Abstract
Human papillomavirus (HPV) is a common sexually transmitted virus that can cause cervical cancer and other diseases. Dynamic transmission models (DTMs) have been developed to evaluate the health and economic impacts of HPV vaccination. These models typically include many parameters, such as natural history of the disease, transmission, demographic, behavioral, and screening. To ensure the accuracy of DTM projections, it is important to parameterize them with the best available evidence. This study aimed to identify and synthesize data needed to parametrize DTMs on the natural history of HPV infection and related diseases. Parameters describing data of interest were grouped by their anatomical location (genital warts, recurrent respiratory papillomatosis, and cervical, anal, vaginal, vulvar, head and neck, and penile cancers), and natural history (progression, regression, death, cure, recurrence, detection), and were identified through a systematic literature review (SLR) and complementary targeted literature reviews (TLRs). The extracted data were then synthesized by pooling parameter values across publications, and summarized using the range of values across studies reporting each parameter and the median value from the most relevant study. Data were extracted and synthesized from 223 studies identified in the SLR and TLRs. Parameters frequently reported pertained to cervical cancer outcomes, while data for other anatomical locations were less available. The synthesis of the data provides a large volume of parameter values to inform HPV DTMs, such as annual progression rates from cervical intraepithelial neoplasia (CIN) 1 to CIN 2+ (median of highest quality estimate 0.0836), CIN 2 to CIN 3+ (0.0418), carcinoma in situ (CIS) 2 to local cancer+ (0.0396), and regional to distant cancer (0.0474). Our findings suggest that while there is a large body of evidence on cervical cancer, parameter values featured substantial heterogeneity across studies, and further studies are needed to better parametrize the non-cervical components of HPV DTMs.
Similar content being viewed by others
Using up-to-date data to parametrize dynamic transmission models (DTMs) that evaluate the health and economic impact of human papillomavirus (HPV) vaccination is important to ensure accuracy and relevancy of models’ predictions. |
Through a series of literature reviews, this study identified and synthesized data related to the natural history of HPV and its related diseases that can be useful to parametrize a wide range of HPV population models, including DTMs. |
Most of the data collected pertained to parameters on cervical disease outcomes, while data for other anatomical locations were less available in the literature. |
Limited non-cervical data and the variability of the available data suggest the need for more studies that generate and report evidence that is conducive to informing the rates at which individuals transition through HPV-related disease stages. |
Introduction
Human papillomavirus (HPV) is the most common sexually transmitted virus worldwide [1], as well as in the USA [2]. HPV accounts for an estimated 5% of cancers worldwide and is associated with substantial clinical and economic burden [3]. It is the cause of nearly all cervical cancers, as well as a common cause of vulvar and vaginal cancers in women, penile cancer in men, and anal and head and neck (H&N) cancers in both sexes, and results from infection with genotypes such as 16/18/31/33/45/52/58 [2]. Additionally, HPV genotypes 6/11 have been associated with up to 5% of penile cancers [4], laryngeal cancers [5,6,7], and are a cause of genital warts and recurrent respiratory papillomatosis (RRP) [2]. The HPV vaccine has been shown to be highly effective in preventing HPV-related disease and cancers, as it offers direct protection through vaccine-derived immunity, and indirect protection through herd immunity [8].
Dynamic transmission models (DTMs) can provide valuable information for regulators and policy makers, as they can be used to assess the long-term population-level health impact of HPV vaccines, and the cost-effectiveness of different HPV vaccination protocols [8,9,10,11,12,13,14,15]. The usefulness and accuracy of model predictions hinges crucially on the values assigned to the parameters that determine how individuals flow through the different model states. Thus, it is important to parametrize these models with the best available evidence.
However, this task presents several challenges. First, these models try to represent reality as closely as possible, and as a result they tend to be structurally large, with hundreds of parameters characterizing clinical, behavioral, and economic aspects of HPV infection and related diseases, as well as vaccine properties. Second, the nature of many of these parameters is such that both clinical and real-world studies (RWS) do not usually collect the necessary data to estimate them, and even when the data are collected, it is often reported in an aggregate way that cannot be directly used to inform the models. For instance, a common parameter in most HPV DTMs is the rate of progression to either pre-cancer or disease (e.g., RRP), which describes the rate at which individuals progress from the time they are infected with HPV to the time they develop pre-cancer or disease. However, when reporting the number of cases per person-time at risk in the treatment and control arms, randomized clinical studies typically include the time when individuals were HPV naïve in the denominator to preserve randomization, and not only the time since HPV infection (which is the relevant time to measure progression from HPV to pre-cancerous stages). Using this approach, the denominators (per-year at risk of transitioning from HPV infection to an HPV-related endpoint) used to derive parameter rates are likely overestimated, and thus the rates may be underestimated. In this study, we aimed to address these challenges by conducting comprehensive literature reviews to identify aggregate data that can be directly or indirectly (via derivations) used to estimate model parameters.
The reference model to establish the parameters of interest for the literature reviews conducted herein was a previously published DTM by Daniels et al. [13]. This instantiation of the model evaluated the health impact and cost-effectiveness of expanded catch-up HPV vaccination in women below 45 years of age, comprehensively including hundreds of parameters that are highly relevant to other HPV vaccine DTMs. In addition, previous versions of the model assessed the implementation of the quadrivalent HPV vaccine in girls. The model has subsequently been updated to consider all HPV-related diseases, as well as the implementation of the more recently approved nonavalent vaccine [8, 14], including a recent assessment of the cost-effectiveness of a one-dose nonavalent vaccination program in the UK [14]. While some of the parameter values that are included in any given model will be country specific (e.g., screening rates for cervical cancer), this model includes parameter definitions that are applicable to a wide range of HPV vaccine DTMs. Therefore, this model served as a reference to ensure that this literature review included the parameters that are most commonly present in HPV-related DTMs.
The set of parameters of interest can be broadly divided into two categories: (1) HPV vaccine efficacy and effectiveness, and (2) HPV natural history. Previous studies have collected evidence for the first category [16, 17]; thus, the current study focuses on identifying model parameter values in the second category, which include the disease’s natural history (e.g., progression and regression rates) and screening (e.g., performance of detection tests) components of HPV vaccine DTMs. This study focuses on identifying parameter values from high-quality studies that can be used in a wide range of models and, therefore, is agnostic to geographical location (e.g., countries, institutions) and the studied population in which the data were collected.
The objectives of this study were to identify and to synthesize data from existing publications with relevant and up-to-date information on the natural history of HPV infection and HPV-related diseases, in order to parametrize HPV vaccine DTMs.
Methods
Study Design
This study was designed to identify the most current input data for parameters describing the natural history of HPV and HPV-related diseases in HPV vaccine DTMs. Relevant model parameters were grouped by their anatomical location (i.e., genital warts, RRP, and cervical, anal, vaginal, vulvar, H&N, penile cancers) and natural history conceptual similarity (i.e., progression, regression, death, cure, recurrence, detection). On the basis of clinical and data availability considerations, parameters were then divided into those to be identified through an SLR (i.e., parameters for which well-crafted electronic search strategies were expected to yield a set of publications with a high percentage of relevant articles, such that identified publications could be efficiently screened) and parameters that could be more effectively identified through targeted literature reviews (TLRs).
