Abstract
Introduction
International treatment guidelines recommend the rapid initiation of antiretroviral therapy (ART) with bictegravir (B)/emtricitabine (F)/tenofovir alafenamide (TAF) and dolutegravir (DTG)-based regimens for treatment-naïve persons living with HIV (PLWH) irrespective of their disease stage. However, we lack evidence of the virological efficacy, virological failure, and tolerability of coformulated B/F/TAF and DTG/ABC/3TC regimens in persons living with advanced HIV (PLWAH; defined as persons with a CD4+ count of < 200 cells/μL or an AIDS-related opportunistic illness [AOI] at or before ART initiation) in the era of rapid ART.
Methods
This retrospective multicenter study enrolled treatment-naïve PLWAH initiating ART with coformulated DTG/ABC/3TC or B/F/TAF in 2019–2020. Viral suppression at week 48 was analyzed using FDA snapshot analysis. Between-regimen differences in time to viral suppression (< 50 copies/mL), virological failure, and regimen discontinuation were examined using a Cox proportional hazards model. Analysis was also performed using time to regimen discontinuation due to adverse reactions (ARs) as the outcome.
Results
We enrolled 162 patients, including 61.1% on DTG/ABC/3TC and 38.9% on B/F/TAF. At week 48 after ART initiation, 73.47% on DTG/ABC/3TC and 85.71% on B/F/TAF achieved viral suppression (P = 0.178). We identified no between-regimen differences in time to viral suppression or virological failure, regardless of pre-ART viral load. Compared with the DTG/ABC/3TC group, regimen discontinuation was less prevalent in the B/F/TAF group (adjusted hazard ratio = 0.23, 95% CI 0.06–0.85, P = 0.027). The main reason for discontinuation in both groups was ARs (61.9% in the DTG/ABC/3TC and 50% in the B/F/TAF, P = 0.877), of which skin manifestations were the most common in both groups (61.5% in the DTG/ABC/3TC and 50% in the B/F/TAF, P = 0.756). DTG/ABC/3TC, same-day ART prescription, and AOI were risk factors for AR or virological failure-related regimen discontinuation.
Conclusion
In the real world, the risk of regimen discontinuation was higher in PLWAH on coformulated DTG/ABC/3TC than in those on B/F/TAF, with no difference in viral suppression or virological failure. Given the findings concerning the effect of same-day ART prescription and AOIs on AR or virological failure-related regimen discontinuation, individualized approaches to PLWAH are necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Although the treatment guidelines recommend the rapid initiation of B/F/TAF and DTG-based regimens for treatment-naïve persons living with HIV (PLWH) irrespective of their disease stage, the recommendation may not be equally suitable for treatment-naïve persons living with advanced HIV (PLWAH) and PLWH because of the overlapping toxicities of concomitant therapies for AIDS-related opportunistic illness (AOI) and the development of immune reconstitution inflammatory syndrome after antiretroviral therapy (ART) initiation among PLWAH. |
However, the between-regimen differences of coformulated B/F/TAF and coformulated DTG-based regimens in terms of clinical outcomes for treatment-naïve PLWAH in the era of rapid ART are lacking. |
We compared the virological efficacy, virological failure, and tolerability of the DTG/ABC/3TC regimen with that of the B/F/TAF regimen in PLWAH in the era of rapid ART. |
What was learned from the study? |
Although no between-regimen differences in time to viral suppression or virological failure were identified, DTG/ABC/3TC, same-day ART prescription, and AOI were risk factors for adverse reactions (AR)-related regimen discontinuation. |
Although B/F/TAF offers advantages over DTG/ABC/3TC in terms of AR-related regimen discontinuation, the associations of same-day ART prescription and AOIs with AR-related regimen discontinuation may indicate the need for individualized approaches to treatment-naïve PLWAH in the era of rapid ART. |
Introduction
Advanced human immunodeficiency virus (HIV) is a critical public health problem. Persons living with advanced HIV (PLWAH) are defined as those with a CD4+ count of < 200 cells/μL or a history of an acquired immune deficiency syndrome (AIDS)-related opportunistic illness (AOI) at presentation [1, 2]. The prevalence of advanced HIV among persons newly diagnosed with HIV is approximately 40% in Taiwan [3].
Since 2007, various types of integrase strand transfer inhibitors (INSTIs), namely raltegravir, dolutegravir (DTG), elvitegravir, and bictegravir (B), have been consecutively approved by the US Food and Drug Administration (FDA) for the treatment of treatment-naïve persons living with HIV (PLWH) [4]. At present, in the era of rapid antiretroviral therapy (ART), second-generation INSTIs (coformulated B/emtricitabine (F)/tenofovir alafenamide (TAF) and DTG-containing regimens) are the preferred anchor drugs for most treatment-naïve PLWH worldwide irrespective of disease stage [5,6,7]. Boosted protease inhibitor- and nonnucleoside reverse transcriptase inhibitor-based regimens are only recommended in certain clinical situations or for persons with a history of pre-exposure prophylaxis with long-acting injectable cabotegravir [5,6,7]. Although coformulated B/F/TAF and DTG-containing regimens have become mainstream in treating HIV infection owing to their potent efficacy [8,9,10,11] and favorable tolerability, as demonstrated in clinical tria≤ls [8,9,10,11] and in nonclinical settings [12, 13], the rapid initiation of INSTI-based ART for treatment-naïve PLWAH may be complicated by the overlapping toxicities of concomitant therapies for AOIs, including pharmacokinetic/pharmacodynamic interactions between combination ART (cART) and non-cART regimens for AOIs and by the development of immune reconstitution inflammatory syndrome (IRIS) after same-day cART initiation [14,15,16,17]. Moreover, in treatment-naïve PLWH who initiate cART with INSTIs, a pre-cART CD4+ count of < 200 cells/μL is still associated with a high risk of treatment failure [15] and is negatively associated with viral suppression [18]. Therefore, the rapid initiation of second-generation INSTI-based ART, as per treatment guidelines, may not be equally suitable for treatment-naïve PLWAH and PLWH. Thus, investigating the between-regimen differences of second-generation INSTI-based ART in terms of clinical outcomes for treatment-naïve PLWAH in the era of rapid ART is essential.
