Abstract
Introduction
Data on real-world effectiveness of subcutaneous (SC) casirivimab and imdevimab (CAS+IMD) for the treatment of coronavirus disease 2019 (COVID-19) are limited. The objective of this study was to assess the effectiveness of SC CAS+IMD versus no antibody treatment among patients with COVID-19.
Methods
This retrospective cohort study linked Komodo Health and CDR Maguire Health and Medical data. Patients diagnosed with COVID-19 in ambulatory settings (August 1–October 30, 2021) treated with SC CAS+IMD were exact- and propensity score-matched to fewer than five untreated treatment-eligible patients and followed for the composite endpoint of 30-day all-cause mortality or COVID-19-related hospitalization. Kaplan–Meier estimators were used to calculate outcome risk overall and across subgroups. Cox proportional-hazards models were used to estimate adjusted hazard ratios (aHR) and 95% confidence intervals (CI).
Results
Of 13,522 patients treated with CAS+IMD, 12,972 were matched to 41,848 untreated patients. The 30-day composite outcome risk was 1.9% (95% CI 1.7–2.2) and 4.4% (95% CI 4.2–4.6) in the treated and untreated cohorts, respectively; treated patients had a 49% lower relative risk of the composite outcome (aHR 0.51; 95% CI 0.46–0.58) and a 67% relative risk of 30-day mortality (aHR 0.33, 95% CI 0.18–0.60). Effectiveness was consistent across vaccination status and various subgroups.
Discussion
Patients with COVID-19 benefitted from treatment with SC CAS+IMD versus untreated patients. The results were consistent across subgroups of patients, including older adults, immunocompromised patients, and patients vaccinated against COVID-19. Results were robust across numerous sensitivity analyses.
Conclusion
SC CAS+IMD is effective in reducing 30-day COVID-19-related hospitalization or mortality in real-world outpatient settings during the Delta-dominant period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Data on real-world effectiveness of SC CAS+IMD in COVID-19 are limited. One study evaluated the effectiveness of SC CAS+IMD and found that patients were 56% less likely to be hospitalized or die than untreated patients but those results reflect the experience at one medical center between July and October 2021 in 652 patients. |
We hypothesized that patients treated with SC CAS+IMD would experience a lower 30-day COVID-19-related hospitalization/all-cause mortality risk compared to untreated patients who were eligible for treatment. |
What was learned from this study? |
SC CAS+IMD reduced the 30-day risk of COVID-19 hospitalization/all-cause mortality relative to no COVID-19 mAb treatment during the Delta-dominant period. |
Patients benefitted from treatment with SC CAS+IMD across all patient subgroups assessed, which included vaccinated and high-risk patients such as older adults and patients who were immunocompromised. |
Introduction
While vaccines remain the primary strategy for control of the coronavirus 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), they require development of active immunity to COVID-19 over time. In contrast, neutralizing monoclonal antibodies (mAbs) against the SARS-CoV-2 spike protein confer immediate passive immunity for SARS-CoV-2 variants that remain sensitive to the mAbs and can be used for pre- or post-exposure prophylaxis or early treatment [1,2,3,4,5,6,7].
In a clinical trial, intravenous (IV) administration of mAbs casirivimab and imdevimab (CAS+IMD; Regeneron Pharmaceuticals, Inc.) was associated with a 71% reduction in all-cause mortality or COVID-19-related hospitalization in patients diagnosed with COVID-19 in ambulatory settings [2]. These mAbs were granted emergency use authorization (EUA) by the US Food and Drug Administration (FDA) for treatment of non-hospitalized patients with COVID-19 who are at risk for severe disease first as IV administration in November 2020 and in June 2021, as subcutaneous (SC) administration when IV infusion is not feasible or would lead to delay in treatment [8]. In January 2022, with the surge in the Omicron variant (B.1.1.529), the FDA amended the EUA for CAS+IMD to exclude its use in geographic regions where infection or exposure is likely due to a variant not susceptible to the treatment [9]. Consequently, it is not currently authorized for use in the USA.
While the majority of real-world studies assessing the effectiveness of CAS+IMD reported 50–78% reductions in the risk of hospitalization [10,11,12,13,14,15,16,17,18,19,20,21,22,23], the effectiveness of SC CAS+IMD was not specifically assessed. A study that did evaluate SC CAS+IMD reported that treated patients were 56% less likely to be hospitalized or die than untreated patients [24]. However, the results reflect the experience at one medical center between July and October 2021 in 652 patients.
