Abstract
Introduction
Bacillus Calmette-Guérin (BCG) vaccination has been reported to be protective against latent tuberculosis infection (LTBI) in the general population. The aim of this study was to investigate the protective effect of BCG vaccination against LTBI in adult patients with end-stage renal disease (ESRD) and renal transplants.
Methods
Patients aged ≥ 20 years with ESRD who received hemodialysis (HD), peritoneal dialysis (PD) or kidney transplant were enrolled from January 2012 to December 2019 at a medical center and a regional hemodialysis center. Patients with active tuberculosis (TB), previously treated TB, active immunosuppressant therapy or human immunodeficiency virus infection were excluded. LTBI status was determined by QuantiFERON-TB Gold In-tube (QFT-GIT).
Results
After the exclusion of indeterminate results of QFT-GIT, 517 participants were enrolled and 97 (18.8%) were identified as having LTBI. Participants with LTBI were older (55.1 ± 11.4 vs. 48.5 ± 14.6 years, p < 0.001) and had a significantly higher proportion receiving HD than those without LTBI (70.1% vs. 56.7%, p = 0.001). The percentage with BCG scars was higher in the non-LTBI group than in the LTBI group (94.8% vs. 81.4%, p < 0.001), whereas the neutrophil-to-lymphocyte ratio (NLR) (≥ 2.68) was significantly higher in the LTBI group (62.8% vs. 45.5%, p = 0.02). By multivariate logistic regression analysis, presence of BCG scar and high NLR were independent protective factors against LTBI [adjusted OR: 0.19 (0.063–0.58, p = 0.001) and 0.50 (0.28–0.89, p = 0.02)].
Conclusion
The prevalence of LTBI was as high as 18.8% in patients with end-stage kidney disease or kidney transplant. BCG vaccination and high NLR might have protective effects against LTBI in patients with renal failure or transplant.
Similar content being viewed by others
There are few reports on the protective effect of the bacillus Calmette-Guérin (BCG) vaccine against latent tuberculosis infection (LTBI) in patients with end-stage renal disease (ESRD). |
The prevalence of LTBI is as high as 18.8% in patients with ESRD or kidney transplant. |
The BCG vaccine might provide protection against LTBI in patients with ESRD or kidney transplant. |
The BCG booster has no additional benefit compared with primary BCG vaccination. |
High neutrophil-to-lymphocyte ration (NLR ≥ 2.68) might also be an independent protective factor against LTBI. |
Introduction
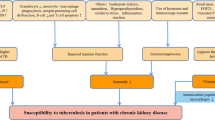
Mycobacterium tuberculosis (MTB) was first identified by Robert Koch in 1821 [1]. Despite the passing of 2 centuries, MTB remains one of the leading pathogens in infectious diseases. It has a profound impact on global health, with the World Health Organization (WHO) reporting 10.6 million new cases of active tuberculosis (TB) and 1.6 million deaths due to TB in 2021, especially in vulnerable populations with low socioeconomic status and crowded living conditions [2]. Patients with end-stage renal disease or transplant are at risk for TB infection and warrant further intervention [3].
The bacillus Calmette-Guérin (BCG) vaccine is currently the only approved vaccine that protects against active TB. Millions of people are vaccinated every year, and global coverage in 2020 was around 85% [4]. It is well known that BCG vaccination has no uniform effect on the diverse types of active TB. Numerous studies have demonstrated clearly that BCG vaccination has the best efficacy, up to 80%, in severe infections, such as meningeal and disseminated active TB [5]. However, the preventive effect of BCG vaccination against pulmonary TB was reported to vary from zero to 90% [5]. In 2022, a meta-analysis reported an efficacy of 18% against all types of active TB [6]. The heterogeneity of the effects of BCG vaccination might be associated with multiple factors. A meta-analysis from Mangtani and colleagues reported that different regions had various BCG vaccination performances [7]. BCG vaccination provided better protection in the regions farther from the equator than in those closer to it, which might be explained partially by environmental non-tuberculosis mycobacteria (NTM) and the diagnostic bias of active TB [7]. In addition, the timing of the inoculation, use of a stringent tuberculin test, waning effect of BCG vaccination and exposure to NTM might confound the protection afforded by BCG vaccination [7,8,9,10].
