Abstract
Introduction
Detection strategies in vulnerable populations such as people experiencing homelessness (PEH) need to be explored to promptly recognize severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreaks. This study investigated the diagnostic accuracy of a rapid SARS-CoV-2 Ag test in PEH during two pandemic waves compared with gold standard real-time multiplex reverse transcription polymerase chain reaction (rtRT-PCR).
Methods
All PEH ≥ 18 years requesting residence at the available shelters in Verona, Italy, across two cold-weather emergency periods (November 2020–May 2021 and December 2021–April 2022) were prospectively screened for SARS-CoV-2 infection by means of a naso-pharyingeal swab. A lateral flow immunochromatographic assay (Biocredit® COVID-19 Ag) was used as antigen-detecting rapid diagnostic test (Ag-RDT). The rtRT-PCR was performed with Allplex™ SARS-CoV-2 assay kit (Seegene). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated as measures for diagnostic accuracy.
Results
Overall, 503 participants were enrolled during the two intervention periods for a total of 732 paired swabs collected: 541 swabs in the first period and 191 in the second. No significant differences in demographic and infection-related characteristics were observed in tested subjects in the study periods, except for the rate of previous infection (0.8% versus 8%; p < 0.001) and vaccination (6% versus 73%; p < 0.001). The prevalence of SARS-CoV-2 in the cohort was 8% (58/732 swabs positive with rtRT-PCR). Seventeen swabs were collected from symptomatic patients (7%). Among them, the concordance between rtRT-PCR and Ag-RDT was 100%, 7 (41.2%) positive and 10 negative pairs. The overall sensitivity of Ag-RDT was 63.8% (95% CI 60.3–67.3) and specificity was 99.8% (95% CI 99.6–100). PPV and NPV were 97.5% and 96.8%, respectively. Sensitivity and specificity did not change substantially across the two periods (65.1% and 99.8% in 2020–2021 vs. 60% and 100% in 2021–2022).
Conclusions
A periodic Ag-RDT-based screening approach for PEH at point of care could guide preventive measures, including prompt isolation, without referral to hospital-based laboratories for molecular test confirmation in case of positive detection even in individuals asymptomatic for COVID-19. This could help reduce the risk of outbreaks in shelter facilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Few studies have been conducted on the implementation of antigen-detecting rapid diagnostic tests (Ag-RDTs) in congregated homeless shelters. | |
This study assessed the performance of a COVID-19 Ag test as a screening tool for SARS-CoV-2 infection in people experiencing homelessness (PEH) to guide shelter access during cold-weather emergency response plan. | |
The adoption of an Ag-RDT in this study was not able to exclude SARS-CoV-2 infection. | |
Given the high specificity, the implementation of this test in PEH requesting a bed shelter can provide timely information to confirm the infection in case of a positive result, even in absence of symptoms. | |
A periodic Ag-RDT-based screening approach at point of care could help control the spread of SARS-CoV-2 infection in PEH, thus reducing the risk of outbreaks in shelter facilities. |
Introduction
People experiencing homelessness (PEH) are particularly exposed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1]. This is due to inadequate and overcrowded living conditions in homeless shelters as well as poor sanitary conditions and limited use of face masks. Moreover, barriers to timely access to healthcare services and high prevalence of comorbidities compared to the general population increase the risk of developing severe coronavirus disease 2019 (COVID-19) [2,3,4]. SARS-CoV-2 infection prevalence in homeless shelters was reported to be up to 67% in San Francisco, California, USA, while in Italy it was slightly above 8%. In both countries a large proportion of asymptomatic cases was observed [5, 6].
