moderate coronavirus disease 2019 during the Omicron variant pandemic. Clin Exp Med. 2023;23(6):2715–23.
Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022;400(10359):1213–22.
Yotsuyanagi H, Ohmagari N, Doi Y, et al. Efficacy and safety of 5-day oral ensitrelvir for patients with mild-to-moderate COVID-19: the SCORPIO-SR randomized clinical trial. JAMA Netw Open. 2024;7(2): e2354991.
Mukae H, Yotsuyanagi H, Ohmagari N, et al. Efficacy and safety of ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study. Clin Infect Dis. 2023;76(8):1403–11.
Mukae H, Yotsuyanagi H, Ohmagari N, et al. A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part. Antimicrob Agents Chemother. 2022;66(10): e0069722.
Sakamaki I, Negoro E, Iwasaki H, Yamauchi T. Ensitrelvir eradicates persistent SARS-CoV-2 infection in a follicular lymphoma patient treated with anti-CD20 antibodies. J Infect Chemother. 2024;30(2):147–9.
Fujimoto A, Shibata K, Miyazawa S, Sonoyama T. Clinical outcomes after administration of ensitrelvir fumaric acid in nonhospitalized patients with COVID-19: a single center, retrospective observational study. BIO Clin. 2023;38(12):1036–42 (in Japanese).
Konishi M. Examination of the pathological condition of outpatient patients with fever during the 8th wave of the new coronavirus infection (COVID-19): evaluation of prognosis and sequelae due to oral antiviral drug administration. J Kyoto Med Assoc. 2023;70(2):32–9 (in Japanese).
Furuya C, Yasuda H, Hiki M, et al. Case report: Ensitrelvir for treatment of persistent COVID-19 in lymphoma patients: a report of two cases. Front Immunol. 2024;15:1287300.
Yamato M, Kinoshita M, Miyazawa S, Seki M, Mizuno T, Sonoyama T. Ensitrelvir in patients with SARS-CoV-2: a retrospective chart review. J Infect Chemother. 2024 (in press).
Ministry of Health, Labour and Welfare. Version 8.1 of the COVID-19 Medical Treatment Guide. https://www.mhlw.go.jp/content/000997789.pdf. Accessed May 30, 2024 (in Japanese).
Ministry of Health, Labour and Welfare. Version 9 of the COVID-19 Medical Treatment Guide. https://www.mhlw.go.jp/content/000936655.pdf. Accessed May 30, 2024 (in Japanese).
Ministry of Health, Labour and Welfare. Version 10 of the COVID-19 Medical Treatment Guide. https://www.mhlw.go.jp/content/001136687.pdf. Accessed May 30, 2024 (in Japanese).
Oshitani H, Komeda T, Fujita S, Asakawa M, Kitanishi Y. Comparison of the incidence of severe outcomes in outpatients with COVID-19 or seasonal influenza without risk factors: retrospective analysis of a health insurance claims-database. J Clin Virol Plus. 2024;4(1): 100175.
Madhi SA, Kwatra G, Myers JE, et al. Population immunity and Covid-19 severity with Omicron variant in South Africa. N Engl J Med. 2022;386(14):1314–26.
Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20(10):1204–14.
Shim SJ, Chan M, Owens L, Jaffe A, Prentice B, Homaira N. Rate of use and effectiveness of oseltamivir in the treatment of influenza illness in high-risk populations: a systematic review and meta-analysis. Health Sci Rep. 2021;4(1): e241.
Miyauchi H, Komeda T, Fujiwara M, et al. Comparison of hospitalization and death frequencies in influenza outpatients treated with baloxavir marboxil or neuraminidase inhibitors: an observational database study. Jpn J Pharmacoepidemiol. 2021;26(1):15–26 (in Japanese).
Puhach O, Meyer B, Eckerle I. SARS-CoV-2 viral load and shedding kinetics. Nat Rev Microbiol. 2023;21(3):147–61.
Kuroda T, Nobori H, Fukao K, et al. Efficacy comparison of 3CL protease inhibitors ensitrelvir and nirmatrelvir against SARS-CoV-2 in vitro and in vivo. J Antimicrob Chemother. 2023;78(4):946–52.
Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–7.
Ministry of Health, Labour and Welfare. D220 Respiratory Heart Rate Monitoring, Neonatal Heart Rate and Respiratory Monitoring, Cardioscope (Heart Scope). Notification from the Insurance Bureau. Implementation Considerations for the Partial Revision of the Calculation Method for Medical Reimbursement. 2022; No.0304-1, Mar 4: 346–347. https://kouseikyoku.mhlw.go.jp/kyushu/000215073.pdf. Accessed May 30, 2024 (in Japanese).
