Abstract
Herpes zoster (HZ) is caused by reactivation of latent infection of varicella zoster virus (VZV) in sensory (cranial, dorsal root) ganglia. Major risk factors for HZ are increasing age and immunosuppression. HZ ophthalmicus (HZO) is a subset of HZ with involvement of the ophthalmic division of the fifth cranial trigeminal nerve. Approximately 4–20% of patients with HZ develop HZO. Approximately 50% of patients with HZO develop ocular disease, among whom up to 25% develop chronic or recurrent disease. Common manifestations of ocular disease include conjunctivitis, keratitis, and uveitis, whereas optic neuropathy and retinitis are uncommon. Due to the potential for vision impairment, ocular involvement requires urgent ophthalmic consultation. Early recognition and timely treatment with antivirals may prevent ocular complications. HZO is preventable by vaccination against HZ. Vaccine efficacy/effectiveness studies have been largely conducted for HZ with few studies assessing HZO. Both the recombinant adjuvanted vaccine (RZV) and live-attenuated vaccine (ZVL) significantly reduce the incidence of HZ and HZO in older adults. RZV is more effective than ZVL. Data on the effectiveness of vaccines for prevention of recurrent disease in patients with HZO are limited; however, vaccination is recommended. Despite recommendations to vaccinate individuals likely to benefit from an HZ vaccine, coverage for adults remains suboptimal. Barriers to vaccination include patient beliefs about HZ or HZ vaccines, and factors related to healthcare providers. In particular, the lack of a recommendation from their primary care physician is often cited by patients as a reason for remaining unvaccinated. By encouraging vaccination against HZ, physicians not only prevent HZ and HZO but also potential vision loss due to HZO.
Graphical abstract available for this article.
Plain Language Summary
Shingles, also known as herpes zoster, is a common and painful rash that develops when the virus that causes chickenpox in children reactivates, most often in adults. When shingles affects the eye or the area surrounding the eye, it is called herpes zoster ophthalmicus, or HZO for short. Up to one-fifth of people with shingles have HZO, and this risk increases with age and in people with other conditions that affect their immune system. Common signs and symptoms include a rash on the face, pain, fever, and headache, as well as symptoms in the eye, such as discomfort, redness, and discharge. HZO has the potential to cause permanent vision loss, and because of this, it is important that people with symptoms are referred to an eye doctor (“ophthalmologist”) as soon as possible. Early diagnosis of HZO is essential for effective treatment and prevention of the more serious complications it can cause. Treatment within 3 days of the symptoms occurring, with medications known as antivirals, can shorten the duration of a shingles episode and help relieve the pain. To help prevent the risk of shingles and its subtypes like HZO, vaccination is recommended. Two vaccines are currently approved for the prevention of shingles in adults. Although these vaccinations are recommended, some people do not have them for various reasons, which include their own personal beliefs about vaccinations or that their doctor has not recommended it to them. It is important that vaccinations against shingles are recommended to all patients eligible to receive one.
AbstractSection Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Herpes zoster ophthalmicus (HZO) is a subset of herpes zoster (HZ) that involves the ophthalmic branch of the trigeminal nerve; approximately 4–20% of patients with HZ develop HZO. |
The clinical signs and symptoms of HZO include a facial herpetic rash associated with, and sometimes preceded by, neuropathic pain, fever, and headache; extension of the rash to the tip of the nose (Hutchinson’s sign) indicates involvement of the nasociliary branch and increases the likelihood of ocular involvement. |
HZO can affect all the structures of the eye from the cornea to the retina and lead to severe complications, including loss of vision; early referral of a patient with a characteristic rash in an ophthalmic area to an ophthalmologist is essential to prevent such long-term irreversible damage. |
Early initiation of antiviral therapy can minimize the risk of ocular complications in patients with HZO. |
Vaccination is recommended to prevent HZ and HZO; it is important that primary care physicians prioritize vaccination of eligible patients as part of a comprehensive vaccination program in adults. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.25737087.
Introduction
Herpes zoster (HZ), or shingles, is caused by reactivation of latent infection with the varicella zoster virus (VZV) in sensory (cranial, dorsal root) ganglia long after primary infection in childhood [1,2,3]. HZ ophthalmicus (HZO) is defined as HZ involvement of the ophthalmic division of the fifth cranial nerve by VZV after reactivation [1]. HZO can result in serious, long-term ocular complications, including permanent moderate or severe vision loss. Therefore, due to the risk of complications, ocular involvement requires urgent ophthalmic consultation. It is increasingly accepted that early recognition of HZO is key to effective treatment and the prevention of severe complications [2].
HZ and HZO can be prevented by vaccination. Without vaccination, 30% of all adults, and 50% of those aged 85 years or older, will develop HZ [4]. However, vaccination with live-attenuated (ZVL) or non-live recombinant adjuvanted vaccine (RZV) significantly reduces the incidence of HZ in older adults who are at high risk of the disease [5,6,7,8]. In a network meta-analysis, RZV was more effective than ZVL and prevented more than 90% of HZ cases in adults aged ≥ 50 years [9].
The aim of this review is to describe the burden and complications associated with HZO and to discuss strategies for recognizing, treating, and preventing HZO. The review is based on a PubMed search for the period January 1, 2013, to February 27, 2024. The search was designed to identify English language articles that contained the terms “zoster” and “ophthalm*” in the title and/or abstract and pertained to adults. Case reports, pediatric studies, and preclinical studies were excluded. A total of 112 citations met these criteria and were reviewed by the authors (Fig. 1). Original research studies, preferably including large numbers of eyes or patients, were preferred. For treatments or preventive interventions, large randomized trials and real-world studies were preferred. Additional articles and treatment guidelines were identified and recommended by individual authors.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Epidemiology
The incidence of HZ increases with age; this age-related increase is related to a decline in VZV-specific cellular immunity [10]. A meta-regression analysis of data from 59 studies in 29 countries estimated the worldwide incidence of HZ to be 5.15/1000 persons among individuals aged 50–54 years and 11.27/1000 persons among those aged 85 years and older [11]. Among patients with HZ, HZO is present in approximately 4–20% of individuals [12,13,14,15,16,17].
Consistent with the epidemiology of HZ, the reported incidence of HZO also increases with age (Fig. 2). In a retrospective US study of data from a large commercial claims database, the incidence of HZO was estimated to range from 13.2 to 32.3 cases per 100,000 person-years among adults aged 21–50 years and from 54.6 to 131.6 per 100,000 person-years in those aged > 50 years [16]. The analysis included 633,474 cases of HZ, of which 7.9% (49,745) contained codes for HZO. Approximately 70% of HZO cases occurred in individuals aged > 50 years. Over the study period, the incidence of HZO increased by 3.6% each year [16].
Incidence rates of herpes zoster ophthalmicus in the USA (1994–2018) by age [16]
HZ and HZO are more common in women than in men [11, 18,19,20]; in the analysis by Kong et al. [16], the annual incidence of HZO in women and men was estimated to be 44.5 and 33.1 cases per 100,000 person-years, respectively.
The occurrence of HZO may vary by ethnicity. For example, in the USA, the incidence of HZO is highest among White individuals and is lower among African American, Asian, and Hispanic individuals [16]. The incidence of HZO has also been reported to be lower in Pacific Islanders compared with non-Pacific Islanders [21].
In terms of patients hospitalized for HZ, a nationwide analysis found that 12% of HZ-related hospitalizations in Denmark were caused by HZO [22].