This study comprehensively covered the available literature on HPV natural history and included both randomized controlled trials (RCTs) and RWS. Consistent with previous SLRs for vaccine efficacy parameters in HPV, the study prioritized the extraction of the most up-to-date data by considering articles published from 2008 and onwards. Data from both SLR- and TLR-identified studies were extracted and synthesized (overall and by sex, age group, and HPV genotype) in a manner conducive to parametrizing HPV vaccination DTMs.
SLR parameters The SLR was conducted to identify studies reporting information relevant to the model parameters characterizing the natural history of HPV infection and HPV-related diseases. Cervical, anal, vaginal, vulvar, head and neck, and penile cancers, as well as genital warts and RRP were considered.
TLR parameters The TLR was conducted to identify studies reporting information on the following parameters: HPV transmission, recovery, reactivation, HPV-vaccine-related waning rates, degree of protection against subsequent infection, relative risk of breakthrough infections, and screening rates of HPV-related diseases.
Inclusion and Exclusion Criteria
SLR The standard Population, Intervention, Comparison, Time, and Study design (PICOTS) elements, as described in the Cochrane Handbook, were used to define the inclusion and exclusion criteria (see Supplement Table 1) [18]. The systematic literature search was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Cochrane review guidelines [19].
All studies that met the following criteria were included in the SLR:
-
Included individuals ≥ 9 years of age (except for RRP, which had no age restriction) that had been infected with HPV as of this study’s baseline
-
Reported data relevant to the model parameters characterizing the natural history of HPV infection and-related diseases. Cervical, anal, vaginal, vulvar, head and neck, and penile cancers, as well as genital warts and RRP were considered HPV-related diseases. Specifically, studies had to report data relevant to at least one of the following six sets of parameters:
-
Progression and regression rates of the different disease stages for each of the HPV-related diseases
-
Progression and breakthrough infection in vaccinated individuals of the different disease stages for each of the HPV-related diseases
-
Death rates for HPV-related endpoints
-
Cure rates for HPV-related endpoints
-
Recurrence rates for HPV-related endpoints
-
Performance of detection tests for HPV-related endpoints
-
-
Were RCTs, RWSs, reviews, meta-analyses, or epidemiological models
-
Published in English language
The SLR was supplemented with manual searches to identify additional studies reporting parameters of interest (i.e., regression, progression, and death rates) in non-cervical anatomical locations (e.g., anal, vaginal, vulvar, head and neck, penile, and warts) for which no data were found in the SLR. For these supplemental searches, the Population criterion was relaxed to include studies in which individuals were not required to be infected with HPV at baseline.
Studies that did not report data for individuals that were HPV-positive at the start of the study or at the time of the outcome measurement or were among individuals younger than 9 years old (except for RRP) were excluded from the SLR. Case reports, letters, guidelines, conference proceedings, and animal and cell studies were also excluded from the SLR.
TLR The TLR included studies that met the following criteria:
-
Reported data relevant to the model parameters characterizing the natural history of HPV and HPV-related diseases. Cervical, anal, vaginal, vulvar, head and neck, and penile cancers, as well as genital warts and RRP were considered HPV-related diseases in the scope of the TLR. The following sets of parameters were included as endpoints:
-
HPV transmission, recovery, reactivation, and waning rates
-
Degree of protection against subsequent infection
-
Relative risk of breakthrough infections
-
Screening rates of HPV-related diseases and performance of detection tests
-
-
Were RCTs, RWSs, reviews, or meta-analyses, or epidemiological models
-
Published in English language
Case reports, letters, guidelines, conference proceedings, and animal and cell studies were excluded from the TLR.
Data Sources
SLR OvidSP was used to identify relevant articles from the following databases: MEDLINE, MEDLINE (R) In-Process & Other Citations, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR). Supplemental manual searches were conducted using PubMed.
Search strategy The SLR search strategy is included in Supplement Table 2. The records in these tables correspond to searches conducted on January 19, 2021.
Study selection All studies were screened using the predefined inclusion and exclusion criteria summarized in Sect. “Inclusion and Exclusion Criteria”.
Level I: screening of titles and abstracts for eligibility Titles and abstracts of all studies identified via the database search were reviewed for relevance to the measures of interest. Titles and abstracts were reviewed manually by two independent experienced reviewers. For all studies meeting the eligibility criteria after screening titles and abstracts, full texts were obtained.
Level II: screening of full texts for eligibility Full-text articles were reviewed to determine relevance based on the same inclusion and exclusion criteria used in the level I screening. Studies that met any exclusion criteria were removed, and the reason for exclusion was recorded. Studies that satisfied the eligibility criteria after full-text screening were selected for data extraction.
A PRISMA diagram was produced to describe the study selection process, reasons for exclusion per level of screening, the list of articles selected for inclusion or exclusion, and associated reasons for excluded articles only after two levels of screening [19].
TLR PubMed was used to identify relevant articles in the TLR [20]. In addition, the TLR was supplemented with relevant references cited in Daniels et al. [13] as well as manual searches on Google Scholar for parameters not identified through PubMed.
Search strategy The structure of the search strategy comprised search strings aimed at identifying studies that reported the outcomes included in the TLR. Supplement Table 3 describes the detailed search strategies used in the TLR. For each term of the TLR search strategy, the top 20 most relevant references according to PubMed were retrieved for further screening and selection. In total, 709 articles were retrieved using the TLR search strategy.
Study selection Studies were selected according to the process described in Supplement 4. A diagram was produced to describe the TLR study selection process for data extraction.
Data Elements
SLR outcomes The outcomes of interest included in the SLR related to the natural history of HPV-related diseases. Cervical, anal, vaginal, vulvar, head and neck, and penile cancers, as well as genital warts and RRP were considered. Supplement Table 5 provides the full description of the outcomes of interest included in the SLR.
TLR outcomes Supplement Table 6 provides the full description of the outcomes of interest included in the TLR.
Data Extraction
Data extraction prioritized studies published on or after 2008Footnote 1 (to focus on recent studies with more up-to-date data since the publication of the original DTM in 2007); reported original data (i.e., data from literature reviews and model-based publications were not extracted); and had more than 30 HPV-positive individuals, in order to increase the reliability of model parameter values.Footnote 2
Data were extracted on study details (e.g., study type, design, follow-up time, geographic region, and HPV-related disease), population characteristics (e.g., age group, sex, HPV genotype, and sample size of HPV population), as well as HPV-related outcomes. Where applicable, multiple data points in the same study for the same HPV-related endpoint (due to, for example, different treatment arms, age group, sex, HPV genotype, or other study-defined stratification) were also extracted.
For the outcomes defined as rates (e.g., progression rates), data were directly extracted from the study if explicitly reported as rates (e.g., rate per person-year). Alternatively, a derived annual rate was calculated for studies that instead reported the proportion or number of individuals transitioning between the relevant health states and the period of transition. The derived annual rate was calculated assuming that the length of time individuals spent in each health state was exponentially distributed (see Supplement 7).
Performance of detection tests for HPV-related endpoints were extracted as sensitivity, specificity, positive predictive value, and negative predictive value, as available in each study.
Data Synthesis
All studies from which data were extracted were included in the data synthesis. The SLR- and TLR-extracted outcomes were synthesized jointly. Outcomes were synthesized overall across all studies, as well as stratified by sex, age group, and HPV genotype. The following synthesis elements were reported for each outcome: estimates of the highest quality study, median of the estimates of the highest quality study (when these reported multiple values for the same parameter), highest quality study ID, and range of all extracted estimates.