Before MTB reactivation, MTB infection is in a latent status, also known as latent TB infection (LTBI). About 5–10% of LTBI progresses to active TB later in life. The detection and treatment of LTBI is an important step to block the transmission of MTB. The use of the BCG vaccine to protect against LTBI has become clearer recently because interferon (IFN)-gamma release assay (IGRA) has been substituted for the tuberculin skin test (PPD test) in many circumstances to diagnose LTBI without cross reaction to BCG vaccination [11]. Studies in the USA, Greenland and UK have reported that BCG vaccination provides 20–75% reduction of LTBI risk in children and young adults [12,13,14]. However, the BCG vaccine cannot prevent the progression of LTBI to active TB [12, 15]. The nature of the immunity against LTBI and active TB conferred by the BCG vaccine still needs to be elucidated.
Notably, the neutrophil-to-lymphocyte ratio of peripheral blood (NLR) has been adopted as a biomarker of systemic immunity [16]. Several studies have shown its usefulness in predicting the severity and prognosis in distinct populations and diseases [17, 18]. However, few studies have investigated the role of NLR in LTBI. In addition, the aforementioned evidence on the effects of the BCG vaccine and NLR was generated mostly from the general population. There is little evidence on these issues in adults at high risk for LTBI, such as patients receiving renal dialysis or renal transplant. To fill this gap, we conducted a cross-sectional study in an area with intermediate incidence of TB (incidence of around 30 per 100,000 people in 2021) [19] to investigate the protective effect of BCG vaccination and the effect of NLR on LTBI in patients with end-stage renal disease (ESRD) and renal transplant.
Methods
Patient Enrollment
This cross-sectional study was conducted in a tertiary referral medical center and a regional hemodialysis center in northern Taiwan. We recruited patients aged ≥ 20 years with ESRD who had received hemodialysis (HD), peritoneal dialysis (PD) or kidney transplant from January 2012 to December 2019. Patients with active TB, previously treated TB or human immunodeficiency virus (HIV) infection were excluded. The information about previous history of TB was collected from the participants and government records. The study proceeded under the approval of the Institutional Review Boards of the Research Ethics Committees of the study hospitals (nos. 201009057R, 201309056RINC and 201709038RINB). All of the enrolled participants signed informed consent forms before the study. The study was conducted in accordance with the Declaration of Helsinki.
Outcome and Determination of Latent Tuberculosis Infection
At the time of enrollment, the presence of LTBI was determined by IGRA test (QuantiFERON-TB Gold In-tube [QFT-GIT], Qiagen, Germany), which was performed according to the manufacturer’s instructions. QFT-GIT is composed of three tubes: a Nil tube for negative control, a Mitogen tube for positive control and an MTB peptides mixture tube. The results of QFT-GIT testing were interpreted according to the manufacturer’s recommendation. The negative control criterion of the Nil tube was IFN-gamma response < 8 IU/ml. The positive control criterion was that the IFN-gamma response of the mitogen tube minus that of the Nil tube was ≥ 0.5 IU/ml. After fulfilling the positive and negative control criteria, the test was interpreted as a positive result if the IFN-gamma response of the MTB peptide mixture minus that of the Nil tube was > 0.35 IU/ml and > 25% of that of the Nil tube. In contrast, a negative result was indicated by the IFN-gamma response of MTB peptides mixture minus that of the Nil tube being < 0.35 IU/ml or > 0.35 IU/ml, but not > 25%. If the positive and negative control criteria were not met, the result was interpretated as indeterminate.
Data Review and Definition
Demographics, clinical characteristics and laboratory data at enrollment were reviewed and recorded in a default case report form by a well-trained research nurse. BCG scar status, which was the indicator of BCG vaccination, was checked by visual confirmation. Ex-smoking was defined as the participants having quit smoking for > 6 months without recurrence at the time of enrollment. Renal replacement therapy was sorted according to whether the subject had received HD, PD or a kidney transplantation at the enrollment. Cardiovascular diseases included hypertension, heart failure, coronary artery disease or arrythmia and were identified by diagnostic code. The information on TB exposure was collected from the participants and defined as ever having household or workplace contacts. Hemograms and serum albumin levels were checked according to the dialysis care protocols of the individual study sites. The laboratory data from routine outpatient care within 1 month before or after enrollment were collected for analysis.
