Abstract
Introduction
The aims of this study were to investigate the risk factors for bacterial infections (BIs) and the association of BIs with the progression to acute-on-chronic liver failure (ACLF) in patients with hepatitis B virus (HBV)-related compensated liver cirrhosis and severe hepatitis flares.
Methods
A total of 237 patients were retrospectively reviewed. Baseline biochemical characteristics were compared between patients with and without the occurrence of BIs and progression to ACLF. Univariate and multivariate logistic regression analyses were used to identify independent risk factors for ACLF before and after 1:1 propensity score matching.
Results
Forty-eight (20.3%) patients progressed to ACLF after admission. Additionally, 136 (57.4%) patients progressed to hepatic decompensation (HD) and 52 (21.9%) patients had BIs before the development of ACLF. Patients with BIs had significantly higher incidences of HD (84.6%) and ACLF (46.2%) than those without BIs (49.7% and 13.0%, respectively; P < 0.01). CTP score (OR 1.660, 95% CI 1.267–2.175) and MELD–Na score (OR 1.082, 95% CI 1.010–1.160) were independent risk factors for BIs. BIs (OR 4.037, 95% CI 1.808–9.061), CLIF-SOFA score (OR 2.007, 95% CI 1.497–2.691), and the MELD-Na score (OR 1.167, 95% CI 1.073–1.260) were independent risk factors for the progression to ACLF. BIs (OR 4.730, 95% CI 1.520–14.718) were also an independent risk factor for the progression to ACLF after propensity score matching.
Conclusion
High CTP and MELD-Na scores are risk factors for BIs, and BIs are risk factors for the progression to ACLF in patients with HBV-related compensated liver cirrhosis and severe hepatitis flares.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Recent studies have suggested that the adverse impacts of bacterial infections (BIs) on patients with compensated liver cirrhosis are limited to those with severe liver injury. |
Whether BIs convey a risk of acute-on-chronic liver failure (ACLF) in patients with HBV-related compensated liver cirrhosis and severe acute hepatitis remains unknown. |
What was learned from the study? |
Fifty-two patients with BIs had a significantly higher incidence of ACLF (46.2%) than those without BIs (13.0%, P < 0.01). BIs (OR 4.730, 95% CI 1.520–14.718) were an independent risk factor for the progression to ACLF after propensity score matching in patients with HBV-related compensated liver cirrhosis and severe acute hepatitis. |
Our study demonstrates that BIs confer a risk of progression to ACLF in patients with HBV-related compensated cirrhosis during severe hepatitis flares. |
Introduction
During the long course of chronic hepatitis B virus (HBV) infection, patients will experience hepatitis flares with various degrees of liver injury [1, 2]. Hepatitis flares occur spontaneously or are triggered by other factors such as co-infection with other hepatitis viruses, treatment and withdrawal of chemotherapy, nucleot(s)ide analogues, and interferon. Spontaneous hepatitis flares occur frequently in patients with chronic HBV infection [2]. Repeated or sustained hepatitis flares will result in liver fibrosis or even liver cirrhosis. Patients with severe hepatitis flares are at high risk of further progression to hepatic decompensation (HD) and acute-on-chronic liver failure (ACLF) [3, 4]. In China and other highly HBV-epidemic countries, severe spontaneous hepatitis flares in patients with or without HBV-related liver cirrhosis are one of the most common precipitating events of ACLF [5].
Patients with chronic HBV infection often have different degrees of liver fibrosis, with compensated liver cirrhosis as a significant stage in this process. When patients with compensated liver cirrhosis progress to HD and ACLF, the complications of liver cirrhosis and extrahepatic organ failures have marked impacts on the prognosis [6]. Therefore, the mechanisms involved in the disease progression before and after ACLF development are different [7]. Most previous studies focused on prognostication in patients with ACLF [8], and the clinical, biochemical, and viral characteristics before the progression to ACLF have not been well elucidated [9].