Although nucleic acid amplification tests (NAATs)—such as real-time multiplex reverse transcription polymerase chain reaction (rtRT-PCR)—remain the gold standard for the diagnosis of SARS-CoV-2 infection, they are expensive and time-consuming and require specialized personnel and equipment [7]. Antigen-detecting rapid diagnostic tests (Ag-RDTs) in nasal swabs represent a quick, easy to use, and less expensive alternative for the diagnosis of SARS-CoV-2 infection at point of care [8]. In particular, Ag-RDTs can help reduce further transmission providing a timely result with rapid isolation and contact tracing [9]. However, as for several other Ag-RDTs, there have been many concerns regarding their sensitivity and high rates of false-positive results (especially in settings with lower prevalence rates and thus low pre-test probability) [9, 10]. The European Centre for Disease Prevention and Control agrees with the World Health Organization minimum performance criteria of ≥ 80% sensitivity and ≥ 97% specificity. Ag-RDTs with a higher specificity (> 98%) are preferable for first-line testing to reduce false-positive results [11, 12]. The performance of the 400 commercially available Ag-RDTs [13] varies in different settings and according to the intended use of the test (diagnosis, screening, surveillance).
Diagnostic accuracy studies and detection strategies in vulnerable, hard-to-reach populations such as PEH need to be explored to promptly recognize outbreaks and avoid further viral spread.
The aim of the study was to investigate the diagnostic accuracy of a rapid SARS-CoV-2 antigen test used as a screening tool in PEH during two pandemic waves compared with gold standard rtRT-PCR.
Methods
Study Population, Setting and Procedures
This study is part of a well-established SARS-COV-2 surveillance program promoted by the Municipality of Verona and the ORCHESTRA project together with the University Hospital of Verona, which targeted key populations to ensure the rapid implementation of public health strategies to contain the spread of SARS-CoV-2. The study discussed in this article was performed in the subgroup of the homeless. During two periods starting from 16 November 2020 to 30 May 2021 and subsequently from 30 December 2021 to 20 April 2022, all PEH ≥ 18 years requesting residence at the available shelters (cold-weather emergency response plan) in Verona, Italy, were prospectively screened for SARS-CoV-2 infection regardless of the presence of symptoms. After obtaining written informed consent, two nasopharyngeal swabs (NPS) were collected from each PEH by professional medical staff trained in NPS techniques, according to manufacturer's recommendations. One NPS was collected to perform the Ag-RDT immediately at point of care, and the other was delivered to the Microbiology Unit of the University Hospital of Verona to perform a rtRT-PCR assay. The following data were self-collected for each participant: demographic characteristics (age, sex, nationality); previous SARS-CoV-2 infection and/or vaccination; presence of current or previous (within 2 weeks) COVID-19 symptoms and type: general body malaise, difficulty breathing, headache, sore throat, running nose, cough, loss of smell or taste, nausea/vomiting, diarrhea, and body aches.
The purpose of administering the Ag-RDT was to screen PEH requesting the assignment of a shelter bed at the access point in Verona. The screening was included in a package of services provided by the Municipality of Verona and Diocesan Caritas, an organisation of the Italian Bishop’s Conference engaged in many welfare activities including assistance to the homeless. All subjects who tested positive on the Ag-RDT were transferred to dedicated isolation centers arranged by the Municipality of Verona. The staff was responsible for the communication with the shelter coordination team in order to implement the official procedures for isolation, protection measures, and contact tracing. The Ag-RDT (index test) result was confirmed using the rtRT-PCR considered the clinical reference (comparator) assay for the diagnosis of SARS-CoV-2 infection. The personnel who processed and performed the rtRT-PCR was not aware of the result (either positive or negative) of the Ag-RDT.
The STARD (standard for reporting of diagnostic accuracy studies) statement was adopted as a guideline for study design and reporting [14].
SARS-CoV-2 Rapid Antigen Test
Biocredit® COVID-19 Ag was used as Ag RDT. This is a lateral flow immunochromatographic assay that adopts dual color system. The test contains a colloid gold conjugate pad and membrane strip pre-coated with antibodies specific to SARS-CoV-2 Ag on the test lines. If SARS-COV-2 Ag is present in the specimen, the complexes between the anti-SARS-CoV-2 conjugate and the virus are captured by specific monoclonal Ab (Ab-Ag-Ab gold conjugate complexes), and a visible black band appears on test line T. The control line (C) serves as a procedural control and should always appear if the test is performed correctly (the sample volume is correct, the membrane functioned correctly, etc.). Reading is carried out at between 5 and 8 min. According to the manufacturer, Biocredit® COVID-19 Ag has been evaluated compared to PCR as reference in three different countries (Europe, South America, and Korea). Overall, the results showed 100% specificity and 90.2 sensitivity (sensitivity range 80–96%) [15].