Studies comparing the virological efficacy, virological failure, and tolerability of B/F/TAF and DTG-based regimens in PLWAH are currently lacking, and healthcare providers have little guidance on treatment regimen selection. Phase 3 randomized clinical trials (RCTs) have revealed that coformulated B/F/TAF and a DTG-containing regimen had comparable virological efficacy, safety, and tolerability. However, < 15% of treatment-naïve participants had a CD4+ count of < 200 cells/μL in these RCTs [8,9,10,11]. Likewise, in a prospective cohort study of treatment-naïve PLWAH conducted from January 1, 2018 to July 31, 2019, B/F/TAF and a DTG-containing regimen demonstrated comparable virological efficacy [13]. However, only 52% of participants in the DTG-based arm received a single-tablet regimen (STR) of specifically coformulated DTG/abacavir (ABC)/lamivudine (3TC). Since STRs have been associated with higher adherence to cART [19] and more effective viral suppression [20, 21] relative to multiple-tablet regimens, the uneven distribution of STR between the B/F/TAF and the three-tablet DTG-based regimen may have confounded between-regimen differences in clinical outcomes in this study [13].
The Taiwanese government has implemented the treat-all policy of the World Health Organization since 2016 and a rapid ART initiation policy since 2018. STRs with second-generation INSTI-based regimens have been recommended as the first-line treatment for treatment-naïve PLWH in Taiwan since mid-2016. Coformulated DTG/ABC/3TC, B/F/TAF, and 3TC/DTG have been available since June 2016, October 2019, and December 2020, respectively. 3TC/DTG was not prescribed during the study period (from January 1, 2019 to December 31, 2020); thus, it was not included in this study. Our primary aim was to compare the virological effectiveness of the B/F/TAF and DTG/ABC/3TC regimens at week 48. Our secondary aim was to compare time to viral suppression, virological failure, regimen discontinuation, and regimen discontinuation due to adverse reactions (ARs) under the two regimes among treatment-naïve PLWAH.
Methods
Study Population and Design
We conducted this retrospective cohort study at six HIV-designated hospitals: Kaohsiung Veterans General Hospital (KVGH), Kaohsiung Chang Gung Memorial Hospital (CGMH), Chi Mei Medical Center (CMMH), Kaohsiung Medical University Chung-Ho Memorial Hospital (KMUH), Kaohsiung Municipal Siaogang Hospital (KMSH), and Kaohsiung Municipal Ta-Tung Hospital (KMTTH). Healthcare personnel at each institution recorded the CD4+ counts and plasma viral load (PVL) of patients at baseline and in follow-up visits.
Ethics
The study protocol was approved by the institutional review boards of the participating hospitals: KMUH, KMSH, and KMTTH (KMUHIRB-SV(I)-20170084); CGMH (202101662A3D001); CMMH (11106-003); and KVGH (VGHKS19-CT4-02 and VGHKS18-CT4-20).
Written informed consent was obtained from patients at KMUH, KMSH, KMTTH, and KVGH. The requirement for informed consent was waived by the institutional review boards of CMMH and CGMH.
Patient Selection
We enrolled treatment-naïve PLWAH (CD4+ count < 200 cells/μL or any episode of AOI at or before initiation of cART) from the participating hospitals between January 1, 2019 and December 31, 2020. PLWAH who initiated cART with coformulated B/F/TAF or DTG/ABC/3TC were included. We excluded PLWAH who had two missing data entries of CD4+ and PVL following cART initiation. The follow-up time was defined as the period from the start of the initial coformulated regimen until 48 weeks after cART commencement, the last visit, modification of the initial regimen, or the patient’s death, whichever occurred first. Each enrolled patient was grouped by initial INSTI regimen (DTG/ABC/3TC or B/F/TAF).