ACLF can develop in patients with different underlying liver diseases [4, 6]. The World Gastroenterology Organization working party suggested that ACLF be classified into three types according to the underlying chronic liver disease as follows: type A from non-cirrhotic liver disease, type B from compensated cirrhosis, and type C from decompensated cirrhosis [10]. Recently, a few studies found that patients with ACLF developed from different underlying diseases had different prognoses [11, 12]; therefore, it is clinically important to understand the clinical characteristics of patients with ACLF developed from different underlying liver diseases.
It is well established that bacterial infections (BIs) are a common trigger for ACLF in patients with decompensated liver cirrhosis [13]; however, the role of BIs in patients with compensated liver cirrhosis has been poorly understood. According to a recent study [14], in patients with compensated liver cirrhosis and clinically significant portal hypertension, BIs were associated with a high prevalence of HD and a poor prognosis during a 36-month follow-up. However, most patients in that study had hepatitis C virus-related liver cirrhosis without hepatitis flares; moreover, whether or not BIs impaired the prognosis of patients by increasing the development of ACLF has not been investigated. In another study, no increased risk of HD was observed in patients with BIs and HBV-related compensated cirrhosis undergoing antiviral therapy [15].
The aforementioned studies suggested that the adverse impacts of BIs on the prognosis of patients with compensated liver cirrhosis are limited to those with severe liver injury; however, whether BIs convey a risk of ACLF in patients with compensated liver cirrhosis and severe acute hepatitis remains unknown. In this study, we retrospectively investigated the risk factors for BIs and the association of BIs with the progression to ACLF in patients with HBV-related compensated liver cirrhosis and severe hepatitis flares.
Methods
Study Population
The flowchart of the study participant selection process is presented in Fig. 1. We included patients with severe hepatitis flares of HBV-related compensated liver cirrhosis who did not fulfill the ACLF diagnostic criteria on admission. A total of 364 hospitalized patients at the Affiliated Hospital of Zunyi Medical University from January 2011 to March 2020 were included. Of these, 127 patients were excluded per the exclusion criteria, which included coinfection with hepatitis A, C, D, or E viruses; coexistence with other liver diseases including alcoholic liver disease, hepatocellular carcinoma (HCC), drug-induced hepatitis, autoimmune hepatitis, Wilson’s disease, or serious diseases involving other organ systems; pregnancy; chemotherapy or nucleot(s)ide analogue (NUC) resistance or withdrawal within 1 year. Patients who were taking proton pump inhibitors before the onset of BIs were also excluded [16]. Finally, a total of 237 patients were included in this study.
Diagnostic Criteria for Severe Hepatitis Flares, ACLF, Liver Cirrhosis, and BIs
A severe hepatitis flare was defined as serum alanine aminotransferase (ALT) levels of > 5 × the normal upper limit (ULN, 200 IU/L) and total bilirubin (TBil) ≥ 5 × ULN (85 μmol/L) or prothrombin activity (PTA) of 40–60% in patients with HBV-related compensated liver cirrhosis.
ACLF was diagnosed according to the criteria proposed by the Asian Pacific Association for the Study of the Liver (APASL), which includes the recent development of jaundice (TBil ≥ 5 × ULN) and coagulopathy (PTA < 40% or an international normalized ratio [INR] ≥ 1.5), complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease [17].
The diagnosis of cirrhosis was based on previous liver biopsy findings or a composite of clinical signs and findings provided by laboratory tests, endoscopy, radiologic imaging, and fibroscanning. HD was defined as the development of ascites, bleeding due to portal hypertensive sources, or overt hepatic encephalopathy [18]. Patients with no previous decompensation were included in this study as participants with compensated liver cirrhosis.