Statistical Analysis
Sample size was calculated as below: α: 0.05, β: 0.2, power: 80%. The percentages of LTBI in the BCG and non-BCG vaccination groups were assumed to be 10% and 25% [20, 21]. The enrollment ratio of the two groups was set to nine. The sample size was estimated as 470. We also estimated that around 10% of cases [20, 21] would have indeterminate results and thus be excluded. The total number of enrollees was 517 in anticipation of exclusion.
Statistical analysis was performed in SPSS version 19 (IBM, Chicago, IL, USA). Categorical items were analyzed by chi-squared test and continuous items by Student’s t-test. One-way analysis of variance (ANOVA) was used for comparison of multiple groups. The cut-off points of the neutrophil-to-lymphocyte ratio (NLR) and ages were determined by Youden index derived from receiver-operating characteristic (ROC) curves. The crude odds ratios (OR) were obtained by univariate logistic regression. If variables had p < 0.05, they entered into the multivariate logistic regression analysis. Subgroup analyses were tested by univariable logistic regression test. Missing data were neglected in the statistical analysis. Statistical significance was set to p < 0.05.
Results
Enrollment of Participants
The flow chart of enrollment is shown in Figure S1. A total of 867 participants were screened, and 548 participants received QFT-GIT tests during the study period. Of these, 33 (6.0%) participants were excluded because of indeterminate QFT-GIT results. Finally, 517 participants were analyzed, and the 97 (18.8%) who had positive QFT-GIT results were assigned to the LTBI group.
Clinical Characteristics of LTBI and Non-LTBI Groups
The participants with LTBI were older than those without LTBI (55.1 ± 11.4 vs. 48.5 ± 14.6 years, p < 0.001), shown in Table 1. Participants were categorized into two age groups, those aged < 60 years and those aged ≥ 60. This categorization was defined by Youden index and the area under the curve (AUC) of ROC. The proportion of participants aged ≥ 60 years was larger in the LTBI group than in the non-LTBI group (42.3% vs. 22.4%, p < 0.001). Sex, BMI, TB exposure and the top three comorbidities, indicating diabetes mellitus (DM), cardiovascular disease and autoimmune diseases, were not different between the LTBI and non-LTBI groups.
In the study population, 92.3% (477/517) had BCG scars. Among the 477 patients with BCG scars, 67.9% (351/477) had 1 BCG scar, while 21.3% and 3.1% had 2 or ≥ 3 BCG scars, respectively. Fewer participants in the LTBI group (79/97, 81.4%) than in the non-LTBI group (394/420, 94.8%) had BCG scars (p < 0.001). Regarding BCG scar status, 45% (18/40) of the participants with no BCG scars had LTBI, whereas only 16.6% (79/477) of those with BCG scars had LTBI (p < 0.001) (Fig. 1). BCG boosters (≥ 2 BCG scars) provided no significant augmentation of LTBI protection over primary BCG inoculation (p = 0.63).
Proportions of LTBI by status of BCG vaccination, low and high NLR groups. The numbers in the bars and error bar represent the proportions of LTBI and standard errors in specific groups. Risk of LTBI was significantly higher in the low NLR subgroup than in the high NLR subgroup. The same findings were also found when comparing the groups with no BCG vaccination and ever BCG vaccination. After a single BCG vaccine inoculation, there was no significant difference in the rate of LTBI regardless of history of BCG vaccine boosters. BCG bacillus Calmette-Guérin vaccine, BCG 1 one dose of BCG, BCG 2 two doses of BCG, BCG ≥ 3 three or more doses of BCG, LTBI latent tuberculosis infection, NLR neutrophil-to-lymphocyte ratio
Compared with the PD and transplant groups, the LTBI group had a significantly higher proportion of HD than the non-LTBI group (70.1% vs. 56.7%, p = 0.001). The prevalences of LTBI were also significantly different among the HD, PD and renal transplant groups. Participants with HD (68/306, 22.2%) had the highest prevalence of LTBI, followed by PD and transplantation (17/104, 16.3% and 12/107, 11.2%, respectively; p = 0.001). However, the HD group had the smallest proportion of BCG scars (273/306, 89.2%), followed by the PD and transplantation groups (104/107, 97.2% and 100/104, 96.2%, respectively, p < 0.001).