Risk Factors
Specific risk factors for HZO have not been reported; however, several risk factors for HZ in general are well established and presumably also apply to HZO. Most cases of HZ (approximately 90%) occur in immunocompetent individuals [13]. A meta-analysis of 88 epidemiologic studies showed that the strongest positive associations (risk factors) with HZ were for immunosuppression via human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS); hematologic malignancy or solid organ malignancy; transplantation or drugs; family history; physical trauma; and increasing age [23]. Lesser positive associations were observed for female sex, psychological stress, and comorbidities, including diabetes mellitus, rheumatoid arthritis, cardiovascular disease, renal disease, systemic lupus erythematosus, and inflammatory bowel disease. Of note, Black race was associated with a low risk of developing HZ [23]. Patients receiving disease-modifying antirheumatic drugs, especially Janus kinase inhibitors, are also at increased risk of HZ/HZO [24, 25].
Severe manifestations of HZO are associated with immunosuppression. For example, HZO is a frequent complication in patients with HIV/AIDS (observed in 22.1%) [26, 27], and although it is a rare complication of HZO, acute retinal necrosis (ARN) and progressive outer retinal necrosis (PORN) may progress rapidly in patients with HIV/AIDS [28, 29]. The presence of uncommon manifestations (including ARN, optic neuritis, orbital apex syndrome, panuveitis, and cellulitis complicated by sepsis) was significantly associated with immunosuppression and diabetes among 50 patients with HZO, verified by polymerase chain reaction (PCR) assays [17].
Two large retrospective cohort studies have shown a small (14–15%) but significant increased risk of HZ after COVID-19 [30, 31]. In addition, some but not all observational studies have reported an increased risk of HZ in general [32,33,34] and HZO in particular [35,36,37,38] after COVID-19 vaccination [39, 40]. A large US healthcare claims database containing data from 1,959,157 patients who received at least one dose of a COVID-19 vaccine showed no increased risk of HZO after receipt of any COVID-19 vaccine [40].
Clinical Presentation
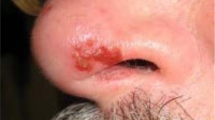
HZ manifests as a painful unilateral vesicular rash with a dermatomal distribution. Clinical signs and symptoms of HZO include a facial herpetic rash, usually associated with but sometimes preceded by neuropathic pain, fever, and headaches (Fig. 3). The rash develops 2–3 days after these early signs and forms blisters that ooze fluid for several days before crusting [1, 2, 41, 42]. Extension of the rash to the tip of the nose (Hutchinson’s sign) indicates involvement of the nasociliary branch and increases the likelihood of ocular involvement because of shared innervation [42].
Ocular involvement develops in approximately 50% of patients with HZO and may result in inflammation of any structures of the eye. Ocular manifestations include components of viral infection; inflammatory and immune reactions; vascular and neural inflammation; and tissue scarring, leading to the clinical syndromes of conjunctivitis, keratitis, uveitis, episcleritis, scleritis, retinitis, retinal necrosis, and optic neuritis (Table 1) [17, 43,44,45,46,47].
Eyelid/Conjunctiva
In patients with HZO, the eyelids and conjunctiva typically become involved within 1–2 weeks after the rash appears [48]. Characteristic vesicles appear on the eyelids, lid margins, and/or bulbar conjunctiva, and are often accompanied by blepharitis. Unilateral conjunctivitis, sometimes with petechial hemorrhages, and periorbital edema may also occur. Chronic inflammation of these structures can lead to late complications such as conjunctival or skin scarring, resulting in dry eye, lid malposition, and trichiasis, in which eyelashes grow toward the eye [49].
Episclera/Sclera
Inflammation of the episclera (episcleritis) or sclera (scleritis) may occur during the first week, presenting as localized redness [48].
Cornea
The cornea may be affected in various ways in patients with HZO [41, 48]. Initially, the epithelium is affected, causing superficial punctate keratitis (SPK) within 1 week of onset, progressing to pseudodendrites. Pseudodendrites resemble raised branching “stuck on” plaques with tapered ends and are distinct from herpes simplex virus (HSV) dendrites, which have terminal bulbs [50].
Later in the disease course, deeper layers of the cornea may be affected [41, 48]. Anterior stromal keratitis may develop within 1–2 weeks, appearing as multiple fine, granular, whitish infiltrates often beneath pre-existing dendrites or SPK as well as coin-shaped (nummular) lesions [41]. Deep stromal keratitis may develop within 1 month to years, featuring stromal inflammation/infiltrates leading to corneal thinning and scarring [41, 48]. Involvement of the endothelial layer (endotheliitis) may lead to corneal edema and is usually associated with anterior chamber inflammation (anterior uveitis), known as keratouveitis when both the cornea and uvea are involved [51].
Neurotrophic keratopathy, marked by reduced corneal sensation, may develop within 1 month to years of the onset of HZO due to neurologic damage, leading to chronic corneal ulcers that do not respond to conventional treatment [41, 48]. A nonhealing ulcer is at risk of secondary bacterial infection and can result in corneal thinning and perforation, necessitating tectonic corneal transplantation [52, 53]. A retrospective study showed that over a median follow-up of 6.3 years, neurotrophic keratopathy developed in 6.7% of patients with HZO, with the highest hazard 1–2 years after the onset of HZO [54]. The occurrence of corneal punctate epithelial erosions in patients with HZO is a risk factor for decreased corneal sensitivity [55].
Uvea
Anterior uveitis may occur either alone or in combination with keratitis (keratouveitis), with an onset typically 2–4 weeks after the onset of HZO [48, 56]. Uveitis often results in elevated intraocular pressure (IOP) upon presentation due to inflammation of the trabeculum, a mesh-like structure found in the eye’s anterior chamber angle that helps regulate the outflow of aqueous humor. Signs and symptoms of anterior uveitis in patients with HZO include ocular pain and hyperemia, blurred vision, ciliary injection, keratic precipitates, detection of “cells” and “flare” in the anterior chamber, and posterior synechia [43]. Chronic uveitis can lead to late-stage sequelae such as iris atrophy or an irregular pupil.
With respect to the posterior segment, HZO may result in ARN, which manifests as patches of peripheral retinitis that merge rapidly, accompanied by occlusive vasculitis and vitreous inflammation [29, 48]. The acute phase of ARN may persist for 6–12 weeks [29] and often leads to retinal detachment within 3 weeks to 5 months of onset (Lau et al. [57] cited in Hoogewoud et al. [58]). As noted above, immunocompromised individuals are at particular risk of PORN [29].
Other Complications
Other ocular complications of HZO include rare cranial nerve palsies, affecting nerves such as the seventh, third (most commonly), fourth, and sixth cranial nerves, which may resolve rapidly or take up to 6 months to improve significantly [59]. Orbital inflammation and myositis, along with optic neuritis, may also occur in patients with HZO, albeit rarely, and may be the first signs of HZO in the absence of a vesicular rash [60]. Postherpetic neuralgia is characterized by dermatomal pain persisting for over 3 months after the rash subsides, often accompanied by symptoms such as allodynia, reduced sensation, and paresthesia.
Differential Diagnosis
Other diseases can imitate HZO, including herpes simplex infection of the eyelid, cornea, or retina; anterior uveitis due to cytomegalovirus; contact dermatitis (from plants); or reactions to active ingredient or excipients in topical medications [2, 29, 61].
The differential diagnosis of HZO can be divided into the following categories, depending on presentation:
-
1.
Neuralgia. If patients experience only pain, tension headache, migraine, cluster headache, and giant cell arteritis must be ruled out.
-
2.
Rash. While a vesicular rash is often associated with HZO, it is crucial to also consider other potential causes like contact dermatitis, impetigo, and HSV infection. Distinguishing between HSV and HZO can pose challenges; while both may present with a vesicular rash, HSV typically affects multiple dermatomes rather than being confined to one. Additionally, classic corneal dendrites are seen in HSV infection, whereas pseudodendrites are characteristic of HZO.