Molecular Detection of SARS-CoV-2
The detection of SARS-CoV-2 was carried out using nasopharyngeal swabs collected from patients using Copan Universal Transport Medium (UTM-RT®) System (Copan Italia Spa, Brescia, Italy). The samples were stored at 4 °C and immediately processed after transport to the laboratory.
The extraction of nucleic acids from samples was carried out using a NIMBUS apparatus, and the amplification and detection of specific SARS-CoV-2 genes were carried out with Allplex™ SARS-CoV-2 assay kit (Seegene, Seoul, Korea) following the manufacturer’s instructions. This multiplex real-time RT-PCR assay detects four viral targets simultaneously including the E, N, RdRp, and S genes with the RdRp and S revealed in the same fluorescence channel. The Ct threshold was considered positive when Ct < 40. All samples were analyzed and interpreted by Seegene Viewer software (Seegene), and the amplification curves could also be visualized and analyszd during and after the run [16].
Statistical Analysis
Means and standard deviations (SD) were calculated for continuous variables, and frequency tables and respective percentages were calculated for categorical variables. Significance of differences between the two study periods were evaluated by chi-square test or Fisher’s exact test for categorical variables and t test for quantitative variables. Measures for diagnostic test accuracy were calculated as follows [17]:
-
Sensitivity = [True Positives/(True Positives + False Negatives)] × 100.
-
Specificity = [True Negatives/(False Positives + True Negatives)] × 100.
-
Positive predictive value (PPV) = [True Positives/(True Positives + False Positives)] × 100.
-
Negative predictive value (NPV) = [True Negatives/(False Negatives + True Negatives)] × 100.
The PPV and NPV were calculated considering the officially reported prevalence of SARS-COV-2 positivity in the same group age of patients in the country in the two different study periods [18]. All analyses were conducted with STATA®, version 17.0 (StataCorp LP, College Station, TX, USA).
Ethical Statement
Ethical approval for this study was obtained from the “Comitato Etico delle Province di Verona e Rovigo” (2948CESC). All procedures were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants.
Results
Overall, 503 participants were enrolled during the two intervention periods for a total of 732 paired swab samples collected. All PEH referred to the access point during the intervention period agreed to undergo both Ag-RDT and molecular tests. The majority (278, 55%) were from sub-Saharan and Northern Africa. The specimens included 541 swabs performed during November 2020–May 2021 (corresponding to the second pandemic wave in Italy) and 191 in the period December 2021–April 2022, during the fourth pandemic wave (Table 1a and b). Average age of subjects was 42 (SD 14) years. No significant differences were reported from subjects tested in the first intervention period compared to the second, except for the rate of previous infection (0.8 vs. 8%) and vaccination (6% vs. 73%; p < 0.001).
Among the 732 swabs collected, 58 resulted positive with rtRT-PCR. Thus, the prevalence of SARS-CoV-2 infection in the cohort was 8%. Overall, 17/732 (2.3%) tests were performed on subjects reporting at least one COVID-related symptom at the time of swab collection. More participants (11/191, 6%) reported symptoms during the period 2021–2022 (p < 0.001). Among the 17 symptomatic subjects, there was a 100% concordance between rtRT-PCR and Biocredit COVID-19 Ag (7 positive, 41.2%, and 10 negative pairs).
Table 2 shows the performance of the Biocredit® COVID-19 Ag compared with the reference rtRT-PCR. Overall, 21 false-negative Biocredit COVID-19 Ag results occurred among the specimens collected while only 1 false-positive result was observed. Sensitivity was 63.8% (95% CI 60.3–67.3); specificity was 99.8% (95% CI 99.6–100). PPV and NPV were 97.5% and 96.8%, respectively. Sensitivity and specificity did not change substantially across the two periods (65.1% and 99.8% in 2020–2021 vs. 60.0% and 100% in 2021–2022; Table 2).