Data Collection
Data on baseline demographic and clinical characteristics (age at HIV diagnosis, sex, comorbidities, occupation, marital status, HIV diagnosis date, and HIV transmission category), ART administration (date of cART initiation, regimen prescription, date of regimen discontinuation, and etiologies of regimen discontinuation), and laboratory results [hepatitis A virus antibody (Ab), hepatitis B virus surface antigen (HBsAg), and hepatitis C virus Ab levels and nontreponemal test] were collected. In addition, data on liver enzymes, total bilirubin, fasting lipid profile, creatinine, glomerular filtration rate estimated by applying the Modification of Diet in Renal Disease equation, CD4+ count, and PVL were collected at HIV presentation, cART initiation, and each follow-up visit. The complete blood count, serum biochemistry panel, HIV-RNA, and CD4+ cell count testing were conducted by the laboratories of each hospital (tests for HIV-RNA and CD4 cell counts at KMSH and KMTTH were conducted by the laboratory of KMUH). Each of the laboratories is accredited every 3 years by the Taiwan Accreditation Foundation, which was founded to provide impartial, objective, and independent third-party accreditation services in compliance with international standards [22]. HIV-RNA was quantified through polymerase chain reaction using a Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 test version 2.0 (Roche Molecular Systems, Pleasanton, CA, USA) (KMUH, CGMH, and KVGH) or an Abbott m2000 RealTime HIV-1 assays (Abbott Molecular Inc., Chicago, IL, USA) (CMMH). The CD4+ cell counts were analyzed using a Cytomics FC500 flow cytometer (Beckman Coulter, Brea, CA, USA) at KMUH, CGMH, and KVGH and using a BD FACS Calibur multiparameter flow cytometer (Becton Dickinson, Mountain View, CA, USA) at CMMH. Pretreatment HIV-RNA levels were categorized as ≤ 500,000 or > 500,000 copies/mL. Baseline CD4+ counts were categorized as ≤ 50 or > 50 cells/μL. PVL and CD4+ counts at each follow-up visit (weeks 4 [± 2], 12 [± 6], 24 [± 6], 36 [± 6], and 48 [± 6]) were collected and anonymized prior to analysis.
Working Definition
Baseline laboratory tests were performed as soon as possible or within 6 months of HIV diagnosis [23]. The pre-cART CD4+ count and PVL data within 3 months prior to the cART initiation date were obtained as close to that date as possible [24]. The CD4+ count and PVL data 48 weeks after cART initiation were defined as the CD4+ count and PVL data at 48 (± 6) weeks after cART initiation through FDA snapshot analysis.
Regimen discontinuation was defined as either a modification of the initial regimen or a gap of > 45 days without any cART prescription [13]. Reasons for regimen discontinuation were derived from the provider’s notes in electronic medical records supplemented with a review of laboratory results, diagnoses, and the prescribed regimen. We classified the reasons for regimen discontinuation into four categories: ARs, virological failure, switching to a two-drug regimen, and other. Reasons that were classified as other included pregnancy, lifestyle and diet considerations, drug–drug interactions, or provider choice. ARs were further categorized as central nervous system toxicity (dizziness, headache, insomnia, abnormal dreams, or vertigo), skin manifestations (pruritus, rash, or hypersensitivity reaction), gastrointestinal or hepatic toxicity (gastrointestinal intolerability, nausea, vomiting, jaundice, or hepatitis), or renal toxicity (declining estimated glomerular filtration rate, electrolyte imbalance, or proteinuria) [25]. Reasons for discontinuation due to ARs were not mutually exclusive.
Definitions of viral suppression and virological failure differ between guidelines [5, 6]. Therefore, we defined time to viral suppression as the time from the start of treatment to the first viral load of < 50 HIV-1 RNA copies/mL. Virological failure was defined as the presence of two consecutive RNA values of > 50 copies/mL after at least 24 weeks of continual treatment, one RNA value of > 50 copies/mL after 24 weeks of treatment followed by treatment change to another drug class, or no viral suppression to < 50 copies/mL after more than 24 weeks of treatment [15].
Outcomes of Interest
Our primary aim was to compare the virological effectiveness of the B/F/TAF and DTG/ABC/3TC regimens at week 48. Our secondary aim was to compare time to viral suppression, virological failure, regimen discontinuation, and regimen discontinuation due to ARs between the two regimens. We also explored the etiologies of regimen discontinuation.
Statistical Analysis
Categorical variables are presented using frequency tables, and continuous variables are presented in terms of median with interquartile range (IQR). Between-regimen differences of several characteristics were analyzed using the Mann–Whitney U test for continuous variables and Fisher’s exact test or a χ2 test for categorical variables.
The unadjusted cumulative probabilities of viral suppression, virological failure, and regimen discontinuation were estimated over time using the Kaplan–Meier method. Univariable and Cox regression analyses with backward selection were performed to examine variables associated with time to viral suppression, time to virological failure, and time to regimen discontinuation. No violations of the proportional hazards assumption were detected when calculating Schoenfeld residuals. A two-tailed P value < 0.05 indicated significance. Analyses were conducted using SAS software (version 9.4; SAS Institute, Cary, NC, USA).
Results
Study Population
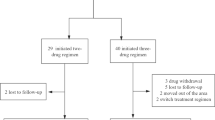
We identified 162 PLWAH, specifically 51 in KMUH, 13 in KMTTH, 5 in KMSH, 36 in KVGH, 33 in CGMH, and 24 in CMMH. The six sites were equally balanced in terms of baseline sociodemographic characteristics (Supplementary Table S1). In total, 99 (61.1%) patients were on DTG/ABC/3TC, and 63 (38.9%) patients were on B/F/TAF (Fig. 1). Most patients were men (98.15%), men who have sex with men (88.27%), and aged ≤ 30 years (48.15%). In total, 36.42% of patients initiated cART on the day of HIV diagnosis (same-day prescription), and 88.27% of patients initiated cART within 14 days of HIV diagnosis. The median baseline CD4+ count (IQR) was 77.0 (31.0–150.0), and the median baseline viral load (IQR) was 5.22 (4.81–5.78) log10 copies/mL.