BIs were diagnosed per the following criteria [19]: (1) spontaneous bacterial peritonitis (SBP): ascitic fluid polymorphonuclear cells > 250/mL or positive ascitic fluid cultures; (2) bacteremia: positive blood cultures without a source of infection; (3) pneumonia: new pulmonary infiltrate with fever (> 38 °C) with any respiratory symptoms (e.g., cough, sputum, and dyspnea) or any findings on auscultation (rales or crepitation), or white blood cell (WBC) counts of > 10 × 109/L or < 4 × 109/L; (4) urinary tract infection (UTI): more than 10 leucocytes per high-power field in urine and positive urine cultures or significant leucocyte counts per field without positive cultures; and (5) other bacterial infections, including skin infections, intra-abdominal infections, and infections of unknown origin. In patients with suspected respiratory infections and infections with unknown origin, the IgM antibody to influenza A and B viruses, parainfluenza virus, adenovirus, mycoplasma, and chlamydia, and DNA of Epstein–Barr virus and cytomegalovirus were detected to exclude the infections induced by these pathogens. SARSCoV testing has been also performed in patients after February 2020. In addition, the diagnosis of BI was made by two independent investigators. If there was any discrepancy between the two investigators, a senior investigator was requested to make the decision.
Candidate Predictor Variables and Treatment Schedules
The demographics, clinical and laboratory variables, and imaging findings of patients on admission were retrospectively collected. The endpoint of this study was the development of ACLF within 28 days after admission. The BIs and HD occurring before the development of ACLF were recorded.
In addition, liver disease severity was assessed using the Model for End-Stage Liver Disease (MELD)-Na score, Child–Turcotte–Pugh (CTP) score, and chronic liver failure–sequential organ failure assessment (CLIF-SOFA) score [6]. Standard medical care, including antiviral therapy with lamivudine, entecavir, or telbivudine, was administered to all patients according to their HBV replication levels and patient willingness. Patients with BI were treated with antibiotics following current recommendations [20]. The protocol conformed to the provisions of the Declaration of Helsinki and was approved by the Human Ethical Committee of the Affiliated Hospital of Zunyi Medical University.
Statistical Analysis
Statistical analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). Patient characteristics were compared between patients with and without progression to ACLF and BIs using the χ2 test for categorical variables, t tests for normally distributed continuous variables, and the Mann–Whitney U test for non-normally distributed continuous variables. Continuous variables are summarized as the mean ± standard deviation (SD) or median (interquartile range, IQR). Categorical variables are displayed as counts and/or percentages (%). Logistic regression was used for univariate and multivariate analyses to identify the risk factors for the progression to BI and ACLF. P < 0.05 was considered statistically significant.
Propensity score matching (PSM) analyses were employed to eliminate confounding bias between patients with BIs and those without BIs. The propensity score was calculated according to age, sex, ALT, aspartate aminotransferase (AST), glutamine transpeptidase (GGT), alkaline phosphatase (ALP), total bilirubin (TBil), albumin (ALB), sodium (Na+), urea nitrogen (BUN), creatinine (Cr), prothrombin time (PT), PTA, lgHBV DNA, CTP score, MELD-Na score, and CLIF-SOFA score. The nearest neighbor 1:1 matching scheme was adopted.
Results
Patient Characteristics
A total of 237 patients with compensated liver cirrhosis and severe hepatitis flares were included in this study. They were mostly male (209, 88.2%), with an average age of 43 years. Forty-eight (20.3%) patients progressed to ACLF within 28 days after admission. A total of 136 (57.4%) patients progressed to HD before progressing to ACLF, including 132 patients who developed ascites and four patients with bleeding due to portal hypertensive sources. The mean number of days between hospital admission and the development of ACLF was 6.1 (range 1–14 days). Although no patient was on NUC treatment at the time of admission, three patients were NUC treatment-experienced and had NUC withdrawal over 1 year.