-
3.
Ocular findings without a rash. In cases of periorbital swelling and ptosis, consider preseptal cellulitis, orbital tumor, and cavernous sinus thrombosis. Other possibilities include corneal abrasion, toxic reaction to active ingredients or preservatives in topical medication, infectious keratitis as Acanthamoeba keratitis also produces pseudodendrites, the different causes of interstitial keratitis, and infectious and non-infectious causes of uveitis including all the different types of Herpesviridae.
Key points regarding HZO are presented in Table 2.
Vision Impairment
Moderate vision loss is not uncommon in patients with HZO, whereas severe vision loss is uncommon and complete loss of vision is rare (Table 1). Multivariate analysis of data from 869 patients treated at a single ophthalmology center over one decade showed that moderate vision loss (seen in 19.8% of eyes) was associated with uveitis, White race, and best corrected visual acuity at the time of presentation, whereas severe vision loss (seen in 7.6% of eyes) was associated with uveitis, immunosuppression, visual acuity at the time of presentation, and older age [46]. In a longitudinal study of patients with HZO, increasing age, positive Hutchinson’s sign, absence of corneal sensation, presence of corneal epithelial lesions, and uveitis were significantly associated with vision loss [62]. Among these factors, uveitis was the best predictor of vision loss in a multivariate analysis [62].
Recurrent and Chronic HZO
A significant proportion of patients with HZO experience chronic or recurrent disease (up to 25% of patients overall) [63, 64]. A retrospective review of data from 130 patients with HZO showed that 24% of patients (31/130) had chronic disease and 15% (19/130) had recurrent ocular complications. In this series, 80% of patients with HZO (n = 104) had ocular manifestations, the most common of which were epithelial keratitis (46%), conjunctivitis (38%), and endothelial keratitis (12%) [63]. Patients with chronic HZO experienced symptoms for a mean of 304 days (range 66–1575 days), compared with 13 days (range 3–50 days) in patients with acute HZO. Most patients with recurrent disease (18/19) also had chronic disease. The most common recurrent manifestations were stromal keratitis (i.e., corneal infiltrates losing transparency; 50%), epithelial keratitis (i.e., pseudodendritic corneal ulcers; 29%), endotheliitis (i.e., corneal edema and loss of transparency; 13%), and conjunctivitis/scleritis (8%) [63]. In this study, through multivariate analysis, recurrent ocular disease was significantly associated with epithelial keratitis, stromal keratitis, increased IOP, and chronic HZO. Of note, sex, age, and immune status were not associated with chronicity or recurrence. The authors did not report whether skin involvement manifested during recurrences.
Recurrence of HZO has been associated with cataract surgery [65]. Among 57 patients with a history of HZO (38 with recurrent disease), 23 patients experienced a recurrence after undergoing cataract surgery. Postsurgical recurrence was associated with more frequent recurrent episodes and shorter periods of disease quiescence prior to surgery [65].
Importance of Early Diagnosis and Referral
HZO is a clinical diagnosis that generally does not require confirmatory laboratory testing. Swabs of skin or ophthalmic lesions for PCR analysis should only be considered when the diagnosis is uncertain, for example in patients with zoster sine herpete or recurrent anterior uveitis. Early referral of a characteristic painful rash in an ophthalmic distribution to an ophthalmologist is essential to help prevent long-term, irreversible damage from complications related to HZO [66].
Patients may present with a history of systemic symptoms such as prodromal symptoms (headache, fever, malaise), skin rash, dermatomal pain, paresthesia, and discomfort, and ocular symptoms such as pain, redness, watering, photophobia, and blurred/decreased vision [48]. A diagnosis of HZO should be considered in patients presenting with a papular or papulovesicular rash in the area innervated by the first division of the fifth cranial nerve, especially if the onset of rash was preceded by tingling and pain exacerbated by touch, accompanied by complaints of a headache and feeling generally unwell [2].
Early signs and symptoms of HZO (itching, tingling, or pain in the affected area) may become apparent several days before the characteristic HZ rash develops [2, 42]. In rare cases, a rash may not develop (termed “zoster sine herpete”) and ocular manifestations of HZO may occur without skin lesions, which increases the difficulty of diagnosing the condition [2, 42, 67]. Ocular symptoms are often non-specific, may include hyperemia, lacrimation, and blurred vision, and usually appear days after the rash [42, 68]. Involvement of the skin supplied by the nasociliary nerve (Hutchinson’s sign) is the main trigger for ophthalmologist referral [66]; however, although this is a traditional teaching, it is not always reliable. Referral to an ophthalmologist should also be triggered by conjunctival or ciliary pain, changes in vision (diplopia, photophobia, or decreased acuity), and/or abnormal extraocular movements suggesting paralysis of the third, fourth, or sixth cranial nerve.
Patients with HZO should undergo a complete ophthalmologic examination including a slit lamp examination, determination of IOP, and cranial nerve examination. Referral times have not been widely reported. Median time from rash to clinical presentation and subsequent referral to an ophthalmologist was 5 days in a New Zealand study [46]. A recent analysis of a US claims database found that 75.8% of patients saw an eye care provider within 7 days of being diagnosed with HZO [69].
Patients with signs or symptoms of herpes zoster oticus (Ramsay Hunt syndrome) should be referred to an otorhinolaryngologist.
Importance of Early and Appropriate Treatment
The general goals of treatment for HZ, including HZO, are to limit the intensity and duration of HZ-associated symptoms, promote the healing of lesions, and improve health-related quality of life [70]. Specific goals of therapy in patients with HZO include limiting the extent of involvement of ocular structures, preserving vision, and preventing recurrence. To these ends, prompt initiation of antiviral therapy (within 72 h of the onset of HZO symptoms) can prevent ocular complications [71, 72]. Ophthalmologic consultation is recommended for all patients with HZO. An algorithm showing management of patients with HZO is shown in Fig. 4.
Treatment algorithm for collaborative care between primary care provider and ophthalmologist [2, 48, 53, 77, 118, 119]. aInvolvement of the skin supplied by the nasociliary nerve. bIdeally ≤ 72 h after onset of rash, but beyond 7 days if new lesions are present in patients with complications, such as herpes zoster ophthalmicus, or at risk of complications, such as immunocompromised patients. cTopical ophthalmic corticosteroids should not be used in the absence of antiviral agents
While initiation of antivirals within 72 h of symptom onset is preferred, initiation of antiviral therapy beyond 72 h after onset is considered to be reasonable (in European consensus guidelines) as long as viral replication is ongoing as indicated by the presence of new lesions in patients with complications or at risk of complications, such as those with ophthalmic disease or those who are immunocompromised [48, 70]. An analysis in New Zealand showed that, although only one-third of patients presented to their general practitioner within 3 days of HZ symptom onset, a higher proportion of patients with HZO did so (45%) when compared with patients with HZ affecting other dermatomes (31%) [73]. A recent analysis of a US claims database showed that nearly 60% of patients with HZO received systemic antiviral therapy within 7 days of the diagnosis [69].