Overall, key baseline characteristics were similar between the B/F/TAF and DTG/ABC/3TC groups; however, some notable differences were observed (Table 1). Compared with those on DTG/ABC/3TC, PLWAH on B/F/TAF were more likely to be women, to have received a diagnosis of HIV in 2020, and to have initiated cART on the day of HIV diagnosis.
Comparison of Virological Trajectories Between DTG/ABC/3TC and B/F/TAF
Overall, 84% of the participants achieved viral suppression by week 48; 73.47% of patients on DTG/ABC/3TC and 85.71% of those on B/F/TAF achieved viral suppression at week 48 (P = 0.178) (Fig. 2).
Time to Viral Suppression
During the 45,135-person-day follow-up period, we observed 128 episodes of viral suppression (103.51 per 100 person-years of follow-up [PYFU], 95% CI 87.07–123.06), with the median time (IQR) to viral suppression being 98 days (40–132). Overall, 79.01% (128/162) of patients reached viral suppression within 48 weeks of cART initiation.
The unadjusted incidence rates of viral suppression in the DTG/ABC/3TC (99.24 per 100 PYFU, 95% CI 78.54–125.40) and B/F/TAF groups (109.18 per 100 PYFU, 95% CI 84.44–141.17) were comparable. The median time (IQR) to viral suppression between the DTG/ABC/3TC (98 [38–126]) and B/F/TAF groups (99 [42–144]) were also comparable.
We identified no between-regimen difference in time to viral suppression (Fig. 3a and Table 2). Compared with a pre-cART viral load of > 500,000 copies/mL, a pre-cART viral load of ≤ 500,000 copies/mL was associated with significantly higher viral suppression (adjusted hazard ratio [aHR] = 2.41, 95% CI 1.55–3.75, P < 0.001). In subgroup analysis where the sample was stratified by pre-cART viral load, the regimens did not significantly differ in time to viral suppression.
Cumulative probability of experiencing viral suppression, virological failure, and regimen discontinuation at 48 weeks stratified by initial cART. a Viral suppression stratified by initial cART. b Virological failure stratified by initial cART. c Regimen discontinuation stratified by initial cART. P values were calculated by using the log-rank test. Significant was indicated by P < 0.05. cART combination ART
Time to Virological Failure
During the 41,995-person-day follow-up, we observed 23 episodes of virological failure (20.00 per 100 PYFU, 95% CI 13.30–30.11). Overall, 14.20% of the patients experienced virological failure, of which 91.30% of failures were attributed to the lack of viral suppression to < 50 copies/mL after more than 24 weeks of cART initiation (Table 3).
The unadjusted incidence rates of virological failure were comparable in the B/F/TAF (18.13 per 100 PYFU, 95% CI 9.43–34.84) and DTG/ABC/3TC groups (21.40 per 100 PYFU, 95% CI 12.68–36.13). The regimens did not significantly differ in the incidence of each category of virological failure (Table 3). A lack of viral suppression to < 50 copies/mL after more than 24 weeks of cART initiation was most frequent in both groups (92.86% in the DTG/ABC/3TC group and 88.89% in the B/F/TAF group, P = 0.332).
The regimens did not significantly differ in terms of time to virological failure (Fig. 3b and Table 2). Compared with a pre-cART viral load of > 500,000 copies/mL, a pre-cART viral load of ≤ 500,000 copies/mL was associated with fewer instances of virological failure (aHR 0.31, 95% CI 0.13–0.73, P = 0.007). In subgroup analysis where the sample was stratified by pre-cART viral load, the regimens did not significantly differ in time to virological failure.
Time to Regimen Discontinuation
During the 45,135-person-day follow-up, we observed 25 episodes of regimen discontinuation (20.05 per 100 PYFU, 95% CI 13.66–29.92), with the median time (IQR) to regimen discontinuation being 55 (29–150) days. Overall, 15.04% of patients experienced regimen discontinuation. Among the 25 episodes of regimen discontinuation, 60% (15/25) were due to ARs, of which 73.3% (11/15) were classified as skin manifestations and 20% were classified as central nervous system toxicity (Table 3).
The unadjusted incidence rate of regimen discontinuation was significantly lower in the B/F/TAF group (7.53 per 100 PYFU, 95% CI 2.83–20.06) than in the DTG/ABC/3TC group (29.77 per 100 PYFU, 95% CI 19.41–45.65). The median time (IQR) to regimen discontinuation was 22 (7–119.5) in the B/F/TAF group and 57 (34–150) in the DTG/ABC/3TC group. Similarly, no between-regimen difference in each reason for regimen discontinuation was identified (Table 3). The most common reason for discontinuation in both groups was ARs (61.9% [13/21] in the DTG/ABC/3TC group and 50% [2/4] in the B/F/TAF group, P = 0.877), and skin manifestations were the most common ARs in both groups (61.5% in the DTG/ABC/3TC group and 50% in the B/F/TAF group, P = 0.756).