Characteristics of the Composition of White Blood Cells
In all, 343 participants (66.3%) had hemogram and serum albumin examinations within 1 month before or after the QFT-GIT test (Table 2). Hemogram and serum albumin levels were similar between the LTBI and non-LTBI groups, except the lymphocyte proportion of white blood cells. The percentages of lymphocytes tended to be higher in the LTBI group than in the non-LTBI group (24.4 ± 8.1 vs. 22.1 ± 8.0, p = 0.05). The neutrophil-to-lymphocyte ratios (NLRs) were not significantly different between the two groups. The cutoff value of NLR, determined by Youden index and AUC of ROC, was 2.68. NLR ≥ 2.68 was defined as high NLR and < 2.68 as low NLR. The number of participants with high NLR was lower in the LTBI group than in the non-LTBI group (45.5% vs. 62.8%, p = 0.02). Furthermore, 21.9% of participants with low NLR had LTBI, but only 12.1% of those with high NLR had it (p = 0.02), as shown in Fig. 1.
Analysis of Risk Factors About LTBI
Univariate analysis showed that age ≥ 60 years [crude OR: 2.54, 95% confidence interval (CI): 1.60–4.04, p < 0.001] and hemodialysis (crude OR: 2.26, 95% CI 1.17–4.37 in reference to PD) were risk factors for LTBI (Table 3). BCG scar (crude OR: 0.24, 95% CI 0.12–0.47, p < 0.001) and high NLR (crude OR: 0.49, 95% CI 0.28–0.88, p = 0.02) were protective factors against LTBI. The interaction between BCG vaccination and age was not significant (p value of interaction was 0.33, Table S1). Because the NLR data were available in 343 participants, multivariate analyses were performed on the total study population and the participants with NLR data, respectively. BCG scar and high NLR were significantly protective factors in both models of multivariate logistic regression analyses (adjusted OR: 0.19, 95% CI 0.063–0.58, p = 0.001 and adjusted OR: 0.50, 95% CI 0.28–0.89, p = 0.02, respectively). In addition, BCG vaccination and NLR were still independent protective factors against LTBI after the inclusion of possible clinically significant factors in the multivariate analyses (Table S2).
The subgroup analyses of the effects of BCG vaccination and NLR on LTBI are shown in Fig. 2A, B. The results from all subgroups, including age, sex, DM and modality of renal placement, mostly suggested positive effects of BCG vaccination and high NLR on protection against LTBI in renal failure patients.
Subgroup analysis of odds ratios of LTBI according to A the presence of BCG vaccination, B high NLR. *343 participants had hemogram data. BCG bacillus Calmette-Guérin vaccine, CI confidence interval, DM diabetes mellitus, HD hemodialysis, NLR neutrophil-to-lymphocyte ratio, OR odds ratio, PD peritoneal dialysis
Discussion
The health policy of Taiwan has promoted BCG vaccination within 1 year of birth since 1965. In 1966, the rate of BCG vaccination was 72.2% in children aged 1 year, progressing to 98.6% by 2020 [22]. The present study demonstrated that the protective efficacy of BCG vaccination against LTBI lasts into adulthood in patients with ESRD or kidney transplants. Meanwhile, a high NLR might also be a protective factor against LTBI.
The evidence that the BCG vaccine protects against TB has mainly been derived from health or household-contact children and young adults without comorbidities. However, certain risk factors of active TB and LTBI, such as HIV, DM and ESRD, cause some degree of impairment of immunity [23]. The performance of the BCG vaccine might be questionable in such conditions. Faurholt-Jepsen and colleagues reported that BCG vaccination in infancy and childhood had a protective effect in adulthood, reducing the risk of active TB by around 70%, irrespective of HIV status [24]. Moreover, a community-based study from Taiwan observed that BCG vaccination in infancy provided a 34% risk reduction of LTBI in adults with DM aged > 40 years [25]. The current study echoes a previous study regarding the effect of BCG in patients with renal transplantation [20] and provides further evidence of the preventive effect of BCG vaccination against LTBI in patients with ESRD.
A meta-analysis by Abubakar and colleagues reported that the effect of BCG against active TB could last for 10 years [26]. One study in Norway claimed that it could persist for 40 years with 42% efficacy, despite the efficacy of the BCG vaccine waning significantly 20 years after the initial inoculation [10]. A study in Taiwan also reported a protective effect of BCG vaccination against LTBI in HIV-free adults in a study population of prison inmates [27]. These studies all imply that the protection afforded by BCG vaccination persists into adulthood but also decreases with age. The present study also found that the LTBI prevalence was lower in participants aged < 60 years than in those age ≥ 60 years. The protection against LTBI provided by BCG vaccination was present in those aged < 60 or ≥ 60 years (Fig. 2A), and the interaction test of age and BCG was not significant. These results might suggest that the protective effect of BCG vaccine could last into adulthood in patients with ESRD, just like the aforementioned findings in the general population. However, the time impacts of BCG vaccination in different age groups could not be clarified in the current study. Further studies will be needed in various regions with appropriately estimated sample sizes and stratification by age.