Antivirals used to treat HZO include oral acyclovir, valaciclovir, and famciclovir [48, 70]. In disseminated HZ or in immunocompromised patients, intravenous (IV) acyclovir is indicated; if the disease is resistant to acyclovir, IV foscarnet may be considered. Early initiation of antiviral therapy limits disease progression in patients with HZO. In a small study (N = 86) in which acyclovir was initiated within 72 h of the onset of rash, 30% of patients developed chronic ocular complications, as compared with ≥ 50% of untreated patients from other studies (Hoang-Xuan et al. [74] cited in Cohen and Kessler [75]). A retrospective analysis of data from patients with HZO who had (n = 202) and had not (n = 121) received antivirals showed that antiviral treatment was associated with a significantly lower incidence of neurotrophic keratitis within 6 months [76]. In addition, serious inflammatory conditions (stromal keratitis, corneal edema, scleritis, uveitis, and glaucoma) were associated with a delay in initiating antiviral therapy among treated patients [76]. A systematic review of data from randomized controlled trials of antiviral treatments for HZO concluded that famciclovir 500 mg three times daily or valacyclovir 1000 mg three times daily are both reasonable alternatives to acyclovir 800 mg five times daily because of the less frequent administration [77]. Occurrence of ocular manifestations was similar across individual trials (famciclovir vs. acyclovir and valacyclovir vs. acyclovir).
In addition to antiviral and analgesic agents, systemic and topical ophthalmic corticosteroids may be prescribed as adjunctive anti-inflammatory agents, but this is controversial [70]. The American Academy of Ophthalmology (AAO) recommends topical corticosteroids be used judiciously with close monitoring for stromal keratitis and uveitis. A survey of US ophthalmologists participating in the Zoster Eye Disease Study (ZEDS) showed that a majority (63.4–69.1%) of specialists used the combination of a topical corticosteroid plus prolonged antiviral therapy for the treatment of stromal keratitis in both recent-onset and chronic HZO [78]. However, corticosteroids should not be used in the absence of antiviral medications because they are immunosuppressants and corticosteroid monotherapy may promote viral replication and result in acute retinal necrosis [70]. The incidence of acute retinal necrosis is fortunately rare (i.e., 1 case per 1.6–2.0 million population per year) [79].
Acute retinal necrosis is a rapidly progressive ophthalmologic emergency and may spread to the contralateral eye [70]. Although no data are available from randomized trials to guide management, immediate treatment of acute retinal necrosis with IV acyclovir followed by oral therapy to complete a 3- to 4-month course of antiviral treatment has been recommended to arrest viral replication and prevent the involvement of both eyes [70, 80, 81]. Intravitreal antiviral agents (foscarnet, ganciclovir) have also been recommended [29, 82]. For optic neuropathy or orbitopathy, IV acyclovir is used at a dosage of 10–15 mg/kg three times daily for 2–3 weeks. The treatment for retinitis is similar, with the addition of several months of oral antiviral therapy [83].
There are no data from randomized, placebo-controlled studies to support the use of long-term suppressive treatment with oral antiviral agents. Prolonged antiviral therapy is being investigated in an ongoing trial (ZEDS; NCT03134196) in the HZO setting [84]. This randomized, double-blind trial has enrolled 652 patients and will determine whether treatment with valacyclovir 1000 mg daily for 1 year reduces the incidence of ophthalmic complications in patients with HZO [85]. Eligible patients are immunocompetent adults who have had an episode of HZO with dendriform epithelial keratitis, endothelial keratitis, stromal keratitis, and/or iritis within the previous year. The study is expected to be completed in 2024 [85].
After the initial diagnosis of HZO, follow-up visits are required on the basis of clinical presentation. If there is no ocular involvement, follow-up for optometric assessment in 10–14 days should be considered [48]. If there is ocular involvement, the follow-up interval is dependent on type of ocular involvement and severity: the AAO recommends following up every 1–7 days during the acute episode, then monitoring at 3- to 12-month intervals thereafter to detect the presence of delayed complications (e.g., ocular hypertension, cataracts, and corneal scarring) [53]. Overall, 48.7% of patients with HZO had at least one follow-up visit within 30 days of the initial diagnosis, and 38.6% of patients with HZO had a follow-up appointment with an ophthalmologist within 1 year of the initial diagnosis [69].
Importance of Prevention
Prevention of HZ in general is an effective means of preventing HZO, given that HZO is a subtype of HZ involving one specific nerve and dermatome. Previously, two vaccines were approved in several countries for the prevention of HZ in adults; a live-attenuated vaccine (zoster vaccine live; ZVL) was licensed in 2006 (Zostavax, Merck & Co., Inc.) [86], and subsequently, an adjuvanted recombinant zoster vaccine (RZV; Shingrix, GSK), which contains the main target of CD4+ T cell-mediated responses to latent VZV (glycoprotein E) and a liposome-based adjuvant (AS01B), was approved in 2017 [87]. Pivotal studies for both vaccines were conducted to assess efficacy in prevention of HZ and did not report on HZO specifically. Effectiveness regarding prevention of HZO has been reported in real-world effectiveness studies.
Efficacy in Preventing HZ
When administered as a single dose in a randomized, multicenter, phase 3 trial, the efficacy of ZVL relative to placebo for prevention of HZ was 69.8% in adults aged 50–59 years [6] and 51.3% in adults aged ≥ 60 years [5]. The efficacy of ZVL waned during a long-term follow-up study of patients enrolled in phase 3 trials and was estimated to be 21.1% at 7–11 years post vaccination [88]. As a live vaccine, ZVL is generally contraindicated in immunocompromised individuals [89]. The most common adverse reactions reported in pivotal clinical trials of ZVL were injection-site reactions (64% in persons aged 50–59 years and 48% in those aged ≥ 60 years) [90]. The most common systemic adverse reactions included headache and pain in the extremities. Most local and systemic adverse reactions were mild in intensity.
A two-dose schedule of RZV is indicated for the prevention of HZ in adults aged ≥ 50 years and also in adults aged ≥ 18 years who are at increased risk of HZ (e.g., immunocompromised individuals) [91, 92]. The efficacy of RZV for prevention of HZ relative to placebo in adults aged ≥ 50 years and ≥ 70 years, respectively, was 97.2% and 91.3% in randomized, controlled, multicenter, phase 3 trials [7, 8]. A combined analysis of data from these trials showed that 1 of 13,881 vaccinated individuals and 7 of 14,035 placebo recipients experienced ophthalmic disease during approximately 4 years of follow-up [93]. Long-term follow-up of patients aged ≥ 50 years vaccinated with RZV in the phase 3 trials has shown that vaccine efficacy against HZ was 81.6% for approximately 6–10 years post vaccination [94]. As a subunit vaccine, RZV can be administered to immunocompromised individuals [91]. As a result, a series of clinical trials have examined the efficacy of the vaccine in this diverse high-risk group of patients. RZV had a vaccine efficacy of 68.2% in preventing HZ in autologous stem cell transplant recipients aged ≥ 18 years [95] and 87.2% in patients with hematologic malignancies according to a post hoc analysis [96].
In individuals aged ≥ 50 years who received RZV in clinical trials, the most common solicited local adverse reactions were pain (78%), redness (38%), and swelling (26%), and the most common solicited general adverse reactions were myalgia (45%), fatigue (45%), headache (38%), shivering (27%), and fever (21%) [91]. Most of these reactions were not long-lasting (median duration of 2–3 days). Reactions reported as severe lasted 1–2 days. In adults ≥ 18 years who are immunodeficient or immunosuppressed as a result of disease or therapy, the safety profile was consistent with that observed in adults ≥ 50 years.
Efficacy/Effectiveness Against HZO
Two meta-analyses have examined the effectiveness of vaccines against HZ and complications. A systematic review of data from 9,536,086 patients in 22 cohort and case–control studies showed that, on the basis of two studies with data on ZVL for the prevention of HZO, ZVL had 30% efficacy in preventing HZO [97]. A network meta-analysis by Tricco et al. [98] including data from a randomized controlled trial of 13,900 patients comparing the efficacy of RZV and placebo in older adults showed that the efficacy of RZV was significantly superior to that of placebo in the prevention of HZO (vaccine efficacy 88%).