We identified a difference in time to regimen discontinuation between regimens (Fig. 3c and Table 2), with the B/F/TAF group having a significantly lower risk of regimen discontinuation than the DTG/ABC/3TC group (aHR 0.23, 95% CI 0.06–0.85, P = 0.027). Age > 30 years (age ≤ 30 years: reference, 31–40 years: aHR 6.97, 95% CI 2.04–23.87; P < 0.001 and ≥ 41 years: aHR 8.67, 95% CI 2.48–30.27; P < 0.001) and same-day prescription (non-same-day prescription: reference, aHR 11.69, 95% CI 4.19–32.63; P < 0.001) were positively associated with the risk of regimen discontinuation. An HIV diagnosis in 2020 was associated with a significantly lower risk of regimen discontinuation (aHR 0.17, 95% CI 0.06–0.52; P = 0.002).
When reasons for regimen discontinuation were limited to ARs or virological failure, same-day prescription (aHR 11.15, 95% CI 3.03–40.98; P < 0.001) and AOI (aHR 4.49, 95% CI 1.23–16.37; P < 0.023) were associated with a significantly higher risk of regimen discontinuation. The B/F/TAF group had a significantly lower risk of regimen discontinuation than the DTG/ABC/3TC group (aHR 0.20, 95% CI 0.04–0.91, P = 0.037).
Discussion
To our knowledge, this is the first observational study to compare two coformulated second-generation INSTIs in terms of time to virological efficacy, virological failure, and regimen discontinuation among treatment-naïve PLWAH in the era of rapid ART. Overall, the two regimens had comparably high rates of viral suppression 48 weeks after cART initiation. The regimens did not significantly differ in time to viral suppression or time to virological failure within 48 weeks of cART initiation, regardless of pre-cART viral load. However, patients on B/F/TAF were 80% less likely to discontinue their regimen because of ARs within 48 weeks than were those on DTG/ABC/3TC. Same-day prescription and AOI were associated with a higher risk of regimen discontinuation due to ARs.
In a prospective study of 961 treatment-naïve PLWAH conducted from 2018 to 2019, time to viral suppression did not significantly differ between coformulated B/F/TAF and DTG-based regimens. However, the study findings were confounded by the uneven distribution of STRs between regimens (only 52% used coformulated DTG/ABC/3TC in the DTG-based regimens) [13]. To control for such confounding, we exclusively enrolled individuals on STRs. Our findings demonstrate that coformulated B/F/TAF and coformulated DTG/ABC/3TC have comparable efficacy with regard to time to viral suppression.
The rates of viral suppression (84%) among PLWAH at week 48 in this study are comparable to those reported in a retrospective multicenter cohort study conducted in Europe, in which 86.1% of the treatment-naïve PLWAH on an INSTI-based regimen reached viral suppression [25]. Although differences in baseline characteristics complicate the direct comparison of viral suppression among studies, the rates of viral suppression at week 48 are generally lower than those reported in a clinical trial on the efficacy of B/F/TAF and DTG/ABC/3TC in 629 treatment-naïve PLWH (missing-as-excluded analysis, 99.3% vs. 97.7%) [11]. Both the high prevalence of AOIs and higher pre-cART PVL characteristic of PLWAH explain why viral suppression at week 48 was poorer in the present study than in the clinical trial. First, the concomitant high prevalence of AOIs among PLWAH may contribute to a higher risk of virological failure due to drug–drug interactions or the development of IRIS [15, 18, 25]. Second, the present study enrolled only PLWAH, who are characterized by a higher pre-cART PVL. A higher pre-cART PVL led to a longer time to viral suppression among treatment-naïve PLWAH in the present study. The enrollment of individuals with a higher pre-cART PVL also explains why a lack of viral suppression to < 50 copies/mL after more than 24 weeks of treatment accounted for the major etiology (91.3%) of virological failure in the present study; this differs from the findings of the Swiss HIV Cohort Study, which examined 1419 treatment-naïve PLWH (19.8% with a baseline CD4+ count of < 200 cells/μL) and reported that only 7.4% of virological failures were due to a lack of viral suppression to < 50 copies/mL after more than 24 weeks of treatment [15].
Both B/F/TAF and DTG/ABC/3TC have strong antiviral properties, a high genetic barrier [26], and well-described rapid viral load decay [25, 27]. However, coformulated B/F/TAF might have advantages over coformulated DTG/ABC/3TC if tolerability is considered. In this study, DTG/ABC/3TC was more likely to be discontinued than was B/F/TAF. The finding is consistent with a study on the OPERA cohort of treatment-naïve PLWAH [13] (a collaboration of 79 US outpatient clinics) and with the findings of US studies on treatment-naïve PLWH initiating cART from 2016 to 2019, regardless of pre-cART CD4+ counts [28, 29]. Among virologically suppressed PLWH switching to DTG/ABC/3TC or B/F/TAF, AR-related discontinuation was also more prevalent for those switching to DTG/ABC/3TC than for those switching to B/F/TAF in a multicenter cohort study conducted in Italy [30].
In the present study, 8.08% of individuals in the DTG/ABC/3TC group experienced regimen discontinuation due to AR, of which skin manifestations were the most common (61.5%). This finding differs from those reported in a clinical trial study [14] and an observational study [25] in which patients are not limited to PLWAH. Those investigations implicated neuropsychiatric and gastrointestinal toxicity as the main causes of AR-related regimen discontinuation. The observational cohort study of a cART containing DTG evaluated the overall efficacy and tolerability of DTG-based regimens in both treatment-naïve and treatment-experienced individuals and also reported neuropsychiatric symptoms as the main cause of regimen discontinuation [31].