Ministry of Health, Labour and Welfare. Number of newly confirmed cases by age (weekly). Visualizing the data: information on COVID-19 infections. https://covid19.mhlw.go.jp/en/. Accessed May 30, 2024 (in Japanese).
ClinicalTrials.gov. Strategies and treatments for respiratory infections & viral emergencies (STRIVE): Shionogi protease inhibitor. NCT05605093. https://clinicaltrials.gov/study/NCT05605093. Accessed May 30, 2024.
Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed May 30, 2024.
Kumamaru H, Togo K, Kimura T, et al. Inventory of real-world data sources in Japan: Annual survey conducted by the Japanese Society for Pharmacoepidemiology Task Force. Pharmacoepidemiol Drug Saf. 2024;33: e5680.
Ministry of Health, Labor and Welfare. Document 2–5: The 98th COVID-19 Countermeasures Advisory Board Meeting of the Ministry of Health, Labor and Welfare (September 7, 2022).. Accessed May 30, 2024 (in Japanese). https://www.mhlw.go.jp/content/10900000/000987057.pdf.
Medical Writing and Editorial Assistance
We thank Tomoko Tsushima and Tsukasa Horiyama (Shionogi & Co., Ltd.) for preparing technical-support documents and Melissa Leffler, MBA, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. This assistance was funded by Shionogi & Co., Ltd.
Funding
This work was supported by Shionogi & Co., Ltd. Shionogi & Co., Ltd. was involved in the study design, data collection, data analysis, and preparation of the manuscript. The journal’s Rapid Service Fee was funded by Shionogi & Co., Ltd.
Author information
Authors and Affiliations
Contributions
Satoki Fujita, Takuji Komeda, Shogo Miyazawa had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Takahiro Takazono, Satoki Fujita, Takuji Komeda, Shogo Miyazawa, Yuki Yoshida, Yoshitake Kitanishi, Masahiro Kinoshita, Satoshi Kojima, Huilian Shen. Acquisition, analysis, or interpretation of data: Takahiro Takazono, Satoki Fujita, Takuji Komeda, Shogo Miyazawa, Yuki Yoshida, Takeki Uehara, Naoki Hosogaya, Naoki Iwanaga, Hiroshi Mukae. Drafting of the manuscript: Satoki Fujita, Yuki Yoshida, Masahiro Kinoshita. Critical revision of the manuscript for important intellectual content: All authors.
Corresponding author
Ethics declarations
Conflict of Interest
Satoki Fujita, Takuji Komeda, Shogo Miyazawa, Yuki Yoshida, Yoshitake Kitanishi, Masahiro Kinoshita, Satoshi Kojima, Huilian Shen, and Takeki Uehara are employees of Shionogi & Co., Ltd. and may hold stocks in the company. Takahiro Takazono has received personal fees from Shionogi & Co., Ltd., MSD K.K., Pfizer Japan Inc., Insmed GK., Asahi Kasei Pharma Corporation, and Kyorin Pharmaceutical Co., Ltd. Hiroshi Mukae has also received personal fees from AbbVie GK., Asahi Kasei Pharma Corporation, Astellas Pharma Inc., AstraZeneca K.K., Bristol-Myers Squibb K.K., Eli Lilly Japan K.K., FUJIFILM Toyama Chemical Co., Ltd., Gilead Sciences Inc., Insmed GK., Janssen Pharmaceutical K.K., Kyorin Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nihon Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Pfizer Japan Inc., Sumitomo Pharma Co., Ltd., Taiho Pharmaceutical Co., Ltd., Taisho Pharma Co., Ltd., Teijin Healthcare Ltd., and Toa Shinyaku Co., Ltd., and grants from Asahi Kasei Pharma Corporation, Astellas Pharma Inc., FUJIFILM Toyama Chemical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Pfizer Japan Inc., Taiho Pharmaceutical Co., Ltd., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., Toa Shinyaku Co., Ltd., and Torii Pharmaceutical Co., Ltd., outside the submitted work. Naoki Hosogaya and Naoki Iwanaga have no conflicts of interest to disclose.
Ethical Approval
This study was conducted according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects. It used anonymized information from an existing database, and informed consent was therefore not required. This study was registered in UMIN Clinical Trials Registry (study ID: UMIN000053217).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior presentation: This study was accepted for presentation at The Conference of The Japanese Association for Infectious Diseases 2024 on June 27th in Kobe, Japan.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Takazono, T., Fujita, S., Komeda, T. et al. Real-World Effectiveness of Ensitrelvir in Reducing Severe Outcomes in Outpatients at High Risk for COVID-19. Infect Dis Ther (2024). https://doi.org/10.1007/s40121-024-01010-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40121-024-01010-4