The highest quality study was determined on the basis of the following decision rules:
-
1.
RWS or RCT: selected RCT if available
-
2.
Sample size: selected largest sample size
-
3.
Year of publication: selected most recent study
-
4.
HPV genotypes: selected study with highest number of genotypes included (e.g., HPV 6/11/16/18 preferred over HPV 16/18)
-
5.
Age group: selected broadest age range, or the youngest range was selected if all ranges were equivalent in lengthFootnote 3
For each parameter, the values for the highest quality study were identified. The decision tree was applied at the study level. Therefore, for studies selected on the basis of the above decision tree that reported multiple values for the same parameter (e.g., due to reporting data from multiple treatment arms, multiple sexes, multiple HPV genotypes), all the reported parameters were included as the highest quality values in the data synthesis. The data synthesis file in Supplement Table 8 provides further detail on the differences between the multiple values.
The synthesis of parameter values was based on a summary of results from all studies for which data were extracted. Resulting ranges combined studies with different study designs and statistical methodologies. The median of the multiple values was computed and reported to provide a single point estimate for high quality studies that reported multiple values for the same parameter.
Data Stratification
Data were synthesized for all studies combined, and were also stratified on the basis of sex, age group, and HPV genotype. The same decision tree described above was reapplied to obtain the highest quality values for each of the stratifications. The following detailed stratifications were included in the data synthesis file in Supplement Table 8:
-
Sex:
-
Female only
-
Male only
-
Male and female—studies that reported data including both male and female
-
Unspecified—studies that did not specify the sex of the study population
-
-
Age:
-
Adults (≥ 26 years old)Footnote 4
-
Young adults (< 26 years old)
-
Young adults (< 26 years old) and adults (≥ 26 years old)—studies that included individuals with ages that ranged from < 26 years old to > 26 years old
-
Unspecified—studies that did not specify the age range of the study population
-
-
HPV genotype (see Supplement 9 for details)
Data synthesis was conducted using R Statistical Software (v4.0.4) [21].
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Identified Studies
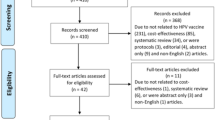
The SLR search strategy yielded a total of 4551 records considered for screening. Of these, 532 studies were included after title/abstract (level 1) screening and 239 studies were included after full-text (level 2) screening. Of these studies, data were extracted from 150 studies according to the prioritization criteria (study published in 2008 or later, reported original data, and included at least 30 HPV-positive individuals) described in Sect. “Data Extraction”. In addition, the supplemental manual searches identified 43 studies reporting parameters of interest (regression, progression, and death rates) in non-cervical anatomical locations (e.g., anal, vaginal, vulvar, head and neck, penile, and warts). In total, the SLR identified 193 studies for data extraction. Figure 1 presents the PRISMA diagram of the selected publications.
PRISMA diagram of the selection of publications in the systematic literature review (SLR). The SLR search strategy yielded a total of 4551 records considered for screening, and 239 studies were included after full-text (level 2) screening. Data were extracted from 150 studies according to the prioritization (study published in 2008 or later, reported original data, and included at least 30 human papillomavirus [HPV]-positive individuals). In addition, supplemental manual searches identified 43 studies reporting parameters of interest in non-cervical anatomical locations (e.g., anal, vaginal, vulvar, head and neck, penile, and warts), leading to a total of 193 studies identified for data extraction. HPV human papillomavirus, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, SLR systematic literature review
The TLR search strategy yielded a total of 709 records considered for selection. Of those, 297 studies were included for title and abstract screening based on the top five most relevant parameters or the cosine similarity between the title/abstract and the TLR parameters. After title and abstract screening, 227 studies were kept, and 26 studies were selected for data extraction after full-text screening. In addition, 23 articles from manual searches on Google Scholar were reviewed, and 4 were selected for data extraction. In total, the TLR identified and selected 30 articles for data extraction and analysis. Figure 2 presents a diagram describing the studies selected for data extraction in the TLR.
Description of the selection of publications in the targeted literature review (TLR). The TLR search strategy yielded 709 records. Of those, 297 studies were included for title and abstract screening, and 26 studies were selected for data extraction after full-text screening. Four additional articles from manual searches on Google Scholar were selected for data extraction, leading to a total of 30 articles for data extraction and analysis. TLR targeted literature review
In total, the SLR and the TLR yielded 223 articles for data extraction, 193 from the SLR, and 30 from the TLR (see Supplement Table 10 for a list of the references selected for data extraction). The selected articles were combined for the purposes of data synthesis.
Characterization of Available Data
Overall, at least one data point was extracted for 96 parameters. Most of the parameters with extracted data pertained to cervical abnormalities. For instance, 12 studies reported regression rates from cervical intraepithelial neoplasia (CIN) 1+ to Normal or HPV, 6 studies from CIN 2+ to Normal or HPV, and 1 study from CIN 3+ to Normal or HPV. Progression rates from CIN 1 to CIN 2+ were found in 14 studies, and data on progression from CIN 2 to CIN 3+ were found in 4 studies.
Death rates were more commonly reported for local cancers, with 26 studies reporting the death rate of local+ H&N cancer, 9 studies reporting the death rate for local+ cervical cancer, 2 for local+ anal cancer and vaginal cancer, and one study each reporting the death rate of local+ vulvar and penile cancer.Footnote 5 Cure rates were more commonly reported for CIN, with 5 studies reporting cure rates of CIN 1+, 4 studies of CIN 2+, and 1 study of CIN 3+. Moreover, the cure rates of H&N cancers were also more commonly reported (10 for local+, 3 for regional+, and 5 for distant H&N cancers) than other HPV-related diseases.
Except for 2 studies that reported the recurrence rate of vulval intraepithelial neoplasia (VIN) 1+, recurrence rates were found only for CIN 1+ (7 studies), CIN 2+ (8 studies), and CIN 3+ (7 studies). Performance of detection tests was found for cervical complications only, with 3 studies reporting at least one measure of performance (i.e., positive predictive value [PPV], negative predictive value [NPV], sensitivity, specificity) of CIN 1+, 21 studies reporting for CIN 2+, 13 studies for CIN 3+, 1 study for carcinoma in situ (CIS)+, and 1 study for local+ cancer.
Regarding warts and RRP, 32 studies reported at least one parameter. The most commonly reported parameters were cure rates of warts and RRP (22 studies), and recurrence rate of warts and RRP (13 studies).
For the parameters on HPV transmission, recovery, reactivation, and waning, 19 studies reported at least one of these parameters. The recovery of HPV infection was the most reported (9 studies).
Data Synthesis
A visual summary of the data synthesis results is provided in Figs. 3, 4, 5, and 6. For each parameter, these figures included a blue dot with the value, or the median of values reported in the highest quality study (with the highest quality study selected according to the decision tree in Sect. “Data Synthesis”), and in orange, all the values extracted for said parameter to depict the range of parameters values reported in the literature. For each parameter, data is presented for all studies combined, as well as stratified on the basis of age group. For the purposes of visualization, the parameters from the highest quality study shown in the age stratification of Figs. 3, 4, 5, and 6 belong to the highest quality study from all data extracted (“all studies”) for each parameter. In addition, Tables 1, 2, 3, 4, 5, 6, 7, 8, and 9 provide the median values and ranges of the highest quality studies of each parameter captured in the literature. Additional details regarding the data synthesis results are in Supplement Table 8, which also includes the synthesis stratified by the sex and HPV genotype.