In June 2021, the Florida Department of Health and Florida Division of Emergency Management deployed COVID-19 mAb therapy treatment sites in Florida. A health disaster management company, CDR Maguire Health and Medical (“CDR Health”), was commissioned to manage the COVID-19 public health crisis. Between August and November 2021, CDR Health treated approximately 115,000 patients with SC CAS+IMD. The objective of this study was to compare the effectiveness of SC CAS+IMD to no COVID-19 mAb treatment on 30-day all-cause mortality or COVID-19–related hospitalization among patients diagnosed with COVID-19 in the ambulatory setting who were eligible to receive treatment under the EUA.
Methods
Data Sources
We conducted a retrospective cohort study using closed administrative claims data from the Komodo Health claims database [25]. Komodo health data is a real-world dataset which integrates various sources of patient-level data to map longitudinal patient journeys. Komodo pulls de-identified, patient-level claims data from clearinghouses, payers (150 or more national and regional payers), and provider consortia data sources to follow patients through the US healthcare system. The database covers all census regions and includes both open and closed (i.e., payer complete) claims data, although only the closed claims were leveraged for this study. As there is no code for distinguishing between SC and IV administration of CAS+IMD in administrative claims data, the Komodo Health data were linked to data from CDR Health, which administered only SC CAS+IMD. The database linkage used Datavant’s encryption and tokenization technology (San Francisco, CA; https://datavant.com) [26].
The dataset resulting from the linkage was used to construct the treatment arm and to identify a control group of patients not treated with COVID-19 mAbs. In addition to date of SC CAS+IMD administration, CDR Health data were used to ensure that patients in the untreated control group had not been treated with SC CAS+IMD.
This study consisted of secondary research using de-identified data licensed from a third party, Komodo, in compliance with 45 CFR 164.514(a)-(c). The data had identifying patient information removed and were coded in such a way that the data could not be linked back to subjects from whom they were originally collected prior to the authors gaining access to it. This research, which used the de-identified licensed data described above, does not require institutional review board or ethics review, as analyses with these data do not meet the definition of “research involving human subjects” as defined within 45 CFR 46.102(f), which stipulates human subjects as living individuals about whom an investigator obtains identifiable private information for research purposes.
Study Population
The treated cohort consisted of patients treated with SC CAS+IMD between August 1, 2021 and October 30, 2021 (index date = date of CAS+IMD administration) who had not received other COVID-19 mAbs (bamlanivimab monotherapy, bamlanivimab and etesevimab, or sotrovimab) within 6 months prior to or on the index date. This period is concurrent with when the Delta variant (B.1.617.2) became dominant in the USA [27] and prior to the spread of Omicron [28]. Patients in the untreated cohort were those diagnosed with COVID-19 (ICD-10: U07.1) during the same period and not treated with COVID-19 mAbs. Given that approximately 90% of SC CAS+IMD–treated patients did not have a documented COVID-19 diagnosis in the 10 days prior to treatment administration in the Komodo claims data, an index date could not be assigned to the untreated patients on the basis of the distribution of days between diagnosis and treatment, as was done previously [29]. For untreated patients, the index date was the COVID-19 diagnosis date; if multiple COVID-19 diagnoses were available, the first diagnosis was selected, and to identify incident COVID-19 diagnoses, patients were required to have no evidence of a prior COVID-19 diagnosis within 30 days pre-index. This approach excludes the immortal time, which would have favored treatment had follow-up started for both groups on the COVID-19 diagnosis date, but introduces survival bias that was adjusted for analytically [30].
Patients in both cohorts were also required to be at least 12 years of age on the index date (in accordance with the age cutoff in the EUA for CAS+IMD), be continuously enrolled in medical and pharmacy benefits for at least 6 months pre-index (i.e., baseline), meet EUA criteria for CAS+IMD treatment at index [8], and have a valid date of death if deceased. Patients who were hospitalized or dead on the index date were excluded.
Outcomes
The primary endpoint was the composite outcome of 30-day all-cause mortality or COVID-19-related hospitalization, and a secondary endpoint was 30-day all-cause mortality. Komodo uses Social Security Administration (SSA) data, a private obituary data source, and a private claims mortality dataset to identify mortality. COVID-19-related hospitalization was defined as a COVID-19 diagnosis as the primary or admitting diagnosis. Patients were followed from the index date until the occurrence of the outcome or a censoring event, which included receipt of another COVID-19 mAb, the end of 30-day risk period, healthcare plan disenrollment, or study end date.