Before the development of ACLF, 52 (21.9%) patients had 57 episodes of BIs, including 33 patients with digestive infections (20 patients with SBP); nine patients with respiratory infections (seven patients with pneumonia); four patients with SBP and pneumonia; three patients with UTIs; one patient with phlegmon; one patient with pneumonia and acute suppurative otitis media; and one patient with brucellosis infection. Forty-one patients had community-acquired infections and 11 had nosocomial infections. Two patients had culture-positive infections, one patient had Klebsiella pneumoniae isolated from phlegm fluid, and another had brucellosis isolated from blood. No patients received steroid therapy before BIs.
Risk Factors Associated with Bacterial Infections in Patients with HBV-Related Compensated Liver Cirrhosis During Severe Hepatitis Flares
As shown in Table 1, patients with BIs had significantly higher levels of ALP, TBil, BUN, PT, INR, WBC, NEUT, NLR, PLT, CTP score, CLIF-SOFA score, and MELD-Na score and significantly lower levels of ALB, and Na+ than those without BIs.
In the logistic regression analyses, we did not include WBC, NEUT, and NLR because significant elevations of these indexes were the results of BIs. Univariate logistic regression analyses revealed that ALB, TBil, Na+, PT, INR, CTP score, CLIF-SOFA, and MELD-Na score were risk factors for BIs. In the multivariate logistic regression analysis, we included CTP score, CLIF-SOFA, and MELD-Na score as ALB, TBil, Na+, PLT, and INR had been included in the calculation of the CTP score, CLIF-SOFA, and the MELD-Na score. Multivariate logistic regression analyses revealed that the CTP score (OR 1.660, 95% CI 1.267–2.175) and the MELD-Na score (OR 1.082, 95% CI 1.010–1.160) were independent risk factors associated with BIs in patients with HBV-related compensated liver cirrhosis during severe hepatitis flares (Table 2).
Bacterial Infections were Associated with Progression to Hepatic Decompensation and Acute-on-Chronic Liver Failure
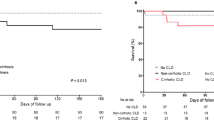
As shown in Fig. 2, among 52 patients with BIs, 24 (46.2%) patients progressed to ACLF within 28 days after admission, which was significantly higher than the proportion in patients without BIs (13.0%, P < 0.01). Before progression to ACLF, 44 (84.6%) patients with BIs had HD, which was also significantly higher than the proportion in patients without BIs (49.7%, P < 0.01).
Bacterial infections were associated with the occurrence of hepatic decompensation and acute-on-chronic liver failure in patients with HBV-related compensated liver cirrhosis during severe hepatitis flares. ACLF acute-on-chronic liver failure, BI bacterial infection, HD hepatic decompensation, HBV hepatitis B virus. #P < 0.01, compared with those with BIs
Bacterial Infections were One of the Risk Factors for Progression to Acute-on-Chronic Liver Failure
As shown in Table 3, the patients who progressed to ACLF after admission had significantly higher levels of ALP, TBil, WBC, INR, CTP score, CLIF-SOFA score, and MELD-Na score and significantly lower levels of GGT, ALB, Na+, PTA, and PLT than those who did not progress to ACLF.
Univariate logistic regression analyses revealed that BIs, TBil, ALB, Na+, PTA, INR, PLT, CTP score, CLIF-SOFA score, and MELD-Na score were risk factors for the progression to ACLF in patients with HBV-related compensated liver cirrhosis during severe hepatitis flares. As the CTP score, CLIF-SOFA score, and MELD-Na score were calculated from TBil, ALB, Na+, PTA, INR, and PLT, in the multivariate logistic regression analysis, we included BIs, the CTP score, the CLIF-SOFA score, and the MELD-Na score. Multivariate logistic regression analyses revealed that BIs (OR 4.037, 95% CI 1.808–9.061), the CLIF-SOFA score (OR 2.007, 95% CI 1.497–2.691), and the MELD-Na score (OR 1.167, 95% CI 1.073–1.260) were independent risk factors for the progression to ACLF (Table 4).