Re-vaccination is one of the methods to enhance self-immunity. Therefore, some regions, including Taiwan, have adopted re-vaccination to improve the efficacy of the BCG vaccine [28]. However, studies in Chile and Malawi have reported that there is no benefit to a BCG booster against active TB [29, 30]. The nationwide data of Finland, which stopped re-vaccination in 1990, were used to compare re-vaccinated and one-shot adolescents, and that study implied that there was no significant difference in protection against active TB [31]. Therefore, the WHO in 1995 formally rescinded its recommendation of a BCG vaccine booster [32]. However, a survey of BCG vaccination and LTBI in Taiwan reported that the LTBI rates of adults stratified by age were inversely correlated with the number of BCG scars [27]. On the other hand, Nemes and colleagues reported that a booster shot of the BCG vaccine in adolescence did not prevent QFT positive conversion in 2 years, but re-vaccination decreased the persistence of QFT positive conversion for 6 months [33]. These findings might imply some efficacy of the BCG booster against LTBI. The current study found that a single dose of the BCG vaccine had a protective effect against LTBI similar to that of boosters in ESRD patients and recipients of renal transplantation. Therefore, the value of BCG revaccination against LTBI might require further validation in large-scale studies recruiting different subgroups.
Despite the abundance of research on NLR, the normal range of NLR has yet to be determined [16]. The normal value of NLR is hard to define partly because factors such as sex and age influence the determination of a cut-off point [34]. Even so, some studies have proposed that the mean value of NLR in the normal population ranges from 1.65 to 2.13 [34, 35]. The current study reported that the mean of NLR was 3.6, which was higher than the aforementioned mean value of NLR in a healthy population. However, it was comparable to the results from research on ESRD [36, 37]. Higher NLR might represent the hyper-inflammatory status in ESRD, which might be associated with atherosclerosis, endothelial dysfunction, anemia or myocardial injury [37].
The immune response to MTB is a complex interaction of innate and adaptive immunity [38]. Neutrophils are crucial for both major types of immunity and play an important role in preventing MTB infection [39, 40]. A study on active pulmonary TB found more neutrophils in respiratory secretions and more MTB bacilli within neutrophils than within lymphocytes [39]. A study in UK reported that neutrophil counts of peripheral blood were inversely correlated with active TB among participants with close contact [41]. Several animal studies found that neutrophils acted as part of the first line of defense as major phagocytes against MTB bacilli. They captured MTB bacilli, migrated to mediastinal lymph nodes and acted with dendritic cells and lymphocytes there to initiate the cascade of MTB immunity [42]. Studies on neutrophils and the effect of the BCG vaccine have also suggested the importance of neutrophils in building protective immunity, as they phagocyte injected BCG and migrated to lymph nodes to produce adaptive immunity [40]. NLR levels in patients with ESRD are also positively correlated with tumor necrosis factor-alpha and interleukin-6, which are crucial cytokines for T-helper 1 and 17 immunity [37]. The BCG vaccine exerts some benefits on bladder cancer, some autoimmune diseases, Parkinsonism and other infections such as SARS-CoV-2 [43]. It does not solely depend on adaptive immunity to specific MTB antigens. One of the proposed mechanisms for the effect beyond MTB is the training of innate immunity and the subsequent more efficient programming of overall immunity [43]. This may explain the benefits of both BCG vaccines and NLR in protecting against MTB in patients with ESRD.
In the absence of a well-established and comprehensive registry system, the presence of a BCG scar is a good indicator of BCG vaccination [44]. Therefore, this indicator has been adopted in many studies [27, 29, 30]. However, a small portion (up to 10%) of BCG vaccinations do not produce a scar [45, 46]. The potential for scar failure might cause errors in categorizing BCG vaccine status. On the other hand, such misclassification would likely occur in both LTBI and non-LTBI groups, which might minimize the bias. Further studies are needed to examine this possibility in assistance of a well-organized vaccine registration system.