Real-world observational studies from the USA have evaluated vaccine effectiveness in the prevention of HZO. In a retrospective cohort study in Medicare beneficiaries aged ≥ 65 years, the effectiveness of ZVL in preventing HZO was estimated to be 31% during the first 3 years post vaccination and 21% after ≥ 4 years [15]. The effectiveness of ZVL in prevention of HZO in adults aged > 50 years was estimated to be 71% during the first years post vaccination, and subsequently decreased to 12% at 8–10 years of follow-up in a prospective study that included 1.5 million individuals [99]. For the 10-year period, effectiveness of ZVL in prevention of HZO was 37% [99].
RZV effectiveness in Medicare beneficiaries aged ≥ 65 years against HZO was reported to be 67% in a study that included 15 million individuals with approximately 7 months of follow-up since vaccination [100]. RZV effectiveness against HZO in adults aged ≥ 50 years was evaluated in two large observational studies of commercial health insurance databases with a median follow-up period of 2 years after vaccination. In one of these analyses, which included approximately 5 million individuals, vaccine effectiveness against HZO was estimated to be 89% in adults aged > 50 years [101]. In the other analysis, the overall incidence of HZO in vaccinated and unvaccinated individuals was estimated to be 11.9 and 72.1 cases per 100,000 person-years, respectively [102].
The effectiveness of HZ vaccines in individuals with a prior history of HZO has not been assessed or reported.
Current Guidance for HZ Prevention
Over the past two decades, recommendations for preventing HZ in older adults with vaccines have evolved. Initially, ZVL was the only vaccine available. For example, in 2008, the US Advisory Committee on Immunization Practices (ACIP) recommended ZVL for all individuals older than 60 years [4]. Subsequently, after the US Food and Drug Administration approval of RZV, ACIP recommended that all adults aged ≥ 50 years receive RZV regardless of whether they had previously received ZVL [4, 103]. ACIP recommends RZV in preference to ZVL because of the greater apparent efficacy of RZV in clinical trials and because of diminished efficacy of ZVL over time [103]. More recently, with the approval RZV in immunocompromised individuals, ACIP recommends RZV for prevention of HZ and related complications in immunodeficient and immunosuppressed adults aged ≥ 19 years [104]. Australian [105], Canadian [106], and German [107] authorities also recommend RZV in preference to ZVL. The AAO recommends vaccination against HZ in patients aged ≥ 50 years and also that ophthalmologists collaborate with primary care physicians, internists, dermatologists, and other healthcare professionals to recommend vaccination against HZ in patients aged ≥ 50 years [108].
Patients with HZ are at risk of recurrent disease. While there are limited data on the effectiveness of vaccines in individuals with a history of HZ, vaccination is recommended; however, guidance varies as to when vaccination should occur. ACIP recommends that vaccination can occur after resolution of acute HZ [103], whereas the Australian Technical Advisory Group on Immunisation (ATAGI) recommends that vaccination is delayed for ≥ 12 months after an episode of HZ (or ≥ 3 months for immunocompromised individuals) [105].
There is concern that vaccination against HZ in individuals with a history of HZO may be associated with exacerbation or recurrence of HZO, with a few cases being reported following ZVL [109, 110] and RZV [111, 112] administration. A recent study reported an increased risk of exacerbation or recurrence in the 56-day period after vaccination with RZV (adjusted hazard ratio 1.64; 95% confidence interval 1.01–2.67; p = 0.04) [113]; there was no significant increase during the 28- or 42-day periods post vaccination [113]. The AAO notes that individuals with a history of HZO may be at risk of recurrent eye disease after vaccination and recommends that these individuals are examined by their ophthalmologist within several weeks before and after vaccination against HZ [108]. Moreover, since cell-mediated immunity is stimulated during and after an episode of HZ, vaccination is not urgent immediately after resolution of symptoms and should be delayed until eye disease is well controlled [108]. As only limited data are available on the use of HZ vaccines in individuals with a history of HZO, clinicians should use professional judgment when considering the resolution of HZO symptoms and immune status of a given patient in light of current recommendations.
An increase in HZ vaccination would be expected to reduce the burden of HZ, including HZO. Despite the recommendations, vaccination coverage for adults, including HZ vaccination, remains suboptimal. Barriers to vaccination against HZ include diverse phenomena that can be categorized as patient beliefs about HZ (low perceived risk of HZ, or the belief that one rarely gets sick or has immunity to HZ already), beliefs about HZ vaccines (concerns about effectiveness, adverse effects, or possible allergic reactions, or the belief that HZ vaccines can cause shingles), and factors related to healthcare providers (such as not having discussed the need for HZ vaccination with their primary care physician or difficulty getting to see a primary care physician) [114]. In particular, a prominent reason cited by patients when declining HZ vaccination is the lack of a recommendation by their primary care physician [115, 116].
Primary care physicians should be proactive and recommend HZ vaccination to all eligible patients rather than waiting for a patient’s request. Patients who regularly receive influenza vaccines have increased uptake of HZ vaccines [117]; therefore, a convenient time to recommend and administer an HZ vaccine would be when a patient is attending a clinic to receive a seasonal influenza vaccine. Practice nurses are ideally situated to identify patients who are eligible for vaccination.
Conclusions
In summary, because HZO is a potentially sight-threatening condition and a significant proportion of patients with HZO experience chronic or recurrent disease, referral to an ophthalmologist for treatment and follow-up is necessary in patients who present with HZ and ocular signs and symptoms or nasociliary branch involvement. Prompt initiation of antiviral treatment and timely follow-up is necessary to limit progression of HZO and complications. Effective vaccines are widely available and recommended for the prevention of HZ and HZO in adults. Primary care physicians should prioritize vaccination of eligible patients as part of a comprehensive vaccination program in adults.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Johnson RW, Alvarez-Pasquin MJ, Bijl M, et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Ther Adv Vaccines. 2015;3(4):109–20.
Tuft S. How to manage herpes zoster ophthalmicus. Community Eye Health. 2020;33(108):71–2.
Gross GE, Eisert L, Doerr HW, et al. S2k guidelines for the diagnosis and treatment of herpes zoster and postherpetic neuralgia. J Dtsch Dermatol Ges. 2020;18(1):55–78.
Tsatsos M, Athanasiadis I, Myrou A, Saleh MG, Ziakas N. Herpes zoster ophthalmicus: a devastating disease coming back with vengeance or finding its nemesis? J Ophthalmic Vis Res. 2022;17(1):123–9.
Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–84.
Schmader KE, Levin MJ, Gnann JW Jr., et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis. 2012;54(7):922–8.
Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96.
Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32.
Xia Y, Zhang X, Zhang L, Fu C. Efficacy, effectiveness, and safety of herpes zoster vaccine in the immunocompetent and immunocompromised subjects: a systematic review and network meta-analysis. Front Immunol. 2022;13:978203.
Herman L, Levin MJ, Rehm S. Shedding light on shingles: the power of prevention. Am J Med. 2016;129(10):1137.
Curran D, Callegaro A, Fahrbach K, et al. Meta-regression of herpes zoster incidence worldwide. Infect Dis Ther. 2022;11(1):389–403.
Ragozzino MW, Melton LJ 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore). 1982;61(5):310–6.
Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–9.
Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833.
Izurieta HS, Wernecke M, Kelman J, et al. Effectiveness and duration of protection provided by the live-attenuated herpes zoster vaccine in the medicare population ages 65 years and older. Clin Infect Dis. 2017;64(6):785–93.