After PSM, we obtained 78 matched case-control pairs of patients. No baseline data difference was found between these two groups of patients except for WBC, NEUT, and NLR (Table 5). There were 16 (41.0%) patients who progressed to ACLF among patients with BIs and 5 (12.8%) patients who progressed to ACLF among patients without BIs. BIs (OR 4.730, 95% CI 1.520–14.718) were also a risk factor for the progression to ACLF in patients with HBV-related compensated liver cirrhosis during severe hepatitis flares.
Discussion
It is well accepted that the progression and the prognosis of patients with liver cirrhosis during acute deterioration depend on both predisposing factors and precipitating factors. Predisposing factors include the severity of liver fibrosis and related portal hypertension, systemic inflammation, immunodeficiency, and gut dysbiosis; precipitating factors include the spontaneous activation of HBV, hepatitis A or E virus infection, drug/alcohol-induced liver injury, and BIs [7]. Therefore, it is difficult to identify the clinical and biochemical characteristics associated with disease progression in patients with different predisposing factors and precipitating factors. To overcome these biases, in this study, we only included patients with HBV-related compensated liver cirrhosis and severe spontaneous hepatitis flare to study the risk factors associated with disease progression before ACLF. The results of this study were more reliable than those of previous studies which included patients with great diversities in their various degrees of liver fibrosis, etiologies of liver cirrhosis, and precipitating factors of acute deterioration [14, 15].
One of the major findings of this study was that BIs occurred in 21.9% of patients, a proportion that was much higher than those reported in compensated liver cirrhosis without severe hepatitis flares (a 4-year cumulative incidence of 12.2% and 5-year cumulative incidence of 12.9%) [14, 15]. A significantly higher level of TBil, PT, INR, NEUT, NLR, CTP, CLIF-SOFA, and the MELD-Na score and significantly lower levels of ALB, Na+, and PLT were observed in patients with BIs than in those without Bis. High CTP and MELD-Na scores were identified as independent risk factors for BIs. Patients with liver cirrhosis have increased susceptibility to BIs. Liver dysfunction and its related complications, such as portal-systemic shunting, bacterial translocation, and liver cirrhosis-associated immune dysfunction, have been implicated in the occurrence of BIs [21, 22]. In patients with severe acute liver inflammation, an excessive hepatic inflammatory response has been found to exhaust the function of immune cells and result in immune paralysis [11]. These studies suggested that susceptibility to BIs is dependent on the severity of acute and chronic liver injury. The prevalence of BIs in patients with compensated liver cirrhosis during severe acute hepatitis flares remains unknown. Our results suggested that severe acute liver inflammation increased the prevalence of BIs in patients with compensated liver cirrhosis.
Most previous studies demonstrated that high HBV DNA levels correlate with the development of HCC and liver cirrhosis in patients with chronic HBV infection [23]. However, whether HBV DNA levels influence short-term outcomes in patients with severe hepatitis flare of chronic HBV infection remains unclear [24]. In a previous study, we had found that high HBV DNA levels were one of the independent risk factors for post-admission progression to ACLF in the patients with acute exacerbation of chronic hepatitis B [25]. However, in another study we found that in patients with more severe liver injury HBV DNA was not a risk factor for ACLF progression [4]. In this study we did not find any difference between patients with and without BIs and between patients with and without progression to ACLF, suggesting that the influence of HBV DNA on disease progression depended on the degree of liver dysfunction. In the patients with more severe liver injury, the deleterious role of HBV DNA may be masked by other risk factors.
Another major finding in this study was that BIs were one of the risk factors for the progression to ACLF. Previous studies demonstrated that BIs and the deterioration of liver cirrhosis have a complex interplay. The outcomes of BIs in patients with liver cirrhosis also depended on the severity of acute and chronic liver injury and the severity of BIs. BIs often trigger extrahepatic organ injuries and failure, and they were one of the most common precipitating factors for the progression to ACLF and death in patients with decompensated liver cirrhosis. In patients with compensated liver cirrhosis, however, the role of BIs in the progression to ACLF has not been studied.