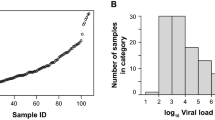
The mean Ct value was 30.4 (SD 5.3) for the RdRp/S viral targets, 30 (SD 5.3) for the E gene, and 27.4 (SD 5.2) for the N gene. The mean S/RdRp Ct value of Ag-RDT-positive samples was 29 (SD 4.7); for the Ag-RDT-negative samples, the mean S gene Ct value was 35 (SD 3.8). To speculate about the difference in SARS-CoV-2 detection by the Ag-RDT at different viral loads, we assessed the sensitivity of the Ag-RDT at three different Ct value ranges of S/RdRp viral targets detected by the rtRT-PCR: ≤ 20, 21–33, and and > 33 ≤ 40. As shown in Table 3, the false-negative results increase with higher Ct values. Sensitivity ranges from 100% (95% CI 100–100%) to 32% (95% CI 28.5–35.5%) for S/RdRP Ct < 20 and > 33 ≤ 40, respectively.
Discussion
This study assessed the analytical performance of the Biocredit® COVID-19 Ag test as a screening tool for SARS-CoV-2 infection in PEH in order to guide shelter access during the cold-weather emergency response plan. The study was conducted in the context of public health surveillance for SARS-CoV-2 of a vulnerable population. Few studies focused on the implementation of Ag-RDTs in congregated homeless shelters. Roland et al. reported a high proportion of SARS-CoV-2 infection among asymptomatic cases, highlighting the importance of testing and isolation measures during the outbreak [19]. A pilot program was carried out in San Francisco, California, offering an Ag-RDT to both residents and staff of congregate-living shelters. Following the pilot phase, the public health department of San Francisco maintained rapid testing in homeless shelters as an alternative to rtRT-PCR [20].
Overall, in our study the sensitivity of the Ag-RDT was low and did not change across the two intervention periods while the specificity remained high Notably, almost 98% of the tests were performed on asymptomatic individuals. The prevalence of SARS-CoV-2 positive rtRT-PCR results was around 8% in both intervention periods.
Several studies have been published on the diagnostic accuracy of Ag-RDTs at point of care, reporting an overall suboptimal sensitivity and high specificity [21,22,23,24].
Notably, these studies mainly included symptomatic individuals. Authors highlighted a clear association between the sensitivity (FN rates) and sample viral load, with the Ct values being significantly lower in the Ag-RDT-positive specimens than in the negative ones. According to the intended use of the index test, in our study we mainly enrolled asymptomatic subjects, and this could partly explain the decreased sensitivity in our cohort. In accordance with other reports, our study showed that the number of Ct values was correlated with sensitivity, reaching 85.7% sensitivity for S gene Ct values < 20. Lower Ct values (< 20) have been shown to be associated with infectivity [25, 26] and with a higher probability of culturing the virus [27]. Therefore, detecting subjects with a higher viral load (lower Ct values), despite being asymptomatic, could help in identifying those individuals who are at higher risk of being infectious.
The latest recommendations on the implementation of the Ag-RDT testing program [8, 11, 12] suggest that Ag-RDTs should be used mainly in symptomatic cases. They can be useful for testing asymptomatic individuals only when the positivity rate is ≥ 10%. Administering Ag-RDTs in a lower prevalence setting could likely result in lower prediction values; however, high PPV rates were achieved in our cohort. On the other hand, the challenge represented by the low sensitivity could be addressed by adopting the so-called “test, re-test, re-test” strategy. This strategy, less expensive and easier to implement compared to one RT-PCR run if the first Ag-RDT is negative, may reduce the probability of false-negative results [28].
The intervention periods of our study correspond to different COVID-19 pandemic waves. According to the epidemiological data released by the national Italian authorities, during the first study period, the alpha variant was the main one in circulation, while during the second period, the omicron variant was dominant [18]. Moreover, also due to the roll-out of the COVID-19 vaccination campaign in Italy, the rate of PEH vaccinated for SARS-CoV-2 during the second period was remarkably higher compared to 1 year before (6% vs. 73%). Our results show that the performance of Ag-RDTs for the diagnosis of SARS-CoV-2 infection was not affected by the viral variant or vaccination status.
The study has some limitations. As per the study design, a NPS was performed as a screening for the access to the shelter but it was not systematically repeated during or after the stay, thus precluding any consideration of the efficacy of the “test, re-test, re-test” strategy. Furthermore, a detailed description of the population in terms of comorbidities was not provided.