The limitations of the study must be considered. First, the data were confined to a tertiary-care hospital and a regional hemodialysis clinic. Thus, they may not be representative of the entire population of patients with ESRD. Second, only about two thirds of the participants had data on hemograms and differential counts of blood leukocytes because hemogram examinations were not required in the study protocol. This may weaken the statement of the protective effect of NLR in patients with ESRD. However, the reported data on NLR in the current study were comparable with those of previous studies [36, 37]. Third, the sequences of BCG, NLR and LTBI acquisition were not clearly identified in this cross-sectional study, so no causal relationship can be confirmed in the present study. In addition, data on the time since BCG vaccination were unavailable, so we could not assess the influence on LTBI. Fourth, the study was conducted in Taiwan, an area with intermediate TB prevalence, so further generalization to other areas or ethnicities should be applied only after validation. Lastly, because there was no evidence of an association between continuous NLR and LTBI, we classified the dichotomy of NLR using the Youden index for clinical decision in ESRD and renal transplantation. The finding of high NLR protecting from LTBI in ESRD was a preliminary result which needs to be further assessed.
Conclusions
BCG vaccination and high NLR level were found to be independent factors protecting against LTBI in patients with ESRD or kidney transplant. BCG vaccination boosters conferred no additional benefit against LTBI compared with one-time vaccination. The NLR of patients with ESRD was higher in those without LTBI and was possibly correlated with reduction in risk of LTBI by nearly half if the level was ≥ 2.68. Further validation is needed.
References
Kaufmann SH. Robert Koch, the Nobel Prize, and the ongoing threat of tuberculosis. N Engl J Med. 2005;353(23):2423–6. https://doi.org/10.1056/NEJMp058131.
WHO Global Tuberculosis Report. 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022/tb-disease-burden.
Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46(6):1563–76. https://doi.org/10.1183/13993003.01245-2015.
Muhoza P, Danovaro-Holliday MC, Diallo MS, et al. Routine vaccination coverage—worldwide, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(43):1495–500. https://doi.org/10.15585/mmwr.mm7043a1.
Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22(6):1154–8. https://doi.org/10.1093/ije/22.6.1154.
Martinez L, Cords O, Liu Q, et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: a systematic review and individual participant data meta-analysis. Lancet Glob Health. 2022;10(9):e1307–16. https://doi.org/10.1016/s2214-109x(22)00283-2.
Mangtani P, Abubakar I, Ariti C, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 2014;58(4):470–80. https://doi.org/10.1093/cid/cit790.
Poyntz HC, Stylianou E, Griffiths KL, Marsay L, Checkley AM, McShane H. Non-tuberculous mycobacteria have diverse effects on BCG efficacy against Mycobacterium tuberculosis. Tuberculosis (Edinb). 2014;94(3):226–37. https://doi.org/10.1016/j.tube.2013.12.006.
Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8(3): e1001012. https://doi.org/10.1371/journal.pmed.1001012.
Nguipdop-Djomo P, Heldal E, Rodrigues LC, Abubakar I, Mangtani P. Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: a retrospective population-based cohort study. Lancet Infect Dis. 2016;16(2):219–26. https://doi.org/10.1016/s1473-3099(15)00400-4.
Abubakar I, Drobniewski F, Southern J, et al. Prognostic value of interferon-γ release assays and tuberculin skin test in predicting the development of active tuberculosis (UK PREDICT TB): a prospective cohort study. Lancet Infect Dis. 2018;18(10):1077–87. https://doi.org/10.1016/s1473-3099(18)30355-4.
Eisenhut M, Paranjothy S, Abubakar I, et al. BCG vaccination reduces risk of infection with Mycobacterium tuberculosis as detected by gamma interferon release assay. Vaccine. 2009;27(44):6116–20. https://doi.org/10.1016/j.vaccine.2009.08.031.
Basu Roy R, Sotgiu G, Altet-Gómez N, et al. Identifying predictors of interferon-γ release assay results in pediatric latent tuberculosis: a protective role of bacillus Calmette-Guerin?: a pTB-NET collaborative study. Am J Respir Crit Care Med. 2012;186(4):378–84. https://doi.org/10.1164/rccm.201201-0026OC.
Michelsen SW, Soborg B, Koch A, et al. The effectiveness of BCG vaccination in preventing Mycobacterium tuberculosis infection and disease in Greenland. Thorax. 2014;69(9):851–6. https://doi.org/10.1136/thoraxjnl-2014-205688.