The high rate of AR-related regimen discontinuation due to skin manifestations in the present DTG/ABC/3TC group, however, may have been overestimated by physicians. ABC-based regimens should only be prescribed in HLA-B*5701-negative PLWH [5, 6] because of the strong association between HLA-B*5701 and ABC-related hypersensitivity reaction (HSR) [32, 33]. However, because PLWH in Taiwan are less likely to express HLA-B*5701 (0.3%) [34] than are individuals in Western countries (5.6%) [33], physicians in Taiwan do not routinely screen for HLA-B*5701 before prescribing ABC-based regimens. Nevertheless, physicians in Taiwan tend to halt DTG/ABC/3TC treatment upon the development of any skin rash, regardless of whether it is related to ABC. Specifically, the development of skin manifestations in the DTG/ABC/3TC group could be due to the cumulative toxicity of non-cART drugs for AOIs, which are prevalent among PLWAH [25]. In this retrospective cohort study, it was not possible to confirm the incidence of AR-related regimen discontinuation due to HSR (the term “HSR” was suspected in the electronic medical records for two patients in the DTG/ABC/3TC group who discontinued treatment). Therefore, prospective cohort studies that standardize the recording of AR-related regimen discontinuation are warranted to determine whether skin manifestations from DTG/ABC/3TC, but not from non-cART drugs for AOIs, are genuinely the main reason for AR-related discontinuation of DTG/ABC/3TC among PLWAH.
DTG-based regimens have been reported to be associated with the development of neuropsychiatric symptoms [35,36,37,38]. However, evidence regarding neuropsychiatric symptom-related discontinuation of B/F/TAF versus DTG/ABC/3TC is inconsistent. In a clinical trial of treatment-naïve PLWH, B/F/TAF and DTG/ABC/3TC were associated with comparable frequencies of central nervous system and psychiatric adverse events [11]. However, in a phase 3 trial on virologically suppressed PLWH, participants on DTG/ABC/3TC were more likely to report symptoms such as sadness, low mood, depression, nervousness, anxiety, and poor sleep quality than were those on B/F/TAF [39]. In the present DTG/ABC/3TC group, 23.1% (3/13) of AR-related regimen discontinuation was due to neuropsychiatric symptoms, compared with 0% in the present B/F/TAF group. However, findings from real-world cohorts are limited by either insufficient power to compare between regimens or by samples that are not preselected for neuropsychiatric symptoms. Therefore, prospective studies that standardize the recording of AR-related regimen discontinuation are warranted to compare AR-related regimen discontinuation due to neuropsychiatric symptoms between these two regimens.
The incidence of AR or virological failure-related regimen discontinuation was also associated with AOIs and same-day ART, possibly as a result of drug–drug interactions between non-cART regimens for AOIs and cART or toxicities attributable to AOI treatment [15, 16, 40]. Same-day cART prescription being a strong risk factor for regimen discontinuation is likely due to the insufficient evaluation of PLWAH before cART initiation with respect to comorbidities, AOIs, and recreational or illicit drug use. These may contribute to drug–drug interactions between non-cART regimens [41] or recreational drugs [41, 42] and cART or may lead to the development of IRIS after same-day cART initiation [14]. Guidelines recommend the immediate initiation of INSTI-based ART for PLWH irrespective of disease stage [5, 6] and despite the risk of IRIS. Herein, AOIs and same-day ART corresponded to a higher risk of AR-related regimen discontinuation among PLWAH.
A strength of this study is that it is the first to compare time to viral suppression, virological failure, and regimen discontinuation in treatment-naïve PLWAH under coformulated B/F/TAF and DTG/ABC/3TC regimens in the era of rapid ART; yet, this study has limitations. First, its retrospective design meant that confounders were not controlled for. Second, its small sample size and short follow-up time limited the findings’ statistical power to indicate between-regimen differences in specific causes of AR-related regimen discontinuation. Further prospective cohort studies that standardize the recording of AR-related regimen discontinuation are warranted to determine the contribution of skin manifestations to the overall incidence of AR-related regimen discontinuation among PLWAH on DTG/ABC/3TC and to compare the incidence of AR-related regimen discontinuation due to neuropsychiatric symptoms between these two regimens. Besides, the finding of skin manifestations as the major etiology of regimen discontinuation among PLWAH on DTG/ABC/3TC may not be generalized to Western countries, where HLA-B*5701 screening is recommended before prescribing ABC-based regimen.
Conclusions
Although the regimens do not differ in time to viral suppression or virological failure, B/F/TAF is preferred over DTG/ABC/3TC for initial therapy in PLWAH because B/F/TAF is more tolerable. Whether skin manifestations contribute to AR-related discontinuation of DTG/ABC/3TC requires further prospective investigation. Further, the strong associations of same-day ART prescription and AOIs with AR or virological failure-related regimen discontinuation may indicate the need for individualized approaches to treatment-naïve PLWAH in the era of rapid ART.
Change history
16 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40121-023-00764-7
References
Antinori A, Coenen T, Costagiola D, et al. Late presentation of HIV infection: a consensus definition. HIV Med. 2011;12:61–4.
IeDEA and COHERE Cohort Collaborations. Global trends in CD4 cell count at the start of antiretroviral therapy: collaborative study of treatment programs. Clin Infect Dis. 2018;66:893–903.