Kong CL, Thompson RR, Porco TC, Kim E, Acharya NR. Incidence rate of herpes zoster ophthalmicus: a retrospective cohort study from 1994 through 2018. Ophthalmology. 2020;127(3):324–30.
Dmitriev AA, Odden J, Mora-Boellstorff D, et al. Herpes zoster ophthalmicus: frequency and risk factors for developing uncommon ocular manifestations. Can J Ophthalmol. 2024;59(3):201–7.
Opstelten W, Van Essen GA, Schellevis F, Verheij TJM, Moons KGM. Gender as an independent risk factor for herpes zoster: a population-based prospective study. Ann Epidemiol. 2006;16(9):692–5.
Alicino C, Trucchi C, Paganino C, et al. Incidence of herpes zoster and post-herpetic neuralgia in Italy: results from a 3-years population-based study. Hum Vaccin Immunother. 2017;13(2):399–404.
Cifuentes-Gonzalez C, Rojas-Carabali W, Fonseca-Mora MA, Mejia-Salgado G, Reyes-Guanes J, de-la-Torre A. Colombian Ocular Infectious Epidemiology Study (COIES): herpes zoster ophthalmicus prevalence and sociodemographic characterization, 2015–2019. Int J Infect Dis. 2022;116:27–33.
Borkar DS, Tham VM, Esterberg E, et al. Incidence of herpes zoster ophthalmicus: results from the Pacific Ocular Inflammation Study. Ophthalmology. 2013;120(3):451–6.
Schmidt SAJ, Kahlert J, Vestergaard M, Schonheyder HC, Sorensen HT. Hospital-based herpes zoster diagnoses in Denmark: rate, patient characteristics, and all-cause mortality. BMC Infect Dis. 2016;16:99.
Marra F, Parhar K, Huang B, Vadlamudi N. Risk factors for herpes zoster infection: a meta-analysis. Open Forum Infect Dis. 2020;7(1):ofaa005.
Dammacco R, Guerriero S, Alessio G, Dammacco F. Natural and iatrogenic ocular manifestations of rheumatoid arthritis: a systematic review. Int Ophthalmol. 2022;42(2):689–711.
Liao TL, Chen YM, Liu HJ, Chen DY. Risk and severity of herpes zoster in patients with rheumatoid arthritis receiving different immunosuppressive medications: a case-control study in Asia. BMJ Open. 2017;7(1):e014032.
Sharew G, Azage M. Predictors of HIV/AIDS related ocular manifestations among HIV/AIDS patients in Felege Hiwot Referral Hospital, Northwest Ethiopia. J Ophthalmol. 2015;2015:965627.
Nithyanandam S, Joseph M, Stephen J. Ocular complications and loss of vision due to herpes zoster ophthalmicus in patients with HIV infection and a comparison with HIV-negative patients. Int J STD AIDS. 2013;24(2):106–9.
Ormerod LD, Larkin JA, Margo CA, et al. Rapidly progressive herpetic retinal necrosis: a blinding disease characteristic of advanced AIDS. Clin Infect Dis. 1998;26(1):34–45 (discussion 6–7).
Taney L, Shah VA, Shah VA, et al. EyeWiki. Acute retinal necrosis. American Academy of Ophthalmology. https://eyewiki.aao.org/Acute_Retinal_Necrosis. Accessed 5 Mar 2024.
Bhavsar A, Lonnet G, Wang C, et al. Increased risk of herpes zoster in adults ≥50 years old diagnosed with COVID-19 in the United States. Open Forum Infect Dis. 2022;9(5):ofac118.
López-Lacort M, Correcher-Martínez E, Muñoz-Quiles C, Díez-Domingo J, Orrico A. Risk of herpes zoster in individuals diagnosed with SARS-CoV2 infection in the Valencia region of Spain: a retrospective cohort population-based study. Abstract P2443 and poster. 33rd European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Copenhagen, Denmark; 2023.
Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–90.
Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21(5):675–84.
Wan EYF, Chui CSL, Wang Y, et al. Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: a self-controlled case series and nested case-control study. Lancet Reg Health West Pac. 2022;21:100393.
Rallis KI, Fausto R, Ting DSJ, Al-Aqaba MA, Said DG, Dua HS. Manifestation of herpetic eye disease after COVID-19 vaccine: a UK case series. Ocul Immunol Inflamm. 2022;30(5):1136–41.
Huang LY, Chiang CC, Li YL, et al. Corneal complications after COVID-19 vaccination: a systemic review. J Clin Med. 2022;11(22):6828.
Ichhpujani P, Parmar UPS, Duggal S, Kumar S. COVID-19 vaccine-associated ocular adverse effects: an overview. Vaccines (Basel). 2022;10(11):1879.
Lotan I, Lydston M, Levy M. Neuro-ophthalmological complications of the COVID-19 vaccines: a systematic review. J Neuroophthalmol. 2022;42(2):154–62.
Parikh R, Yousefi M, Curran D, Widenmaier R. The impact of the COVID-19 pandemic on the incidence of herpes zoster: a narrative literature review. Infect Dis Ther. 2024;13(3):447–61.
Akpandak I, Sechrist SJ, Claire Miller D, et al. Risk of herpes zoster ophthalmicus after COVID-19 vaccination in a large US health care claims database. Am J Ophthalmol. 2024;258:139–44.
Minor M, Payne E. Herpes zoster ophthalmicus. Treasure Island (FL): StatPearls; 2023.
Cohen EJ, Jeng BH. Herpes zoster: a brief definitive review. Cornea. 2021;40(8):943–9.
Harding SP, Lipton JR, Wells JC. Natural history of herpes zoster ophthalmicus: predictors of postherpetic neuralgia and ocular involvement. Br J Ophthalmol. 1987;71(5):353–8.
Yawn BP, Wollan PC, St Sauver JL, Butterfield LC. Herpes zoster eye complications: rates and trends. Mayo Clin Proc. 2013;88(6):562–70.
Szeto SKH, Chan TCY, Wong RLM, Ng ALK, Li EYM, Jhanji V. Prevalence of ocular manifestations and visual outcomes in patients with herpes zoster ophthalmicus. Cornea. 2017;36(3):338–42.
Niederer RL, Meyer JJ, Liu K, Danesh-Meyer HV. Herpes zoster ophthalmicus clinical presentation and risk factors for loss of vision. Am J Ophthalmol. 2021;226:83–9.
Chakrabarti R, George G, Wells K, Crock C, Fahy E. Characteristics, treatment and complications of herpes zoster ophthalmicus at a tertiary eye hospital. Med J Aust. 2020;213(5):226–7.
The Royal Victorian Eye and Ear Hospital. Clinical practice guideline. Emergency department. Herpes zoster ophthalmicus. https://eyeandear.org.au/wp-content/uploads/2021/11/Herpes-Zoster-Ophthalmicus-Clinical-Practice-Guideline1.pdf. Accessed 5 Mar 2024.
Yu X, Jia X, Zhang Z, et al. Meibomian gland morphological changes in ocular herpes zoster patients based on AI analysis. Front Cell Dev Biol. 2022;10:1094044.
Hu AYH, Strauss EC, Holland GN, Chan MF, Yu F, Margolis TP. Late varicella-zoster virus dendriform keratitis in patients with histories of herpes zoster ophthalmicus. Am J Ophthalmol. 2010;149(2):214–20.e3.
Reijo A, Antti V, Jukka M. Endothelial cell loss in herpes zoster keratouveitis. Br J Ophthalmol. 1983;67(11):751–4.
Hassan OM, Farooq AV, Soin K, Djalilian AR, Hou JH. Management of corneal scarring secondary to herpes zoster keratitis. Cornea. 2017;36(8):1018–23.