A few previous studies investigated risk factors for the development of ACLF in patients with different degrees of liver fibrosis and inflammation and found different results [4, 25]. However, and as we know, no study had included BIs as a risk factor for ACLF progression [25,26,27]. To the best of our knowledge, our study is the first to demonstrate that BIs were a risk factor associated with the progression to ACLF in pre-ACLF stages in patients with severe hepatitis flare of HBV-related compensated liver cirrhosis. This finding is clinically relevant in the prevention and treatment of liver cirrhosis and ACLF.
Because most patients in this study had BIs and HD on admission, we could not determine which of them occurred first; therefore, we could not decide whether BIs were the result of HD or a precipitating factor of HD. However, we found a strong association between HD and BIs in this study. According to a previous study [14], in patients with compensated liver cirrhosis, BI mostly occurred before HD, suggesting that BIs are a trigger for HD in patients with compensated liver cirrhosis.
The robustness of this study was that we included a large group of patients with great homogeneity in both predisposing factors and precipitating factors to study the risk factors for ACLF. In addition, we only studied the association of BIs with ACLF development, which might reflect the direct impacts of BIs on ACLF development. Our study also had several limitations. At first, the diagnosis of BIs was mostly based on clinical data; there were few patients with culture-positive BIs in this study. This can be partly explained by the fact that for most patients, BIs had occurred before their admission, and antibiotics had been used in local hospitals, and we only recorded the BIs that occurred before ACLF development. However, as we diagnosed BIs on the basis of strictly established criteria and used examinations to exclude infections by common pathogens, we believed most patients with BIs had been correctly captured in this study. Secondly, it was a retrospective, single-center study, and the findings of this study need to be verified in a multicenter prospective study.
Conclusions
High CTP and MELD-Na scores are risk factors for BIs, and BIs are a risk factor for the progression to ACLF in patients with HBV-related compensated liver cirrhosis and severe hepatitis flares.
References
Kumar M, Chauhan R, Gupta N, et al. Spontaneous increases in alanine aminotransferase levels in asymptomatic chronic hepatitis B virus-infected patients. Gastroenterology. 2009;136:1272–80.
Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: pathogenesis, natural course and management. J Hepatol. 2014;61:1407–17.
Trebicka J, Macnaughtan J, Schnabl B, Shawcross DL, Bajaj JS. The microbiota in cirrhosis and its role in hepatic decompensation. J Hepatol. 2021;75:S67–81.
Yuan L, Zeng BM, Liu LL, et al. Risk factors for progression to acute-on-chronic liver failure during severe acute exacerbation of chronic hepatitis B virus infection. World J Gastroenterol. 2019;25:2327–37.
Shi Y, Yang Y, Hu Y, et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. 2015;62:232–42.
Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038–47.
Gustot T, Stadlbauer V, Laleman W, Alessandria C, Thursz M. Transition to decompensation and acute-on-chronic liver failure: role of predisposing factors and precipitating events. J Hepatol. 2021;75:S36–48.
Wu T, Li J, Shao L, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181–91.
Chang ML, Liaw YF. Hepatitis B flare in hepatitis B e antigen-negative patients: a complicated cascade of innate and adaptive immune responses. Int J Mol Sci. 2022;23:1552.
Yang F, Liu Y, Zeng B, et al. Noninvasive assessment of liver fibrosis for predicting acute-on-chronic liver failure in patients with chronic hepatitis B. Hepatol Int. 2021;15:593–601.
Thanapirom K, Teerasarntipan T, Treeprasertsuk S, et al. Impact of compensated cirrhosis on survival in patients with acute-on-chronic liver failure. Hepatol Int. 2022;16:171–82.
Liu X, Zhang J, Wei X, et al. HBV-related acute-on-chronic liver failure with underlying chronic hepatitis has superior survival compared to cirrhosis. Eur J Gastroenterol Hepatol. 2021;33:e734–9.