World Health O. BCG vaccine: WHO position paper, February 2018—recommendations. Vaccine. 2018;36(24):3408–10. https://doi.org/10.1016/j.vaccine.2018.03.009.
Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci. 2022;23(7):3636. https://doi.org/10.3390/ijms23073636.
Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil–lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91(3):181–4. https://doi.org/10.1002/jso.20329.
Song M, Graubard BI, Rabkin CS, Engels EA. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep. 2021. https://doi.org/10.1038/s41598-020-79431-7.
Epidemiologic report of TB in Taiwan. https://daily.cdc.gov.tw/stoptb/CareMagChart.aspx.
Shu CC, Tsai MK, Lin SW, Wang JY, Yu CJ, Lee CY. Latent tuberculosis infection increases in kidney transplantation recipients compared with transplantation candidates: a neglected perspective in tuberculosis control. Clin Infect Dis. 2020;71(4):914–23. https://doi.org/10.1093/cid/ciz851.
Shu CC, Hsu CL, Lee CY, et al. Comparison of the prevalence of latent tuberculosis infection among non-dialysis patients with severe chronic kidney disease, patients receiving dialysis, and the dialysis-unit staff: a cross-sectional study. PLoS ONE. 2015;10(4): e0124104. https://doi.org/10.1371/journal.pone.0124104.
The rates of vaccination in 2020, Taiwan. https://www.cdc.gov.tw/File/Get?q=t9WnCInvvVMS9kUboNEwG_6RBxlcu7zZLxAE35XnFjWuefk1FMRnbyPMynRql8xM7V82gsfJDgik_TDLxcZ-XlzKIIOnGiy78tLT6HaZMR9F_xj_6H474cK-AFG_HoX3.
Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. https://doi.org/10.1164/rccm.200604-571st.
Faurholt-Jepsen D, Range N, Praygod G, et al. BCG protects against tuberculosis irrespective of HIV status: a matched case–control study in Mwanza, Tanzania: Table 1. Thorax. 2013;68(3):288–9. https://doi.org/10.1136/thoraxjnl-2012-201971.
Lin CH, Kuo SC, Hsieh MC, et al. Effect of diabetes mellitus on risk of latent TB infection in a high TB incidence area: a community-based study in Taiwan. BMJ Open. 2019;9(10): e029948. https://doi.org/10.1136/bmjopen-2019-029948.
Abubakar I, Pimpin L, Ariti C, et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis. Health Technol Assess. 2013;17(37):1–372. https://doi.org/10.3310/hta17370. (v–vi).
Chan PC, Yang CH, Chang LY, et al. Lower prevalence of tuberculosis infection in BCG vaccinees: a cross-sectional study in adult prison inmates. Thorax. 2013;68(3):263–8. https://doi.org/10.1136/thoraxjnl-2012-202208.
Anonymous. A review of the tuberculosis control program in Taiwan, 1949–1989: chronological development of the program, BCG vaccination. Taipei: Provincial Chronic Disease Control Bureau; 1991. p. 4–37.
Sepulveda RL, Parcha C, Sorensen RU. Case–control study of the efficacy of BCG immunization against pulmonary tuberculosis in young adults in Santiago, Chile. Tuber Lung Dis. 1992;73(6):372–7. https://doi.org/10.1016/0962-8479(92)90043-j.
Karonga Prevention Trial Group. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Karonga Prevention Trial Group. Lancet. 1996;348(9019):17–24.
Tala-Heikkilä MM, Tuominen JE, Tala EO. Bacillus Calmette-Guérin revaccination questionable with low tuberculosis incidence. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1324–7. https://doi.org/10.1164/ajrccm.157.4.9706037.
Global Tuberculosis Programme and Global Programme on Vaccines. Statement on BCG revaccination for the prevention of tuberculosis. Wkly Epidemiol Rec. 1995;70(32):229–31.
Nemes E, Geldenhuys H, Rozot V, et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. 2018;379(2):138–49. https://doi.org/10.1056/NEJMoa1714021.
Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck CHJ, Stricker BH. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep. 2018;8(1):10566. https://doi.org/10.1038/s41598-018-28646-w.
Forget P, Khalifa C, Defour J-P, Latinne D, Van Pel M-C, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017. https://doi.org/10.1186/s13104-016-2335-5.
Okyay GU, İnal S, Öneç K, et al. Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren Fail. 2013;35(1):29–36. https://doi.org/10.3109/0886022x.2012.734429.