Study Variables
Baseline demographic variables included age as a continuous variable and categorized by age groups (12–17, 18–54, 55–64, ≥ 65 years), sex, and geographic region (Florida resident vs. not). The Charlson Comorbidity Index (CCI) [31] was derived by identifying the presence of comorbidities over the baseline period. In addition to body mass index (BMI), which was categorized as not overweight, overweight, obese, severely obese, morbidly obese, or missing, specific comorbidities included cardiovascular disease (myocardial infarction, hypertension, atrial fibrillation, heart failure; ischemic/hemorrhagic stroke, or venous thromboembolism), chronic respiratory disease (asthma, chronic obstructive pulmonary disease, emphysema, obstructive sleep apnea, pulmonary fibrosis, or cystic fibrosis), chronic kidney disease stage 3–5 or renal failure requiring dialysis, B cell deficiency (primary, secondary, or drug-induced; Table S1 in the supplementary material), and diabetes (type 1 or 2). The occurrence of at least one hospitalization and emergency room/urgent care visit for any reason during the baseline period was also captured.
The following risk factors for use of CAS+IMD under the EUA were identified during the baseline period up to and including the index date: ≥ 65 years of age on index date; 12–17 years of age on index date with BMI ≥ 85th percentile for age and sex based on CDC growth charts [32]; BMI > 25 kg/m2; pregnancy; diabetes; chronic lung disease; immunosuppressive disease; history of immunosuppressive treatment; cardiovascular disease, hypertension, or congenital heart disease; sickle cell disease; and neurodevelopmental disorders. Since EUA risk factors of chronic kidney disease, cardiovascular disease or hypertension, and use of medical-related technological dependence could be an outcome of COVID-19 infection, their presence was assessed over the baseline period only.
Statistical Analysis
Matching
To derive adjusted estimates, both exact and probabilistic matching methods were used. Propensity scores (PS), derived using logistic regression, predicted the probability of CAS+IMD treatment versus no treatment given age (as a continuous variable), sex, index month, three-digit zip code (Florida residents) or state (non-Florida residents), individual EUA criteria, BMI category, CCI score (as a continuous variable), prior COVID-19 vaccination (at least one COVID-19 vaccine during baseline), and baseline healthcare resource utilization (prior all-cause hospitalization and all-cause emergency room/urgent care visits). Each treated patient was matched to up to five untreated patients on a caliper width of 0.2 of the standard deviation (SD) of the logit of the estimated PS [33] and exact-matched on index month and three-digit zip code (Florida residents) or state (non-Florida residents). Standardized mean differences (SMD) were used to assess balance between groups; SMD > 0.1 indicated imbalance and unbalanced variables were included directly in the outcome model [34].
Primary Analysis
Baseline characteristics were reported for SC CAS+IMD and untreated EUA-eligible patients using means (SD) and medians (Q1–Q3) for continuous variables and number and frequency for categorical variables. Kaplan–Meier estimators were used to estimate the 30-day risk of the composite outcome and mortality among the matched patients [35], with 95% confidence bands constructed using the Hall–Wellner method [36] and log-rank tests used to compare survival distributions.
Adjusted hazard ratios (aHR) with 95% confidence intervals (CI) were derived using Cox proportional-hazard models that fit the model to the matched pairs, and used robust sandwich variance estimators to account for clustering within matched sets [37]. Since starting follow-up on the date of treatment for treated patients and the COVID-19 diagnosis for untreated patients excludes immortal time and can introduce a survival bias, a correction factor was derived to account for this bias [30]. This bias was removed by dividing the aHR as well as the 95% upper and lower CI values by the correction factor T0/T0+TIT; TIT denotes the observed immortal person time which, in our study, is the number of days between the COVID-19 diagnosis and the date of treatment among treated patients, and T0 is person time in the untreated group. The correction factor was derived assuming that TIT was at most 10 days (the maximum number of permissible days between symptom onset and treatment, per the EUA) but could be shorter if the patient experienced the outcome or was censored. The actual number of days between the COVID-19 diagnosis and treatment date was used for matched treated patients with a non-missing COVID-19 diagnosis date. For T0, matched EUA-eligible untreated patients contributed up to 30 days, the occurrence of the event, or censoring, whichever came first relative to T0. The survival bias was then accounted for by dividing aHR and 95% CIs by the correction factor.
Subgroup Analyses
Subgroups of interest included age stratified as 12–17, 18–54, 55–64, and ≥ 65 years; elevated risk defined as ≥ 65 years of age or 55–64 years of age with BMI ≥ 35 kg/m2, type 2 diabetes, chronic obstructive pulmonary disease, or chronic kidney disease; immunocompromised patients, overall and by type of B cell deficiency (primary, secondary, or drug-induced); and prior COVID-19 vaccination. The same matched set of patients was used to evaluate effectiveness across subgroups. Kaplan–Meier estimators were used to determine 30-day outcome risks for subgroups. Adjusted HRs comparing treated versus untreated cohorts across subgroups were derived using Cox proportional-hazard models and including the interaction term between treatment and the subgroup. The adjusted estimates of subgroups were derived using the same matched set of patients as the primary endpoint and accounted for clustering of patients. No adjustments were made for multiplicity. As in the primary analysis, a correction factor was applied to aHRs and 95% CIs to account for potential survival bias.