Conclusions
Considering the low cost, ease of use, and turnaround time, from a public health perspective our findings suggest that Ag-RDTs can be useful for specific population screening programs, especially in high-prevalence settings or when the epidemic curve rises. This study suggests that detecting asymptomatic subjects with a higher viral load could be crucial to identify those individuals who are at higher risk of being contagious and allow for early intervention in terms of public health measures. Considering the low rate of false-positive results, a periodic Ag-RDT-based screening approach at point of care could reliably guide preventive measures, including prompt isolation without referral to hospital-based laboratories for molecular test confirmation in case of positive results. This could help control the spread of SARS-CoV-2 infection in this vulnerable population, thus reducing the risk of outbreaks in shelter facilities.
References
Karb R, Samuels E, Vanjani R, Trimbur C, Napoli A. Homeless shelter characteristics and prevalence of SARS-CoV-2. West J Emerg Med. 2020;21:1048–53.
Babando J, Quesnel DA, Woodmass K, Lomness A, Graham JR. Responding to pandemics and other disease outbreaks in homeless populations: a review of the literature and content analysis. Health Soc Care Community. 2022;30(1):11–26.
Baggett TP, Liauw SS, Hwang SW. Cardiovascular disease and homelessness. J Am Coll Cardiol. 2018;71:2585–97.
Fazel S, Geddes JR, Kushel M. The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. 2014;384(9953):1529–40.
Mosites E, Parker EM, Clarke KEN, Gaeta JM, Baggett TP, Imbert E, et al. Assessment of SARS-CoV2 infection prevalence in homeless shelters—four U.S. Cities, March 27–April 15, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:521–2.
Ralli M, De-Giorgio F, Pimpinelli F, Cedola C, Shkodina N, Morrone A, et al. SARS-CoV-2 infection prevalence in people experiencing homelessness. Eur Rev Med Pharmacol Sci. 2021;25(20):6425–30.
Esbin MN, Whitney ON, Chong S, Maurer A, Darzacq X, Tjian R. Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection. RNA. 2020;26(7):771–83.
Dinnes J, Sharma P, Berhane S, van Wyk SS, Nyaaba N, Domen J, et al. Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2022;7(7):CD013705.
Centre for Disease Control. Interim guidance for antigen testing for SARS-CoV-2. In: National center for immunization and respiratory diseases (NCIRD), Division of Viral Diseases. 2020.
Peeling RW, Olliaro PL, Boeras DI, Fongwen N. Scaling up COVID-19 rapid antigen tests: promises and challenges. Lancet Infect Dis. 2021;21(9):e290–5.
WHO. Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays. In: Interim guidance. Emergencies Preparedness, WHO Headquarters (HQ). 2020; p. 9.
ECDC. Options for the use of rapid antigen detection tests for COVID-19 in the EU/EEA – first update. Technical report. 26 October 2021.
Foundation for Innovative New Diagnostics. Test directory (https://www.finddx.org/test-directory/). Accessed 5 Dec 2022.
Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11):e012799.
Rapigen Biocredit® COVID-19 Ag. https://biott.it/wp-content/uploads/2020/11/01-IFU-Biocredit-Covid-19-Ag-Rapid-ita-1.pdf. Accessed 9 Mar 2023.
So MK, Park S, Lee K, Kim SK, Chung HS, Lee M. Variant prediction by analyzing RdRp/S gene double or low amplification pattern in Allplex SARS-CoV-2 assay. Diagnostics (Basel, Switzerland). 2021;11(10):1854.
Watson J, Richter A, Deeks J. Testing for SARS-CoV-2 antibodies. BMJ. 2020;370:m3325.
Istituto Superiore di Sanità. https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati. Accessed 5 Dec 2022.
Roland M, Ben Abdelhafidh L, Déom V, Vanbiervliet F, Coppieters Y, Racapé J. SARS-CoV-2 screening among people living in homeless shelters in Brussels, Belgium. PLoS One. 2021;16(6):e0252886.
Aranda-Díaz A, Imbert E, Strieff S, Graham-Squire D, Evans JL, Moore J, et al. Implementation of rapid and frequent SARS-CoV2 antigen testing and response in congregate homeless shelters. PLoS One. 2022;17(3):e0264929.