Feldman BH, Bunya VY, Woodward MA, et al. EyeWiki. Herpes zoster ophthalmicus. American Academy of Ophthalmology. https://eyewiki.aao.org/Herpes_Zoster_Ophthalmicus. Accessed 5 Mar 2024.
Meyer JJ, Liu K, McGhee CNJ, Danesh-Meyer HV, Niederer RL. Neurotrophic keratopathy after herpes zoster ophthalmicus. Cornea. 2022;41(11):1433–6.
Kim M, Chun YS, Moon NJ, Kim KW. Clinical factors associated with the early reduction of corneal sensitivity in herpes zoster ophthalmicus. Korean J Ophthalmol. 2022;36(2):147–53.
O'Keefe GD, Patel N, Hurzhii O. EyeWiki. Herpes zoster uveitis. American American Academy of Ophthalmology. https://eyewiki.aao.org/Herpes_Zoster_Uveitis. https://eyewiki.aao.org/Herpes_Zoster_Uveitis. Accessed 27 Mar 2024.
Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis: features, management, and outcomes. Ophthalmology. 2007;114(4):756–62.e1.
Hoogewoud F, Rossi DC, Stappler T, Guex-Crosier Y. Acute retinal necrosis: a mini review. Front Ophthalmol. 2022. https://doi.org/10.3389/fopht.2022.916113.
Tsau PW, Liao MF, Hsu JL, et al. Clinical presentations and outcome studies of cranial nerve involvement in herpes zoster infection: a retrospective single-center analysis. J Clin Med. 2020;9(4):946.
Bak E, Kim N, Khwarg SI, Choung HK. Case series: herpes zoster ophthalmicus with acute orbital inflammation. Optom Vis Sci. 2018;95(4):405–10.
Terada Y, Kaburaki T, Takase H, et al. Distinguishing features of anterior uveitis caused by herpes simplex virus, varicella-zoster virus, and cytomegalovirus. Am J Ophthalmol. 2021;227:191–200.
Nithyanandam S, Stephen J, Joseph M, Dabir S. Factors affecting visual outcome in herpes zoster ophthalmicus: a prospective study. Clin Exp Ophthalmol. 2010;38(9):845–50.
Lee SM, Han J, Yang CM, et al. Chronic and recurrent herpes zoster ophthalmicus. Medicina (Kaunas). 2021;57(10):999.
Tran KD, Falcone MM, Choi DS, et al. Epidemiology of herpes zoster ophthalmicus: recurrence and chronicity. Ophthalmology. 2016;123(7):1469–75.
Lu LM, McGhee CNJ, Sims JL, Niederer RL. High rate of recurrence of herpes zoster-related ocular disease after phacoemulsification cataract surgery. J Cataract Refract Surg. 2019;45(6):810–5.
Werner RN, Nikkels AF, Marinovic B, et al. European consensus-based (S2k) guideline on the management of herpes zoster—guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), part 1: diagnosis. J Eur Acad Dermatol Venereol. 2017;31(1):9–19.
Al-Ani HH, Niederer RL. Zoster sine herpete: a disease that ophthalmologists should be aware of. Korean J Pain. 2020;33(4):403–4.
Goswami M, Bhattacharya S, Bandyopadhyay M. Ocular manifestation and visual outcomes in herpes zoster ophthalmicus: a prospective study from a tertiary hospital of Eastern India. Int J Ophthalmol. 2021;14(12):1950–6.
Lu A, Sun Y, Porco TC, Arnold BF, Acharya NR. Practice patterns in the initial management of herpes zoster ophthalmicus in the United States. Cornea. 2024;43(1):6–12.
Werner RN, Nikkels AF, Marinovic B, et al. European consensus-based (S2k) guideline on the management of herpes zoster—guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), part 2: treatment. J Eur Acad Dermatol Venereol. 2017;31(1):20–9.
Wood MJ, Kay R, Dworkin RH, Soong SJ, Whitley RJ. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis. 1996;22(2):341–7.
Jackson JL, Gibbons R, Meyer G, Inouye L. The effect of treating herpes zoster with oral acyclovir in preventing postherpetic neuralgia. A meta-analysis. Arch Intern Med. 1997;157(8):909–12.
Wallis KA, Hood LJ, Rao K. Herpes zoster: when do patients present and who gets antiviral treatment? J Prim Health Care. 2014;6(2):108–13.
Hoang-Xuan T, Büchi ER, Herbort CP, et al. Oral acyclovir for herpes zoster ophthalmicus. Ophthalmology. 1992;99(7):1062–71.
Cohen EJ, Kessler J. Persistent dilemmas in zoster eye disease. Br J Ophthalmol. 2016;100(1):56–61.
Severson EA, Baratz KH, Hodge DO, Burke JP. Herpes zoster ophthalmicus in olmsted county, Minnesota: have systemic antivirals made a difference? Arch Ophthalmol. 2003;121(3):386–90.
Fan S, Stojanovic D, Malvankar-Mehta MS, Hutnik C. Treatment of herpes zoster ophthalmicus: a systematic review and Canadian cost-comparison. Can J Ophthalmol. 2018;53(2):117–23.
Lo DM, Jeng BH, Gillespie C, Wu M, Cohen EJ. Current practice patterns and opinions on the management of recent-onset or chronic herpes zoster ophthalmicus of Zoster Eye Disease Study Investigators. Cornea. 2019;38(1):13–7.
Muthiah MN, Michaelides M, Child CS, Mitchell SM. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol. 2007;91(11):1452–5.
Wong RW, Jumper JM, McDonald HR, et al. Emerging concepts in the management of acute retinal necrosis. Br J Ophthalmol. 2013;97(5):545–52.
Pleyer U, Chee SP. Current aspects on the management of viral uveitis in immunocompetent individuals. Clin Ophthalmol. 2015;9:1017–28.
Mayer CS, Blobner K, Storr J, Baur ID, Khoramnia R. Acute retinal necrosis: signs, treatment, complications and outcome. Diagnostics (Basel). 2022;12(2):386.
Le P. Herpes zoster infection. BMJ. 2019;364:k5095.
Cohen EJ, Hochman JS, Troxel AB, Colby KA, Jeng BH; ZEDS Trial Research Group. Zoster Eye Disease Study: rationale and design. Cornea. 2022;41(5):562–71.
Zoster Eye Disease Study (ZEDS). ClinicalTrials.gov ID NCT03134196. https://clinicaltrials.gov/study/NCT03134196. Accessed 28 Feb 2024.
Cohen EJ. Incidence rate of herpes zoster ophthalmicus. Ophthalmology. 2020;127(3):331–2.
Pan CX, Lee MS, Nambudiri VE. Global herpes zoster incidence, burden of disease, and vaccine availability: a narrative review. Ther Adv Vaccines Immunother. 2022;10:25151355221084535.
Morrison VA, Johnson GR, Schmader KE, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60(6):900–9.
Merck & Co., Inc. Zostavax (Zoster Vaccine Live), suspension for subcutaneous injection. Highlights of Prescribing Information. https://www.fda.gov/media/82524/download#:~:text=Administer%20ZOSTAVAX%20as%20a%20single,region%20of%20the%20upper%20arm.&text=subcutaneously.&text=ADMINISTER%20IMMEDIATELY%20AFTER%20RECONSTITUTION%20to,not%20used%20within%2030%20minutes. Accessed 19 Oct 2023.
Zostavax. Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/zostavax-epar-product-information_en.pdf. Accessed 27 Mar 2024.
Shingrix (Zoster Vaccine Recombinant, Adjuvanted), suspension for intramuscular injection. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Shingrix/pdf/SHINGRIX.PDF. Accessed 24 Apr 2023.