Sensitivity Analyses
Sensitivity analyses conducted to assess robustness of the results included (1) deriving the correction factor using the subset of matched SC CAS+IMD-treated patients with a non-missing COVID-19 diagnosis date in the Komodo claim database; (2) modifying the definition of COVID-19-related hospitalization to COVID-19 as the primary diagnosis only; (3) modifying inclusion/exclusion criteria so that only the untreated patients were required to meet the EUA criteria on the index date; and (4) requiring 3 months (vs. 6 months) of continuous healthcare plan enrollment pre-index.
The analytic file was created and all analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC).
Results
Linkage and Cohort Creation
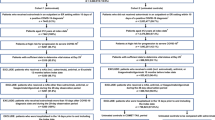
Among 90,133 patients treated with SC CAS+IMD in the CDR Health database, 79,295 patients (88.0%) were successfully linked to the Komodo claims, and 13,522 of these were eligible for matching (Fig. S1 in the supplementary material). After applying inclusion/exclusion criteria to 5,132,798 patients in the Komodo database with a COVID-19 diagnosis during the study period, we determined that 828,087 patients were eligible for matching (Fig. S1 in the supplementary material). A total of 12,972 of the 13,522 (95.9%) SC CAS+IMD–treated patients were exact- and PS-matched to 41,848 EUA-eligible untreated patients.
Prior to matching, cohorts were imbalanced on several variables; notably, the treated cohort was older with a higher proportion at elevated risk (Table S2 in the supplementary material). After matching, per the SMDs, all variables except region and index month were balanced (Table 1). The matched populations were 57–58% female), with mean age between 50 and 52 years and 34–36% at elevated risk (Table 1).
Primary Analysis
The 30-day risk of the composite outcome was 1.9% (95% CI 1.7–2.2) in the SC CAS+IMD-treated cohort (247 events) and 4.4% (95% CI 4.2–4.6) in the EUA-eligible untreated cohort (1822 events) (Fig. 1a). The 30-day mortality risk was lower in the treated cohort versus the untreated cohort: 0.1% (95% CI 0.1–0.2; 11 deaths) and 0.3% (95% CI 0.3–0.4; 128 deaths), respectively (Fig. 1b).
In the adjusted model after applying the correction factor, treatment with SC CAS+IMD was associated with a 49% lower risk of the composite endpoint versus the untreated patients (aHR 0.51; 95% CI 0.46–0.58) (Fig. 2). Treatment was also associated with a 67% lower 30-day risk of all-cause mortality (aHR 0.33, 95% CI 0.18–0.60).
Adjusted hazard ratios of 30-day all-cause mortality or COVID-19-related hospitalization among patients diagnosed with COVID-19 in the outpatient setting. Square size corresponds to the total sample available for analysis. aHR adjusted hazard ratio, CAS+IMD casirivimab and imdevimab, CI confidence interval, EUA emergency use authorization, SC subcutaneous
The results of the sensitivity analyses were consistent; patients treated with SC CAS+IMD experienced 49–54% lower adjusted 30-day risk of mortality or COVID-19-related hospitalizations compared to EUA-eligible untreated patients (Fig. 2).
Subgroup Analyses
The 30-day risk of the composite outcome generally increased with older age and was higher among those at elevated risk or who were immunocompromised (B cell deficient) or unvaccinated relative to those without these risk factors (Table 2). The aHRs for treatment with SC CAS+IMD versus no treatment were generally consistent across subgroups (Fig. 3). While the CIs were wide in the subgroups defined by 12–17 years of age and secondary B cell deficiency, the point estimates suggested that SC CAS+IMD treatment was beneficial. Results were inconclusive among patients with primary B cell deficiencies as a result of a lack of observed events.