Albert E, Torres I, Bueno F, Huntley D, Molla E, Fernández-Fuentes MÁ, et al. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect. 2021;27(3):472.e7-472.e10.
Lee H, Kang H, Cho Y, Oh J, Lim TH, Ko BS, et al. Diagnostic Performance of the rapid antigen test as a screening tool for SARS-CoV-2 infection in the emergency department. J Pers Med. 2022;12(7):1172.
Giberti I, Costa E, Domnich A, Ricucci V, De Pace V, Garzillo G, et al. High diagnostic accuracy of a novel lateral flow assay for the point-of-care detection of SARS-CoV-2. Biomedicines. 2022;10(7):1558.
Rohde J, Himmel W, Hofinger C, Lâm TT, Schrader H, Wallstabe J, et al. Diagnostic accuracy and feasibility of a rapid SARS-CoV-2 antigen test in general practice - a prospective multicenter validation and implementation study. BMC Prim Care. 2022;23(1):149.
Coyle PV, Al Molawi NH, Kacem M, El Kahlout RA, Al Kuwari E, Al Khal A, et al. Reporting of RT-PCR cycle threshold (Ct) values during the first wave of COVID-19 in Qatar improved result interpretation in clinical and public health settings. J Med Microbiol. 2022. https://doi.org/10.1099/jmm.0.001499.
Kampf G, Lemmen S, Suchomel M. Ct values and infectivity of SARS-CoV-2 on surfaces. Lancet Infect Dis. 2021;21(6): e141.
Rao SN, Manissero D, Steele VR, Pareja J. A systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis Ther. 2020;9(3):573–86.
Ramdas K, Darzi A, Jain S. “Test, re-test, re-test”: using inaccurate tests to greatly increase the accuracy of COVID-19 testing. Nat Med. 2020;26(6):810–1.
Acknowledgements
We thank all the participants of the study. We also thank Giuseppe Turrini, Lorenzo Marasca, Beatrice Callegaro, and all the personnel working with the Diocesan Caritas, “Cooperativa Salute e Territorio,” and social services of the Municipality of Verona for their support to this research, especially in the coordination and logistics. We are grateful to Ruth Joanna Davis for language editing.
Funding
The study has been partly funded by the ORCHESTRA project, which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 101016167 and partly by the Municipality of Verona. The journal’s Rapid Service fees are funded by the Municipality of Verona.
Author Contributions
Pasquale De Nardo, Maela Tebon, and Evelina Tacconelli conceived the study. Pasquale De Nardo, Alessia Savoldi, Maela Tebon, and Nicola Soriolo designed the study and enrolled the patients. Pasquale De Nardo wrote the first draft of the manuscript. Elisa Danese and Denise Peserico, Riccardo Cecchetto, Annarita Mazzariol, and Davide Gibellini were responsible for all laboratory procedures. Elisa Gentilotti and Matteo Morra conducted the statistical analyses. Gulser Caliskan, Pierpaolo Marchetti, and Giuseppe Verlato contributed to the study design and reviewed the statistical analysis. All the authors reviewed and approved the final version of the manuscript.
Disclosures
Pasquale De Nardo, Maela Tebon, Alessia Savoldi, Nicola Soriolo, Elisa Danese, Denise Peserico, Matteo Morra, Elisa Gentilotti, Gulser Caliskan, Pierpaolo Marchetti, Riccardo Cecchetto, Annarita Mazzariol, Giuseppe Verlato, Davide Gibellini, and Evelina Tacconelli all confirm that they have no conflicts of interest to declare.
Compliance with Ethics Guidelines
Ethical approval for this study was obtained from the “Comitato Etico delle Province di Verona e Rovigo” (2948CESC). All procedures were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The informed consent was obtained from all participants.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
De Nardo, P., Tebon, M., Savoldi, A. et al. Diagnostic Accuracy of a Rapid SARS-CoV-2 Antigen Test Among People Experiencing Homelessness: A Prospective Cohort and Implementation Study. Infect Dis Ther 12, 1073–1082 (2023). https://doi.org/10.1007/s40121-023-00787-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00787-0