Summary of data synthesis for parameter annual rates in cervical diseases. The parameters extracted as rates for cervical diseases (e.g., progression rates) are shown using a logarithmically transformed scale (using log10 where Inf. is abbreviated for parameter values with an infinite upper bound) to enhance the visual differentiation of estimates clustered around low values, and for plotting a wide range of values. For each parameter, the value, or the median of values, reported in the highest quality study is shown in blue, and all values extracted for the parameter are in orange. Each study’s population sample size is denoted using the size of the data point. A circle was used to denote data from clinical trials, and a diamond was used to denote data from real-world studies. Data is presented for all studies combined, as well as stratified on the basis of age group. The “Other” age group includes parameters defined for populations whose age range spanned < 26 years through 26+ years as well as studies reporting unspecified age ranges. The parameters from the highest quality study shown in the age stratification belong to the highest quality study from all data extracted (“all studies”) for each parameter. CIN cervical intraepithelial neoplasia, CIS carcinoma in situ, HPV human papillomavirus
Summary of data synthesis for parameter annual rates in head and neck (H&N), anal, penile, vaginal, and vulvar diseases. The parameters extracted as rates for H&N, anal, penile, vaginal, and vulvar disease are shown using a logarithmically transformed scale (using log10 where Inf. is abbreviated for parameter values with an infinite upper bound) to enhance the visual differentiation of estimates clustered around low values, and for plotting a wide range of values. For each parameter, the value, or the median of values, reported in the highest quality study is shown in blue, and all values extracted for the parameter are in orange. Each study’s population sample size is denoted using the size of the data point. A circle was used to denote data from clinical trials, and a diamond was used to denote data from real-world studies. CIS carcinoma in situ, HPV human papillomavirus, H&N head and neck, IEN intraepithelial neoplasia
Summary of data synthesis for parameter annual rates in warts and recurrent respiratory papillomatosis (RRP). The parameters extracted as rates for warts and RRP are shown using a logarithmically transformed scale (using log10 where Inf. is abbreviated for parameter values with an infinite upper bound) to enhance the visual differentiation of estimates clustered around low values, and for plotting a wide range of values. For each parameter, the value, or the median of values, reported in the highest quality study is shown in blue, and all values extracted for the parameter are in orange. Each study’s population sample size is denoted using the size of the data point. A circle was used to denote data from clinical trials, and a diamond was used to denote data from real-world studies. HPV human papillomavirus, RRP recurrent respiratory papillomatosis
Summary of data synthesis for performance of detection tests of cervical disease parameters. The performance of detection tests (i.e., positive predictive value [PPV], negative predictive value [NPV], sensitivity, and specificity) related to cervical complications parameters are reported using percentages (0–100%). For each parameter, the value, or the median of values, reported in the highest quality study is shown in blue, and all values extracted for the parameter are in orange. Each study’s population sample size is denoted using the size of the data point. A circle was used to denote data from clinical trials, and a diamond was used to denote data from real-world studies. Data is presented for all studies combined, as well as stratified on the basis of the available age groups. The “Other” age group includes parameters defined for populations whose age range spanned < 26 years through 26+ years as well as studies reporting unspecified age ranges. The parameters from the highest quality study shown in the age stratification belong to the highest quality study from all data extracted (“all studies”) for each parameter. CIN cervical intraepithelial neoplasia, CIS carcinoma in situ, NPV negative predictive value, PPV positive predictive value
Cervical cancer Figure 3 summarizes the parameters extracted as rates for cervical cancer (e.g., progression rates). A logarithmically transformed scale was used for visualization purposes to enhance the visual differentiation of estimates clustered around low values and allow for plotting a wide range of values. The left panel shows the results for all studies with available information in each parameter, and the remaining panels separate the parameter values by age subgroup (the “Other” age group includes parameters defined for populations whose age range spanned < 26 years through 26+ years as well as studies that did not report age). The median value for parameters reported in highest quality study, based on all studies, is in blue, while the values for all studies are in orange. Furthermore, the figure also provides a distinction between clinical trials (using a circle) from RWS (using a diamond), and the sample size of each study is proportional to the size of the circles and diamonds. For each parameter, the range of values is shown as a line using all extracted data. Table 1 provides the median value and the corresponding ranges of the highest quality studies (for all studies) for each parameter extracted as annual rates for cervical cancer.
Overall, Fig. 3 displays a wide range of values for most rates related to cervical cancer, both across studies, but also within the highest quality study. Focusing on all studies combined, data from the highest quality studies (Table 1) show rates of progression from CIN 1 to CIN 2+ (median of highest quality estimate 0.0836), CIN 2 to CIN 3+ (median 0.0418), CIS 2 to local cancer+ (median 0.0396), and regional to distant cancer (median 0.0474). For persistently infected individuals, the synthesis shows progression rates from HPV to CIN 1+ (median 0.0414), and lower rates to CIN 2+ (median 0.0240) and CIN 3+ (median 0.0089). For transiently infected individuals, the synthesis shows progression rates from HPV to CIN 1+ (median 0.0323), and lower rates to CIN 2+ (median 0.0182) and CIN 3+ (median 0.0064). For once-vaccinated individuals with breakthrough transient infections, the synthesis shows progression rates from HPV to CIN 1+ (median 0.0440), CIN 2+ (median 0.0245), and CIN 3+ (median 0.0615).
Annual regression rates were synthesized as regression to HPV/normal from CIN 1+ (median 0.6106), CIN 2+ (median 0.2513), and CIN 3+ (median 0.000), as well as from CIN 2+ to CIN 1 (median 0.3364) and from CIN 3+ to CIN 1 (median 0.2245). In addition, Table 1 shows recurrence rates of CIN 1+ (median 0.0078), CIN 2+ (median 0.0040), and CIN 3+ (median 0.0626).
The bottom two panels of Fig. 3 display the annual death rates and cure rates of cervical cancers. Death rates of local (median 0.0548) and regional cervical cancer (median 0.0451) were synthesized. The synthesis also shows cure rates of CIN 1+ (median 11.0011), and lower rates of CIN 2+ (median 5.6860), CIN 3+ (median 0.4034), and treated local cervical cancer (median 0.0793).
H&N, anal, penile, vaginal, and vulvar cancers Figure 4 summarizes the parameters extracted as annual rates for H&N, anal, penile, vaginal, and vulvar cancers, in a logarithmically transformed scale. Each panel corresponds to one HPV-related disease. As a result of the limited information available in the literature, no age stratification is shown. All other visual elements shown are as described above. The figure suggests that parameters for these types of cancer are not as well reported in the literature. Tables 2, 3, 4, 5, and 6 provide the corresponding median values for the highest quality studies, where each table refers to each of the non-cervical related cancers.
Progression rates for all studies combined were synthesized using the highest quality study for anal intraepithelial neoplasia (AIN) 1 to AIN 2/3+ (median of highest quality study 0.4542), and vaginal intraepithelial neoplasia (VAIN) 1 to VAIN 2/3+ (median 0.0317). Furthermore, progression rates were available for CIS 2 to local cancer (median value for anal 0.0093, vaginal 0.0184, vulvar 0.0102, penile 0.0044), local to regional cancer (median value for anal 0.1107, vaginal 0.1308, H&N 0.0325, penile 0.0581), and regional to distant cancer (median value for anal 0.0269, vaginal 0.0657, H&N 0.0320, penile 0.4385). Recurrence rates were synthesized for VIN 1 + (median 0.0636).