Cao ZJ, Liu YH, Zhu CW, et al. Bacterial infection triggers and complicates acute-on-chronic liver failure in patients with hepatitis B virus-decompensated cirrhosis: a retrospective cohort study. World J Gastroenterol. 2020;26:645–56.
Villanueva C, Albillos A, Genescà J, et al. Bacterial infections adversely influence the risk of decompensation and survival in compensated cirrhosis. J Hepatol. 2021;75:589–99.
Nahon P, Lescat M, Layese R, et al. Bacterial infection in compensated viral cirrhosis impairs 5-year survival (ANRS CO12 CirVir prospective cohort). Gut. 2017;66:330–41.
Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64:1265–72.
Sarin SK, Kedarisetty CK, Abbas Z, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453–71.
European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–60.
Bajaj JS, O’Leary JG, Reddy KR, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) experience. Hepatology. 2012;56:2328–35.
Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL special conference 2013. J Hepatol. 2014;60:1310–24.
Piano S, Brocca A, Mareso S, Angeli P. Infections complicating cirrhosis. Liver Int. 2018;38(Suppl 1):126–33.
Bajaj JS, Kamath PS, Reddy KR. The evolving challenge of infections in cirrhosis. N Engl J Med. 2021;384:2317–30.
Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–99.
Wong VW, Chan HL. Severe acute exacerbation of chronic hepatitis B: a unique presentation of a common disease. J Gastroenterol Hepatol. 2009;24:1179–86.
Ren Y, Liu L, Li Y, et al. Development and validation of a scoring system to predict progression to acute-on-chronic liver failure in patients with acute exacerbation of chronic hepatitis B. Hepatol Res. 2018;48:692–700.
Tsubota A, Arase Y, Suzuki Y, et al. Lamivudine monotherapy for spontaneous severe acute exacerbation of chronic hepatitis B. J Gastroenterol Hepatol. 2005;20:426–32.
Zhang Q, Han T, Li Y, Nie C, Liu H. Predictors of progression into acute-on-chronic liver failure from acute deterioration of pre-existing chronic liver disease. Hepatol Res. 2016;46:320–8.
Acknowledgements
Thanking Patient Participants
The authors thank all patients and members of staff who made this study possible.
Funding
This work was supported by the Funds of Chinese National Natural Science Foundation Project (81860114). The Journal’s Rapid Service Fee was funded by the authors.
Author Contributions
Conceptualization: Shide Lin; Methodology: Jun Chu, Yanqing Yang, and Yujuan Liu; Formal analysis and investigation: Jun Chu, Yanqing Yang, Lingqi Pei, Yihong Zhou, Tao Lu, and Yin Zhang; Writing–original draft preparation: Shide Lin, Han Hu, and Ying Li; Writing–review and editing: Shide Lin, Jun Chu, Yanqing, and Yang Fangwan Yang; Funding acquisition: Shide Lin; Supervision: Shide Lin.
Disclosures
Jun Chu, Yanqing Yang, Yujuan Liu, Lingqi Pei, Yihong Zhou, Tao Lu, Yin Zhang, Han Hu, Ying Li, Fangwan Yang, and Shide Lin declare no competing interests.
Compliance with Ethics Guidelines
The protocol of this study conformed to the tenets of the Declaration of Helsinki and was approved by the Human Ethical Committee of the Affiliated Hospital of Zunyi Medical University. Written informed consent for participation was not required for this study per the national legislation and institutional requirements.
Data Availability
The datasets supporting the conclusions of this article are included in the article. These data are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chu, J., Yang, Y., Liu, Y. et al. Bacterial Infections Confer a Risk of Progression to Acute-on-Chronic Liver Failure in Patients with HBV-Related Compensated Cirrhosis During Severe Hepatitis Flares. Infect Dis Ther 11, 1839–1851 (2022). https://doi.org/10.1007/s40121-022-00695-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00695-9