Turkmen K, Guney I, Yerlikaya FH, Tonbul HZ. The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients. Ren Fail. 2012;34(2):155–9. https://doi.org/10.3109/0886022x.2011.641514.
Ulrichs T, Kaufmann SH. New insights into the function of granulomas in human tuberculosis. J Pathol. 2006;208(2):261–9. https://doi.org/10.1002/path.1906.
Eum S-Y, Kong J-H, Hong M-S, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137(1):122–8. https://doi.org/10.1378/chest.09-0903.
Trentini MM, de Oliveira FM, Kipnis A, Junqueira-Kipnis AP. The role of neutrophils in the induction of specific Th1 and Th17 during vaccination against tuberculosis. Front Microbiol. 2016;7:898. https://doi.org/10.3389/fmicb.2016.00898.
Martineau AR, Newton SM, Wilkinson KA, et al. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117(7):1988–94. https://doi.org/10.1172/jci31097.
Blomgran R, Ernst JD. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J Immunol. 2011;186(12):7110–9. https://doi.org/10.4049/jimmunol.1100001.
Dow CT, Kidess L. BCG vaccine-the road not taken. Microorganisms. 2022;10(10):1919. https://doi.org/10.3390/microorganisms10101919.
Floyd S, Ponnighaus JM, Bliss L, et al. BCG scars in northern Malawi: sensitivity and repeatability of scar reading, and factors affecting scar size. Int J Tuberc Lung Dis. 2000;4(12):1133–42. https://docserver.ingentaconnect.com/deliver/connect/iuatld/10273719/v4n12/s7.pdf?expires=1665238905&id=0000&titleid=3764&checksum=15A6F6C29C0FB9DAC6BBC24662D1BC1B&host=https://www.ingentaconnect.com.
Rani SH, Vijayalakshmi V, Sunil K, Lakshmi KA, Suman LG, Murthy KJ. Cell mediated immunity in children with scar-failure following BCG vaccination. Indian Pediatr. 1998;35(2):123–7.
Dhanawade SS, Kumbhar SG, Gore AD, Patil VN. Scar formation and tuberculin conversion following BCG vaccination in infants: a prospective cohort study. J Family Med Prim Care. 2015;4(3):384–7. https://doi.org/10.4103/2249-4863.161327.
Acknowledgements
We thank the participants in the study. We would also like to acknowledge Dr. Chin-Hao Chang for statistical support and the staff of the Department of Medical Research of National Taiwan University Hospital for their support.
Funding
This study was partially supported by a research grant from National Taiwan University Hospital (nos. 110-T07 and 111-T0014) and Far Eastern Memorial Hospital (FEMH-2023-C-055). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The rapid service fee was funded by Far Eastern Memorial Hospital (FEMH-2023-C-055).
Medical Writing and/or Editorial Assistance
John P. Ring, Head coordinator at the Language Training and Testing Center, Taipei, provided editorial assistance. The source of funding for this assistance was Far Eastern Memorial Hospital (FEMH-2023-C-055).
Author Contributions
Chin-Chung Shu was responsible for the conception, design and critical review. Ping-Huai Wang and Chin-Chung Shu were involved in data analysis. Ping-Huai Wang and Chin-Chung Shu prepared and wrote the manuscript. Shu-Yung Lin, Hung-Hsiang Liou, Chien-Chia Chen, Chih-Yuan Lee and Meng-Kun Tsai assisted in case enrollment. Chong-Jen Yu was responsible for critical review.
Compliance with Ethics Guidelines
Approval for the study was granted by the respective Research Ethics Committees (nos. 201009057R, 201309056RINC and 201709038RINB). All participants signed informed consent before commencement of the study. The study was conducted in accordance with the Declaration of Helsinki.
Disclosures
Ping-Huai Wang, Shu-Yung Lin, Hung-Hsiang Liou, Chien-Chia Chen, Chin-Chung Shu, Chih-Yuan Lee, Meng-Kun Tsai and Chong-Jen Yu all have nothing to disclose.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, PH., Lin, SY., Liou, HH. et al. Protective Effect of BCG and Neutrophil-to-Lymphocyte Ratio on Latent Tuberculosis in End Stage Renal Disease. Infect Dis Ther 12, 1907–1920 (2023). https://doi.org/10.1007/s40121-023-00839-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00839-5