Death rates were available and synthesized for local+ H&N (median 0.0202), anal (median 0.1090), penile (median 0.1205), vaginal (median 0.0729), and vulvar (median 0.0397) cancers, as well as regional+ H&N cancer (median 0.0764) and distant H&N (median 0.0347), regional+ vaginal cancer (median 0.1174) and distant+ vaginal cancer (median 0.2983), and distant+ anal (median 0.1683) cancer.
Cure rates were synthesized for VAIN 1+ (median 0.1915), VAIN 2+ (median 0.1962), VIN 1+ (median 0.9463), local+ (median 0.3006) and regional+ (median 0.4828) H&N cancer, and distant H&N (median 0.4231) and anal (median 0.3630) cancers.
Genital warts and RRP Figure 5 and Table 7 summarize the parameters extracted as rates for genital warts and RRP, shown in a logarithmically transformed scale in the figure. As a result of the limited information available in the literature, no age stratification is shown. The synthesis shows the progression rate from HPV to genital warts (median of the highest quality study 0.0087), the regression rates of symptomatic (median 4.9460) and asymptomatic genital warts (median 1.7080), as well as the recurrence (median 0.2897) and cure (median 3.0632) rates of genital warts and RRP, and the RRP-associated death rate (median 0.002).
Performance of detection tests Figure 6 shows the performance of detection tests (i.e., PPV, NPV, sensitivity, and specificity) related to cervical complications parameters, including both the synthesis for all studies with available datapoints, as well as the age subgroups described above. Parameter values are reported using percentages (0–100%). Estimates were primarily available for CIN 1+ (sensitivity highest quality estimate 57.4%; specificity highest quality median 97.1%), CIN 2+ (sensitivity median 78.0%; specificity median 71.4%), CIN 3+ (sensitivity median 80.6%; specificity median 77.2%), and local+ cervical cancer (sensitivity median 54.3%; specificity median 67.4%). Table 9 provides the medians of the highest quality studies for all parameters extracted on the performance of detection tests.
Other parameters Table 8 summarizes HPV transmission, recovery, reactivation, and waning parameters. The synthesis includes the transmission rate from men to women (median 0.0876) and women to men (median 0.1476), as well as the rate of recovery from HPV infection (median 0.4856), the rate of seroconversion following HPV clearance (median 2.8972), and the reactivation rate following seroconversion (median 0.0075).
Stratification of parameters by sex Non-sex-specific parameters for which at least one value was reported for both male and female patients were identified. These included cure rate for both treated and untreated genital warts and RRP (annual rate for male = 2.3364; annual rate for female = 3.4627) [22], rate of progression from HPV infection to genital warts (0.0013; 0.0087) [23, 24], and rate of regression from asymptomatic genital warts (1.3289; 2.1297) [22], in all cases indicating higher rates for female patients, although the sparsity of the available data makes it difficult to derive more definitive conclusions.
Discussion
In this study, a series of systematic and targeted searches of the literature were conducted to comprehensively identify relevant publications and synthesize information needed to parametrize HPV vaccine DTMs with the most current data related to the natural history of HPV infection and HPV-related diseases.
DTMs and other population-based models are increasingly critical to inform policy making and vaccination strategies for HPV vaccines [25, 26]. Infectious disease modelers rely on HPV natural history studies and other real-world studies to help parametrize the rates at which individuals transition between the health states of these models. For the reference model in this paper, we chose a previously published HPV DTM that included a comprehensive range of HPV vaccine targeted genotypes (6/11/16/18/31/33/45/52/58) and HPV-related diseases (cervical cancer and intraepithelial neoplasia, vaginal cancer, vulvar cancer, anal cancer, penile cancer, head and neck cancer, genital warts, and RRP) [13]. Therefore, in contrast to past reviews and meta-analyses of the natural history of HPV disease [27,28,29,30], this paper critically reviewed a broader range of parameters and gathered more comprehensive data for HPV DTMs and population-based models in general. Furthermore, to help synthesize the large volume and heterogenous nature of data extracted, a set of decision rules for each parameter was developed to identify the study most likely to contain the highest quality data based on features such as study type, sample size, age groups, and HPV genotypes.
This study used both an SLR and a TLR to identify the availability of hundreds of parameters used in HPV DTMs. In total, this study identified 4551 records in the SLR and 709 records in the TLR. Of these, 223 studies were selected for data extraction. To take full advantage of the data reported in the literature, the parameter values of interest were extracted either directly from the publications (when feasible) or were derived from related data reported in the publications (even when the underlying publications were not directly designed to inform population-based models). For instance, most studies report risk of event, e.g., progression or regression of disease, rather than a rate. However, DTMs and other population-based models typically use rates to describe individuals’ transitions between the model’s health states. Thus, similar to previous studies [31], we transformed risks (proportions) into rates assuming the risks follow an exponential distribution, thereby allowing us to provide parameter values that would not otherwise be available in the literature. Furthermore, to provide readily available parameter values that can be directly incorporated in HPV DTMs, the values were synthesized across publications, as well as stratified by sex, age group, and HPV genotype. Moreover, for parameters that were reported by more than one study, a study-quality assessment was also conducted to identify studies with the most reliable data for use in the DTMs of HPV vaccination, and the median value and the corresponding range for such studies were reported.
The results indicate that the most commonly reported parameters pertained to cervical cancer outcomes. In particular, the data showed a pattern of higher progression rates from HPV to CIN for lower CIN grades—a pattern that was consistent for both persistently and transiently HPV-infected individuals. Conversely, the regression rates of CIN to HPV were higher for lower grades of CIN. In addition, cure rates for cervical diseases decreased as disease progressed through CIN grades and local cancer.
The results also indicated that the data for parameters associated with anatomical locations other than cervical were scarcer. Consequently, the parametrization of HPV DTMs can largely rely on direct evidence/data for the cervical components of the model, whereas many of the parameter values characterizing the rates associated with non-cervical complications may need to be obtained by other means (e.g., estimated via calibration and/or assumptions), which may introduce limitations to the results of the DTMs. For example, the estimation of non-cervical parameters based on calibration can lead to parameter values that are dependent on model structures, particularly for complex DTMs that are typical in HPV modeling, and may therefore yield different parameter values for different models (i.e., nonidentifiability). Moreover, relying on assumptions, such as the equivalency of rates for non-cervical diseases that are unavailable in the literature to those of cervical ones, may be inadequate for some parameters, especially when the distribution of HPV genotypes is different for different cancers. Therefore, relying on these methods to parametrize some portions of DTMs may affect the accuracy of its health and cost-effectiveness predictions, and thus its public health and policy implications.
For most parameters, the values were found to vary substantially both across studies, and within the highest quality study reporting multiple values. The main drivers of this variability were the differences in the studied populations (e.g., different geographic regions and/or population subgroups), and treatment arms for which the parameter values were reported, as well as differences in parameter definitions across studies. This heterogeneity in parameter values underscores the importance of conducting sensitivity analyses to assess the impact of varying influential model parameters that feature high variability on model outcomes when conducting policy evaluations.