Shingrix. Summary of Product Characteristics. https://www.ema.europa.eu/en/medicines/human/EPAR/shingrix. Accessed 24 Apr 2023.
Kovac M, Lal H, Cunningham AL, et al. Complications of herpes zoster in immunocompetent older adults: incidence in vaccine and placebo groups in two large phase 3 trials. Vaccine. 2018;36(12):1537–41.
Strezova A, Diez-Domingo J, Al Shawafi K, et al. Long-term protection against herpes zoster by the adjuvanted recombinant zoster vaccine: interim efficacy, immunogenicity, and safety results up to 10 years after initial vaccination. Open Forum Infect Dis. 2022;9(10):ofac485.
Bastidas A, de la Serna J, El Idrissi M, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322(2):123–33.
Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988–1000.
Mbinta JF, Nguyen BP, Awuni PMA, Paynter J, Simpson CR. Post-licensure zoster vaccine effectiveness against herpes zoster and postherpetic neuralgia in older adults: a systematic review and meta-analysis. Lancet Healthy Longev. 2022;3(4):e263–75.
Tricco AC, Zarin W, Cardoso R, et al. Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta-analysis. BMJ. 2018;363:k4029.
Klein NP, Bartlett J, Fireman B, et al. Effectiveness of the live zoster vaccine during the 10 years following vaccination: real world cohort study using electronic health records. BMJ. 2023;383:e076321.
Izurieta HS, Wu X, Forshee R, et al. Recombinant zoster vaccine (Shingrix): real-world effectiveness in the first 2 years post-licensure. Clin Infect Dis. 2021;73(6):941–8.
Lu A, Sun Y, Porco TC, Arnold BF, Acharya NR. Effectiveness of the recombinant zoster vaccine for herpes zoster ophthalmicus in the United States. Ophthalmology. 2021;128(12):1699–707.
Sun Y, Jackson K, Dalmon CA, et al. Effectiveness of the recombinant zoster vaccine among Kaiser Permanente Hawaii enrollees aged 50 and older: a retrospective cohort study. Vaccine. 2021;39(29):3974–82.
Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103–8.
Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(3):80–4.
Australian Technical Advisory Group on Immunisation (ATAGI). Clinical Advice. Version 2.1 Issue date 26 April 2022. Statement on the clinical use of sozter vaccines in adults in Australia. https://www.health.gov.au/sites/default/files/documents/2022/05/statement-on-the-clinical-use-of-zoster-vaccine-in-older-adults-in-australia-statement-on-the-clinical-use-of-zoster-vaccine-in-older-adults-in-australia.pdf. Accessed 5 Dec 2023.
Government of Canada. Herpes zoster (shingles) vaccine: Canadian Immunization Guide. Public Health Agency of Canada. Date modified 2022–01–20. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-8-herpes-zoster-(shingles)-vaccine.html#a5. Accessed 15 May 2023.
Siedler A, Koch J, Garbe E, et al. Background paper to the decision to recommend the vaccination with the inactivated herpes zoster subunit vaccine: statement of the German Standing Committee on Vaccination (STIKO) at the Robert Koch Institute. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2019;62(3):352–76.
American Academy of Ophthalmology. Policy statement. Recommendations for herpes zoster vaccine for patients 50 years of age and older. Revised June 2018. https://www.aao.org/education/clinical-statement/recommendations-herpes-zoster-vaccine-patients-50-#:~:text=Ophthalmologists%20should%20recommend%20strongly%20that,recommend%20vaccination%20strongly%20against%20herpes. Accessed 10 Mar 2024.
Hwang CW Jr., Steigleman WA, Saucedo-Sanchez E, Tuli SS. Reactivation of herpes zoster keratitis in an adult after varicella zoster vaccination. Cornea. 2013;32(4):508–9.
Jastrzebski A, Brownstein S, Ziai S, Saleh S, Lam K, Jackson WB. Reactivation of herpes zoster keratitis with corneal perforation after zoster vaccination. Cornea. 2017;36(6):740–2.
Altukhaim F, Mutlaq M, Alghamdi M, Hakami S. Reactivation of herpes zoster after recombinant vaccine (Shingrix): a case report. Cureus. 2023;15(1):e34431.
Richards PJ, Wingelaar MJ, Armbrust KR, Kopplin LJ. Uveitis reactivation following recombinant zoster vaccination. Am J Ophthalmol Case Rep. 2021;23:101115.
Walia A, Sun Y, Acharya NR. Risk of herpes zoster ophthalmicus recurrence after recombinant zoster vaccination. JAMA Ophthalmol. 2024;142(3):249–56.
Litt J, Cunningham T, Van Buynder P. Update on herpes zoster. Healthed expert monograph, issue 18. 2018. https://www.healthed.com.au/wp-content/uploads/2018/01/Monograph-No-18-Final-Updated.pdf. Accessed 2 Aug 2023.
Litt JCB, Kim S, Woodman R, MacIntyre R, Cunningham T. Australian zoster study: GP and patient views about herpes zoster (shingles), its complications, and the likely acceptance of a zoster vaccine (Zostavax). Int J Infect Dis. 2014;21:436–7.
Litt J, Booy R, Bourke D, et al. Early impact of the Australian national shingles vaccination program with the herpes zoster live attenuated vaccine. Hum Vaccin Immunother. 2020;16(12):3081–9.
Litt J, Cunningham AL. Herpes zoster. Improving protection in older people. Med Today. 2019;20(2 Suppl):16–22.
Institut national d’excellence en santé et en services sociaux Québec. Herpes zoster ophthalmicus. https://www.inesss.qc.ca/fileadmin/doc/INESSS/Outils/GUO/Zona/Guide_ZonaOphtalmique_web_EN_VF.pdf. Accessed 5 Mar 2024.
Cohen EJ. Management and prevention of herpes zoster ocular disease. Cornea. 2015;34(Suppl 10):S3–8.
Kahloun R, Attia S, Jelliti B, et al. Ocular involvement and visual outcome of herpes zoster ophthalmicus: review of 45 patients from Tunisia. North Africa J Ophthalmic Inflamm Infect. 2014;4:25.
Zaal MJ, Völker-Dieben HJ, D’Amaro J. Visual prognosis in immunocompetent patients with herpes zoster ophthalmicus. Acta Ophthalmol Scand. 2003;81(3):216–20.
Medical Writing Assistance
Medical writing support was provided by Blair Jarvis, a contract writer working on behalf of Luna, OPEN Health Communications, funded by GSK, in accordance with Good Publication Practice 3 (GPP) guidelines (www.ismpp.org/gpp-2022).
Funding
This article and the Rapid Service Fee was supported by GSK.
Author information
Authors and Affiliations
Contributions
John Litt, Anthony L. Cunningham, Francisco Arnalich-Montiel, and Raunak Parikh all contributed to the literature search, analysis, and review strategy; and all reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
John Litt has received payment and/or honoraria from GSK, Merck, Pfizer, Sanofi, and Seqirus, and served as a member of the Immunisation Coalition Scientific Advisory Committee. Anthony L. Cunningham’s institution has received payment and/or honoraria from AbbVie, Curevo, GSK, HealthEd, Merck, Moderna, and Seqirus. Francisco Arnalich-Montiel has no conflicts of interest to disclose. Raunak Parikh is an employee of GSK and holds stock or stock options.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Litt, J., Cunningham, A.L., Arnalich-Montiel, F. et al. Herpes Zoster Ophthalmicus: Presentation, Complications, Treatment, and Prevention. Infect Dis Ther (2024). https://doi.org/10.1007/s40121-024-00990-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40121-024-00990-7