Lee CY, Lin YP, Wang SF, Lu PL. Late cART initiation consistently driven by late HIV presentation: a multicenter retrospective cohort study in Taiwan from 2009 to 2019. Infect Dis Ther. 2022;11:1033–56.
Scarsi KK, Havens JP, Podany AT, Avedissian SN, Fletcher CV. HIV-1 integrase inhibitors: a comparative review of efficacy and safety. Drugs. 2020;80:1649–76.
ClinicalInfo. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. 2022. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new-guidelines. Accessed 03 Aug 2022
EACS Guidelines. 2021. https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf. Accessed 03 Aug 2022.
Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of hiv infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324:1651–69.
Wohl DA, Yazdanpanah Y, Baumgarten A, et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6:e355–63.
Stellbrink HJ, Arribas JR, Stephens JL, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6:e364–72.
Orkin C, DeJesus E, Sax PE, et al. Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir-containing regimens for initial treatment of HIV-1 infection: week 144 results from two randomised, double-blind, multicentre, phase 3, non-inferiority trials. Lancet HIV. 2020;7:e389–400.
Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. 2017;390:2063–72.
Peñafiel J, de Lazzari E, Padilla M, et al. Tolerability of integrase inhibitors in a real-life setting. J Antimicrob Chemother. 2017;72:1752–9.
Mounzer K, Brunet L, Fusco JS, et al. Advanced HIV infection in treatment-naïve individuals: effectiveness and persistence of recommended 3-drug regimens. Open Forum Infect Dis. 2022;9:ofac018.
Uthman OA, Okwundu C, Gbenga K, et al. Optimal timing of antiretroviral therapy initiation for HIV-infected adults with newly diagnosed pulmonary tuberculosis: a systematic review and meta-analysis. Ann Intern Med. 2015;163:32–9.
Pyngottu A, Scherrer AU, Kouyos R, et al. Predictors of virological failure and time to viral suppression of first-line integrase inhibitor-based antiretroviral treatment. Clin Infect Dis. 2021;73:e2134–41.
Manzardo C, Zaccarelli M, Agüero F, Antinori A, Miró JM. Optimal timing and best antiretroviral regimen in treatment-naive HIV-infected individuals with advanced disease. J Acquir Immune Defic Syndr. 2007;46(Suppl 1):S9-18.
Manzardo C, Guardo AC, Letang E, Plana M, Gatell JM, Miro JM. Opportunistic infections and immune reconstitution inflammatory syndrome in HIV-1-infected adults in the combined antiretroviral therapy era: a comprehensive review. Expert Rev Anti Infect Ther. 2015;13:751–67.
Armenia D, Bouba Y, Gagliardini R, et al. Evaluation of virological response and resistance profile in HIV-1 infected patients starting a first-line integrase inhibitor-based regimen in clinical settings. J Clin Virol. 2020;130:104534.
de Melo MG, Varella I, Gorbach PM, et al. Antiretroviral adherence and virologic suppression in partnered and unpartnered HIV-positive individuals in southern Brazil. PLoS ONE. 2019;14:e0212744.
Scott Sutton S, Magagnoli J, Hardin JW. Impact of pill burden on adherence, risk of hospitalization, and viral suppression in patients with HIV infection and AIDS receiving antiretroviral therapy. Pharmacotherapy. 2016;36:385–401.
Cohen CJ, Meyers JL, Davis KL. Association between daily antiretroviral pill burden and treatment adherence, hospitalisation risk, and other healthcare utilisation and costs in a US Medicaid population with HIV. BMJ Open. 2013;3:e003028.
Taiwan Accreditation Foundation. 2021. https://www.taftw.org.tw/en/aboutTAF/introduction/. Accessed 3 Aug 2022.
Lee CY, Wu PH, Tsai JJ, Chen TC, Chang K, Lu PL. Cascade analysis of anonymous voluntary HIV counseling and testing among patients with HIV infection in Taiwan. AIDS Patient Care STDS. 2020;34:303–15.
Lin KY, Cheng CY, Li CW, et al. Trends and outcomes of late initiation of combination antiretroviral therapy driven by late presentation among HIV-positive Taiwanese patients in the era of treatment scale-up. PLoS ONE. 2017;12:e0179870.
Schuettfort G, Boekenkamp L, Cabello A, et al. Antiretroviral treatment outcomes among late HIV presenters initiating treatment with integrase inhibitors or protease inhibitors. HIV Med. 2021;22:47–53.
Spagnuolo V, Castagna A, Lazzarin A. Bictegravir. Curr Opin HIV AIDS. 2018;13:326–33.
Min S, Sloan L, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS. 2011;25:1737–45.
Cohen JPWX, Wade RL, Cuervo HD, Dionne DM. Persistence of guideline-recommended antiretroviral therapy regimens among persons living with HIV newly initiating treatment in the US. Open Forum Infect Dis. 2020;7(Suppl 1):S548-549.
Sutton S WX, Diaz-Cuervo H, Magagnoli J. Persistence of antiretroviral therapy regimens among veterans with HIV newly initiating treatment in the US. In: International Congress on Drug Therapy in HIV Infection, Glasgow; 2020.
Lagi F, Botta A, Ciccullo A, et al. Early discontinuation of DTG/ABC/3TC and BIC/TAF/FTC single-tablet regimens: a real-life multicenter cohort study. HIV Res Clin Pract. 2021;22:96–101.