This study is subject to potential biases and/or limitations that are typical of most SLRs. For instance, the nature of the parameter definitions in DTMs or population-based models may differ or be more specific than what is reported in most studies. Consequently, data were not extracted in cases where some data related to a parameter of interest were available, but were insufficient to calculate/derive the parameter value (e.g., a study reported the proportion of individuals with complete cancer remission for a specific treatment, but it did not report the follow-up time to achieve remission). Furthermore, extracted estimates are subject to limitations in the data reported by those studies. Additionally, despite our best efforts to conduct thorough reviews, our search strategies may have missed some relevant publications. It should be noted that our synthesis of the data aimed to provide a qualitative description of the extracted data. It is not intended to replace more rigorous statistical analyses such as meta-analyses that can provide a quantitative description of the point estimates of each parameter of interest while accounting for variabilities within and across studies [32]. While the present study focused on comprehensively identifying and describing the broad range of parameter values related to the natural history of HPV infection and its related diseases, in future work, we are planning to conduct full statistical analyses of the present extracted data.
Conclusion
This literature review identified a large volume of data to inform the parametrization of HPV vaccine DTMs. Overall, our findings suggest that there is a large body of evidence to directly inform cervical cancer-related parameters, especially the progression between HPV and pre-cancer stages. Nevertheless, further studies are needed to help parametrize the non-cervical components of HPV DTMs or other HPV populations-based models.
Data availability
Data collected or analyzed during this study are reported in published articles, and are also included in this article or as Supplementary Material files. Further inquiries can be directed to the corresponding author.
Notes
This criterion was not applied in the supplemental manual searches for non-cervical studies.
This criterion was not applied in the supplemental manual searches for non-cervical studies.
For some studies, the age group was not a closed interval or range, but rather an open-ended interval as a range without one of the limits (e.g., age was reported as “larger than X” or “lower than Y”). To create a closed age range for these studies as to allow for the categorization of studies by the length of said range, the unreported limit was imputed with the 25th (if the lower limit was missing) or 75th (if the upper limit was missing) percentile of all extracted lower and upper limits for the purposes of study selection.
This age cutoff was determined on the basis of the Centers for Disease Control and Prevention (CDC) guidance, according to which HPV vaccination is recommended for everyone through age 26 years (https://www.cdc.gov/hpv/parents/vaccine-for-hpv.html).
The “+” suffix is used throughout the results to denote any set of subsequent states (e.g., CIN 1+ denotes CIN 1/2/3, and local+ cancer denotes local/regional/distant cancer).
References
Bruni L, Albero G, Rowley J, et al. Global and regional estimates of genital human papillomavirus prevalence among men: a systematic review and meta-analysis. Lancet Glob Health. 2023;11(9):e1345–62.
Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1–30.
Tota JE, Chevarie-Davis M, Richardson LA, Devries M, Franco EL. Epidemiology and burden of HPV infection and related diseases: implications for prevention strategies. Prev Med. 2011;53(Suppl 1):S12-21.
Alemany L, Cubilla A, Halec G, et al. Role of human papillomavirus in penile carcinomas worldwide. Eur Urol. 2016;69(5):953–61.
Sun J, Xiong J, Zhen Y, Chen ZL, Zhang H. P53 and PCNA is positively correlated with HPV infection in laryngeal epitheliopapillomatous lesions in patiets with different ethnic backgrounds in Xinjiang. Asian Pac J Cancer Prev. 2012;13(11):5439–44.
Weiss D, Heinkele T, Rudack C. Reliable detection of human papillomavirus in recurrent laryngeal papillomatosis and associated carcinoma of archival tissue. J Med Virol. 2015;87(5):860–70.
Lam EWH, Chan MMH, Wai CKC, et al. The role of human papillomavirus in laryngeal cancer in Southern China. J Med Virol. 2018;90(6):1150–9.
Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13(1):28–41.
Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–67.
Burger EA, Campos NG, Sy S, Regan C, Kim JJ. Health and economic benefits of single-dose HPV vaccination in a Gavi-eligible country. Vaccine. 2018;36(32 Pt A):4823–9.
Kim JJ. Could 1 dose be less efficacious than 2 doses but still be a great public health intervention? HPV World. https://www.hpvworld.com/media/29/media_section/0/5/1605/kim.pdf. Accessed 28 Aug 2023.
Drolet M, Laprise JF, Martin D, et al. Optimal human papillomavirus vaccination strategies to prevent cervical cancer in low-income and middle-income countries in the context of limited resources: a mathematical modelling analysis. Lancet Infect Dis. 2021;21(11):1598–610.
Daniels V, Prabhu VS, Palmer C, et al. Public health impact and cost-effectiveness of catch-up 9-valent HPV vaccination of individuals through age 45 years in the United States. Hum Vaccin Immunother. 2021;17(7):1943–51.
Daniels V, Saxena K, Patterson-Lomba O, et al. Modeling the health and economic implications of adopting a 1-dose 9-valent human papillomavirus vaccination regimen in a high-income country setting: an analysis in the United Kingdom. Vaccine. 2022;40(14):2173–83.
Rihan FA. Delay differential equations and applications to biology. Singapore: Springer; 2021.
Wang WV, Kothari S, Skufca J, et al. Real-world impact and effectiveness of the quadrivalent HPV vaccine: an updated systematic literature review. Expert Rev Vaccines. 2022;21(12):1799–817.
Goodman E, Reuschenbach M, Kaminski A, Ronnebaum S. Human papillomavirus vaccine impact and effectiveness in six high-risk populations: a systematic literature review. Vaccines (Basel). 2022;10(9):1543.
Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:142.
Moher D, Liberati A, Tetzlaff J, Altman D, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
PubMed. https://pubmed.ncbi.nlm.nih.gov/about/. Accessed 28 Aug 2023.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2021.
Tatti S, Stockfleth E, Beutner KR, et al. Polyphenon E: a new treatment for external anogenital warts. Br J Dermatol. 2010;162(1):176–84.
Anic GM, Lee JH, Stockwell H, et al. Incidence and human papillomavirus (HPV) type distribution of genital warts in a multinational cohort of men: the HPV in men study. J Infect Dis. 2011;204(12):1886–92.
Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–14.
Pitman R, Fisman D, Zaric GS, et al. Dynamic transmission modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-5. Value in Health. 2012;15(6):828–34.
Ng SS, Hutubessy R, Chaiyakunapruk N. Systematic review of cost-effectiveness studies of human papillomavirus (HPV) vaccination: 9-Valent vaccine, gender-neutral and multiple age cohort vaccination. Vaccine. 2018;36(19):2529–44.
Cantor SB, Atkinson EN, Cardenas-Turanzas M, Benedet JL, Follen M, MacAulay C. Natural history of cervical intraepithelial neoplasia: a meta-analysis. Acta Cytol. 2005;49(4):405–15.
Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92(4 Pt 2):727–35.
Ostör AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12(2):186–92.
Syrjänen KJ. Spontaneous evolution of intraepithelial lesions according to the grade and type of the implicated human papillomavirus (HPV). Eur J Obstet Gynecol Reprod Biol. 1996;65(1):45–53.
Insinga RP, Dasbach EJ, Elbasha EH. Epidemiologic natural history and clinical management of human papillomavirus (HPV) disease: a critical and systematic review of the literature in the development of an HPV dynamic transmission model. BMC Infect Dis. 2009;9:119.
Daly C, Dias S, Welton N, Anwer S, Ades A. NICE Guidelines Technical Support Unit. Meta-analysis: guideline methodology document 1. 2021. https://www.bristol.ac.uk/media-library/sites/social-community-medicine/documents/mpes/gmd-1-meta-analysis-jan2021.pdf. Accessed 28 Aug 2023.
Rosales R, López-Contreras M, Rosales C, et al. Regression of human papillomavirus intraepithelial lesions is induced by MVA E2 therapeutic vaccine. Hum Gene Ther. 2014;25(12):1035–49.
Harper DM, Nieminen P, Donders G, et al. The efficacy and safety of Tipapkinogen Sovacivec therapeutic HPV vaccine in cervical intraepithelial neoplasia grades 2 and 3: Randomized controlled phase II trial with 2.5 years of follow-up. Gynecol Oncol. 2019;153(3):521–9.
de Cremoux P, de la Rochefordière A, Savignoni A, et al. Different outcome of invasive cervical cancer associated with high-risk versus intermediate-risk HPV genotype. Int J Cancer. 2009;124(4):778–82.
Lei J, Ploner A, Lagheden C, et al. High-risk human papillomavirus status and prognosis in invasive cervical cancer: a nationwide cohort study. PLoS Med. 2018;15(10):e1002666.
Haupt RM, Wheeler CM, Brown DR, et al. Impact of an HPV6/11/16/18 L1 virus-like particle vaccine on progression to cervical intraepithelial neoplasia in seropositive women with HPV16/18 infection. Int J Cancer. 2011;129(11):2632–42.
Insinga RP, Perez G, Wheeler CM, et al. Incident cervical HPV infections in young women: transition probabilities for CIN and infection clearance. Cancer Epidemiol Biomark Prev. 2011;20(2):287–96.
Matsumoto K, Oki A, Furuta R, et al. Predicting the progression of cervical precursor lesions by human papillomavirus genotyping: a prospective cohort study. Int J Cancer. 2011;128(12):2898–910.
Grimm C, Polterauer S, Natter C, et al. Treatment of cervical intraepithelial neoplasia with topical imiquimod. Int J Gynecol Cancer. 2011;21(Suppl 3):46.
Jaisamrarn U, Castellsagué X, Garland SM, et al. Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PLoS ONE. 2013;8(11):e79260.
Okonogi N, Kobayashi D, Suga T, et al. Human papillomavirus genotype affects metastatic rate following radiotherapy in patients with uterine cervical cancer. Oncol Lett. 2018;15(1):459–66.
Cao M, Shah W, Qi J, Zhou Y, Wang Y, Chen H. Prognostic significance of human papillomavirus viral load in correlation with different therapeutic modalities in cervical cancer patients. Pathol Res Pract. 2016;212(9):804–10.
Garland SM, Paavonen J, Jaisamrarn U, et al. Prior human papillomavirus-16/18 AS04-adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: post-hoc analysis from a randomized controlled trial. Int J Cancer. 2016;139(12):2812–26.
Hildesheim A, Gonzalez P, Kreimer AR, et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am J Obstet Gynecol. 2016;215(2):212e1 –e15.
Centers for Disease Control and Prevention (CDC). Cancer screening-United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(3):41–5.
Bernard-Tessier A, Jeannot E, Guenat D, et al. Clinical validity of HPV circulating tumor DNA in advanced anal carcinoma: an ancillary study to the epitopes-HPV02 trial. Clin Cancer Res. 2019;25(7):2109–15.
Kim S, Meurisse A, Spehner L, et al. Pooled analysis of 115 patients from updated data of Epitopes-HPV01 and Epitopes-HPV02 studies in first-line advanced anal squamous cell carcinoma. Ther Adv Med Oncol. 2020;12:1758835920975356.
Baricevic I, He X, Chakrabarty B, et al. High-sensitivity human papilloma virus genotyping reveals near universal positivity in anal squamous cell carcinoma: different implications for vaccine prevention and prognosis. Eur J Cancer. 2015;51(6):776–85.
Liu Y, Sigel K, Gaisa MM. Human papillomavirus genotypes predict progression of anal low-grade squamous intraepithelial lesions. J Infect Dis. 2018;218(11):1746–52.
Faber MT, Frederiksen K, Palefsky JM, Kjaer SK. A nationwide longitudinal study on risk factors for progression of anal intraepithelial neoplasia grade 3 to anal cancer. Int J Cancer. 2022;151(8):1240–7.
Oehler-Jänne C, Huguet F, Provencher S, et al. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol. 2008;26(15):2550–7.
Scholefield JH, Ogunbiyi OA, Smith JH, Rogers K, Sharp F. Treatment of anal intraepithelial neoplasia. Br J Surg. 1994;81(8):1238–40.
Mathews WC, Agmas W, Cachay ER, Cosman BC, Jackson C. Natural history of anal dysplasia in an HIV-infected clinical care cohort: estimates using multi-state Markov modeling. PLoS ONE. 2014;9(8):e104116.
Lin CT, Qiu JT, Wang CJ, et al. Topical imiquimod treatment for human papillomavirus infection in patients with and without cervical/vaginal intraepithelial neoplasia. Taiwan J Obstet Gynecol. 2012;51(4):533–8.
Huang J, Cai M, Zhu Z. Survival and prognostic factors in primary vaginal cancer: an analysis of 2004–2014 SEER data. Transl Cancer Res. 2020;9(11):7091–102.
Kim MK, Lee IH, Lee KH. Clinical outcomes and risk of recurrence among patients with vaginal intraepithelial neoplasia: a comprehensive analysis of 576 cases. J Gynecol Oncol. 2018;29(1):e6.
Frank SJ, Deavers MT, Jhingran A, Bodurka DC, Eifel PJ. Primary adenocarcinoma of the vagina not associated with diethylstilbestrol (DES) exposure. Gynecol Oncol. 2007;105(2):470–4.
Ao M, Zheng D, Wang J, Gu X, Xi M. Risk factors analysis of persistence, progression and recurrence in vaginal intraepithelial neoplasia. Gynecol Oncol. 2021;162(3):584–9.
Westermann C, Fischer A, Clad A. Treatment of vulvar intraepithelial neoplasia with topical 5% imiquimod cream. Int J Gynaecol Obstet. 2013;120(3):266–70.
Kortekaas KE, Bastiaannet E, van Doorn HC, et al. Vulvar cancer subclassification by HPV and p53 status results in three clinically distinct subtypes. Gynecol Oncol. 2020;159(3):649–56.
Thuijs NB, van Beurden M, Bruggink AH, Steenbergen RDM, Berkhof J, Bleeker MCG. Vulvar intraepithelial neoplasia: Incidence and long-term risk of vulvar squamous cell carcinoma. Int J Cancer. 2021;148(1):90–8.
Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005;106(6):1319–26.
Posner M, Lorch J, Goloubeva O, et al. Five-year survival (OS) and patterns of failure for human papillomavirus (HPV) positive and negative oropharynx cancer (OPC) in the TAX 324 clinical trial: results of sequential therapy. Ann Oncol. 2010;21(Suppl 8):Abstract 1004O.
Samuels SE, Vainshtein J, Spector ME, et al. Impact of retropharyngeal adenopathy on distant control and survival in HPV-related oropharyngeal cancer treated with chemoradiotherapy. Radiother Oncol. 2015;116(1):75–81.
Fujita A, Buch K, Truong MT, et al. Imaging characteristics of metastatic nodes and outcomes by HPV status in head and neck cancers. Laryngoscope. 2016;126(2):392–8.