Ciccullo A, Baldin G, Borghi V, et al. Real-life impact of drug toxicity on dolutegravir tolerability: clinical practice data from a multicenter Italian cohort. Viruses. 2022;14:163.
Tangamornsuksan W, Lohitnavy O, Kongkaew C, et al. Association of HLA-B*5701 genotypes and abacavir-induced hypersensitivity reaction: a systematic review and meta-analysis. J Pharm Pharm Sci. 2015;18:68–76.
Mallal S, Phillips E, Carosi G, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–79.
Sun HY, Hung CC, Lin PH, et al. Incidence of abacavir hypersensitivity and its relationship with HLA-B*5701 in HIV-infected patients in Taiwan. J Antimicrob Chemother. 2007;60:599–604.
Hoffmann C, Welz T, Sabranski M, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. 2017;18:56–63.
Ciccullo A, Baldin G, Borghi V, et al. Overall tolerability of integrase inhibitors in clinical practice: results from a multicenter Italian cohort. AIDS Res Hum Retroviruses. 2021;37:4–10.
Allen Reeves A, Fuentes AV, Caballero J, Thomas JE, Mosley Ii JF, Harrington C. Neurotoxicities in the treatment of HIV between dolutegravir, rilpivirine and dolutegravir/rilpivirine: a meta-analysis. Sex Transm Infect. 2021;97:261–7.
Ciccullo A, Baldin G, Putaggio C, Di Giambenedetto S, Borghetti A. Comparative safety review of recommended, first-line single-tablet regimens in patients with HIV. Expert Opin Drug Saf. 2021;20:1317–32.
Wohl D, Clarke A, Maggiolo F, et al. Patient-reported symptoms over 48 weeks among participants in randomized, double-blind, phase III non-inferiority trials of adults with HIV on co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus co-formulated abacavir, dolutegravir, and lamivudine. Patient. 2018;11:561–73.
Krentz HB, Gill MJ. The impact of non-antiretroviral polypharmacy on the continuity of antiretroviral therapy (ART) among HIV patients. AIDS Patient Care STDS. 2016;30:11–7.
Lee CY, Wu PH, Chen TC, Lu PL. Changing pattern of chemsex drug use among newly diagnosed HIV-positive Taiwanese from 2015 to 2020 in the era of treat-all policy. AIDS Patient Care STDS. 2021;35:134–43.
Funke B, Spinner CD, Esser S, et al. High prevalence of recreational and illicit drug use in German people living with HIV with a potential for drug-drug interactions with antiretroviral therapy. Int J STD AIDS. 2021;32:75–82.
Acknowledgements
We thank the participants of the study.
Funding
The design of the study, editorial writing support, the collection, analysis, interpretation of data, and the Rapid Service Fee, were supported by the Ministry of Science and Technology, Taiwan, R.O.C. (grant number MOST 108-2314-B-037-050), Kaohsiung Municipal Siaogang Hospital, Taiwan, R.O.C. (grant number H-108-006, H109-009), and Kaohsiung Medical University Hospital, Taiwan, R.O.C. (grant number KMUH107-7R21, KMUH110-0M22).
Medical Writing, Editorial, and Other Assistance
This manuscript was edited by Wallace Academic Editing.This study is supported partially by Kaohsiung Medical University Research Center Grant (KMU-TC109B05).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Concept and study design: all authors. Data acquisition: all authors. Analyses of the data: Chun-Yuan Lee, Yi-Pei Lin. Statistics: Chun-Yuan Lee, Yi-Pei Lin. Supervision: Sheng-Fan Wang, Po-Liang Lu. Manuscript preparation: Chun-Yuan Lee, Yi-Pei Lin. Review and approval: all authors.
Disclosures
The authors declare that they have no competing interests.
Compliance with Ethics Guidelines
The study protocol was approved by the institutional review boards of the participating hospitals: Kaohsiung Medical University Chung-Ho Memorial Hospital, Kaohsiung Municipal Siaogang Hospital, and Kaohsiung Municipal Ta-Tung Hospital [approval no. KMUHIRB-SV(I)-20170084]; Kaohsiung Chang Gung Memorial Hospital [approval no. 202101662A3D001]; Chi Mei Medical Center [approval no. 11106-003]; and Kaohsiung Veterans General Hospital [approval nos. VGHKS19-CT4-02 and VGHKS18-CT4-20]. The investigators obtained signed informed consent forms from patients from Kaohsiung Medical University Chung-Ho Memorial Hospital, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Municipal Ta-Tung Hospital, and Kaohsiung Veterans General Hospital. The requirement for informed consent was waived by the institutional review boards of Chi Mei Medical Center and Kaohsiung Chang Gung Memorial Hospital. The study was carried out according to the principles expressed in the Declaration of Helsinki of 1964 and its later amendments.
Data Availability
All data containing relevant information to support the study findings are provided in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lee, CY., Lee, CH., Tang, HJ. et al. Comparison of Virological Efficacy of DTG/ABC/3TG and B/F/TAF Regimens and Discontinuation Patterns in Persons Living with Advanced HIV in the Era of Rapid ART: A Retrospective Multicenter Cohort Study. Infect Dis Ther 12, 843–861 (2023). https://doi.org/10.1007/s40121-022-00734-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00734-5