Miah AB, Schick U, Bhide SA, et al. A phase II trial of induction chemotherapy and chemo-IMRT for head and neck squamous cell cancers at risk of bilateral nodal spread: the application of a bilateral superficial lobe parotid-sparing IMRT technique and treatment outcomes. Br J Cancer. 2015;112(1):32–8.
Mendenhall WM, Morris CG, Amdur RJ, et al. Definitive radiotherapy for tonsillar squamous cell carcinoma. Am J Clin Oncol. 2006;29(3):290–7.
Sims JR, Van Abel K, et al. Management of recurrent and metastatic HPV-positive oropharyngeal squamous cell carcinoma after transoral robotic surgery. Otolaryngol Head Neck Surg. 2017;157(1):69–76.
Hernandez BY, Goodman MT, Unger ER, et al. Human papillomavirus genotype prevalence in invasive penile cancers from a registry-based United States population. Front Oncol. 2014;4:9.
Kravvas G, Ge L, Ng J, et al. The management of penile intraepithelial neoplasia (PeIN): clinical and histological features and treatment of 345 patients and a review of the literature. J Dermatolog Treat. 2022;33(2):1047–62.
Langsenlehner T, Mayer R, Quehenberger F, et al. The role of radiation therapy after incomplete resection of penile cancer. Strahlenther Onkol. 2008;184(7):359–63.
Sudenga SL, Torres BN, Fulp WJ, et al. Country-specific HPV-related genital disease among men residing in Brazil, Mexico and the United States: the HIM study. Int J Cancer. 2017;140(2):337–45.
Necchi A, Lo Vullo S, Perrone F, et al. First-line therapy with dacomitinib, an orally available pan-HER tyrosine kinase inhibitor, for locally advanced or metastatic penile squamous cell carcinoma: results of an open-label, single-arm, single-centre, phase 2 study. BJU Int. 2018;121(3):348–56.
Stockfleth E, Beti H, Orasan R, et al. Topical Polyphenon E in the treatment of external genital and perianal warts: a randomized controlled trial. Br J Dermatol. 2008;158(6):1329–38.
Jardine D, Lu J, Pang J, et al. A randomized trial of immunotherapy for persistent genital warts. Hum Vaccin Immunother. 2012;8(5):623–9.
Xiao Y, Zhang X, Ma L, Wang J. Long-term outcomes of juvenile-onset recurrent respiratory papillomatosis. Clin Otolaryngol. 2021;46(1):161–7.
Park H, Lee SW, Lee IH, et al. Rate of vertical transmission of human papillomavirus from mothers to infants: relationship between infection rate and mode of delivery. Virol J. 2012;9:80.
Turek EM, Fairley CK, Bradshaw CS, et al. Are genital examinations necessary for STI screening for female sex workers? An audit of decriminalized and regulated sex workers in Melbourne, Australia. PLoS ONE. 2020;15(4):e0231547.
Lee SL, Tameru AM. A mathematical model of human papillomavirus (HPV) in the United States and its impact on cervical cancer. J Cancer. 2012;3:262–8.
Nyitray AG, Lin HY, Fulp WJ, et al. The role of monogamy and duration of heterosexual relationships in human papillomavirus transmission. J Infect Dis. 2014;209(7):1007–15.
Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007;26(3):201–9.
Johnson HC, Elfström KM, Edmunds WJ. Inference of type-specific HPV transmissibility, progression and clearance rates: a mathematical modelling approach. PLoS ONE. 2012;7(11):e49614.
Joura EA, Garland SM, Paavonen J, Ferris DG, Perez G, Ault KA, et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ. 2012;344:e1401.
Zhao S, Hu S, Xu X, et al. Impact of HPV-16/18 AS04-adjuvanted vaccine on preventing subsequent infection and disease after excision treatment: post-hoc analysis from a randomized controlled trial. BMC Infect Dis. 2020;20(1):846.
Leinonen M, Nieminen P, Kotaniemi-Talonen L, et al. Age-specific evaluation of primary human papillomavirus screening vs conventional cytology in a randomized setting. J Natl Cancer Inst. 2009;101(23):1612–23.
Giorgi Rossi P, Carozzi F, Ronco G, et al. p16/ki67 and E6/E7 mRNA accuracy and prognostic value in triaging HPV DNA-positive women. J Natl Cancer Inst. 2021;113(3):292–300.
Cuzick J, Adcock R, Carozzi F, et al. Combined use of cytology, p16 immunostaining and genotyping for triage of women positive for high-risk human papillomavirus at primary screening. Int J Cancer. 2020;147(7):1864–73.
Ogilvie GS, Krajden M, van Niekerk DJ, et al. Primary cervical cancer screening with HPV testing compared with liquid-based cytology: results of round 1 of a randomised controlled trial—the HPV FOCAL Study. Br J Cancer. 2012;107(12):1917–24.
Gage JC, Schiffman M, Solomon D, et al. Risk of precancer determined by HPV genotype combinations in women with minor cytologic abnormalities. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1095–101.
Granados R, Tellez-Safina H, Solis I, et al. Cervical cancer screening cotesting with cytology and MRNA HPV E6/E7 yields high rates of CIN2+ lesions in young women. Diagn Cytopathol. 2017;45(12):1065–72.
De Strooper LMA, Berkhof J, Steenbergen RDM, et al. Cervical cancer risk in HPV-positive women after a negative FAM19A4/mir124-2 methylation test: a post hoc analysis in the POBASCAM trial with 14 year follow-up. Int J Cancer. 2018;143(6):1541–8.
Mandal R, Ghosh I, Banerjee D, et al. Correlation between p16/Ki-67 expression and the grade of cervical intraepithelial neoplasias. Int J Gynecol Pathol. 2020;39(4):384–90.
Zhou Q, Zhang F, Sui L, Zhang H, Lin L, Li Y. Application of 2011 International Federation for Cervical Pathology and Colposcopy Terminology on the Detection of Vaginal Intraepithelial Neoplasia. Cancer Manag Res. 2020;12:5987–95.
Santoso JT, Likes W. Colposcopic acetowhitening of vulvar lesion: a validity study. Arch Gynecol Obstet. 2015;292(2):387–90.
Acknowledgements
Medical Writing, Editorial, and Other Assistance.
Editorial assistance in the preparation of this article was provided by Roxanne Wosu of Analysis Group.
Authorship.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The study sponsors were involved in several aspects of the research, including the study design, interpretation of data, and writing of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Bruno Martins, Oscar Patterson-Lomba, Andres Gomez-Lievano, Abigail Zion, and Ryan Simpson. The first draft of the manuscript was written by Bruno Martins and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Ibrahim Diakite, Kwame Owusu-Edusei, Cody Palmer, Vincent Daniels, and Elamin Elbasha are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and shareholders in Merck & Co., Inc., Rahway, NJ, USA. Bruno Martins, Oscar Patterson-Lomba, Andres Gomez-Lievano, Abigail Zion, and Ryan Simpson are employees of Analysis Group, Inc., that received funding from Merck Sharp & Dohme LLC to conduct this study.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Diakite, I., Martins, B., Owusu-Edusei, K. et al. Structured Literature Review to Identify Human Papillomavirus’s Natural History Parameters for Dynamic Population Models of Vaccine Impacts. Infect Dis Ther (2024). https://doi.org/10.1007/s40121-024-00952-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40121-024-00952-z