Adjusted hazard ratios of 30-day all-cause mortality or COVID-19-related hospitalization in subgroups of patients diagnosed with COVID-19 in the outpatient setting. Square size corresponds to the total sample available for analysis. aElevated risk defined as ≥ 65 years of age, or ≥ 55 years of age with body mass index ≥ 35 kg/m2, type 2 diabetes, chronic obstructive pulmonary disease, or chronic kidney disease. aHR adjusted hazard ratio, CAS+IMD casirivimab and imdevimab, CI confidence interval, EUA emergency use authorization, N/A not available, SC subcutaneous
Discussion
This real-world study found that SC CAS+IMD is effective in the treatment of patients diagnosed with COVID-19 and managed in the outpatient setting [24], and further supports the benefits of treatment reported in clinical trials and smaller real-world studies [2, 14, 15, 19, 23]. Importantly, this study was conducted during the Delta-dominant period, supporting the effectiveness of SC CAS+IMD against this variant. As expected in the EUA-eligible untreated control group, worse COVID-19 outcomes were observed that generally increased with age and were highest in those who were at elevated risk, immunocompromised (i.e., B cell deficient), or unvaccinated. Treatment with SC CAS+IMD resulted in a 49% reduction in the risk of hospitalization/mortality compared with the EUA-eligible untreated patients and these findings were robust across sensitivity analyses. This study also demonstrated that the effect of SC CAS+IMD treatment was maintained across subgroups of patients at greater risk of poor COVID-19 outcomes.
The observed reduction is consistent with a 56% reduction in 28-day mortality/hospitalization reported in a previous study of 652 patients treated with SC CAS+IMD [24]. While the hospitalization outcome of that study was for any cause, it also evaluated IV CAS+IMD and suggested no significant difference in outcomes between IV and SC administration. The results are also consistent with real-world effectiveness reported in claims-based analyses, including one that encompassed a period when the Delta variant was dominant, although SC administration was not specifically evaluated [25, 29].
Results of the subgroup analyses are further concordant with the previous claims database studies [25, 29]; the risk of outcomes was reduced among CAS+IMD–treated patients across all subgroups, demonstrating effectiveness regardless of age or vaccination status and in patients who were at elevated risk or immunocompromised. In particular, treatment effects in vaccinated patients were similar to those who were not vaccinated, suggesting the utility of therapy in EUA-eligible patients with breakthrough infections and in those who are unwilling to be vaccinated or for whom COVID-19 vaccines are less effective. Of clinical relevance is that the largest observed treatment effect was among patients who were immunocompromised, specifically those with secondary B cell deficiencies, who are at increased risk for severe COVID-19 and poorer outcomes [38, 39]. However, given the limited number of patients with primary B cell deficiencies, the benefit of treatment could not be confirmed in this subgroup, although prior real-world studies have demonstrated that this patient subgroup also benefits from CAS+IMD treatment [25, 29].
Strengths and Limitations
To our knowledge, this is the largest study evaluating the real-world effectiveness CAS+IMD, and by linking the Komodo Health data to the CDR Health data, this is also the largest study evaluating effectiveness of SC CAS+IMD. Results were also consistent across numerous sensitivity analyses conducted to evaluate the robustness of our findings and the appropriateness of our assumptions. The results of this study, however, must be interpreted in the context of its limitations, including that viral load and symptoms, which are indicative of COVID-19 severity and may be predictive of outcomes [40,41,42], were not captured. If untreated EUA-eligible patients had less severe disease, which may be why they are untreated, the lack of information on symptoms and confounders may have resulted in bias against CAS+IMD and thus underestimated the treatment effect. Another limitation regards how BMI was captured, since being overweight or obese is a strong risk factor for poor outcomes [43,44,45,46]. Since categorization of BMI was based on ICD-10 codes and most patients did not have their BMI recorded using an ICD diagnosis code, the results may be subject to residual confounding. Residual confounding may also have arisen because vaccination status, a potentially important confounder, is undercaptured in the Komodo Health data. Additionally, given that a correction factor was applied to derive unbiased aHRs, the crude risks between treated and untreated patients are not directly comparable. Furthermore, to estimate the correction factor for SC CAS+IMD-treated patients without a recorded COVID-19 diagnosis, it was assumed that 10 days had elapsed between symptom onset and treatment, which may have inflated the correction factor and resulted in underestimating the treatment effect. While the study period did not overlap with emergence of the Omicron variant, CAS+IMD is not expected to be active against Omicron [9]. Additionally, although Delta was the most prevalent variant (i.e., greater than 95%) in circulation during the study period [47], we cannot rule out that the distribution of variants may have been different in the state of Florida compared to the USA. Finally, the untreated control group consisted of patients who were never treated with COVID-19 mAbs during the study period, potentially resulting in a healthier cohort of patients that could bias results against treatment.
Conclusion
This study reports the real-world benefits of SC CAS+IMD among patients diagnosed with COVID-19 in the ambulatory setting during the Delta-dominant period. Overall, SC CAS+IMD was associated with a 49% reduction in the risk of COVID-19-related hospitalization/mortality compared with EUA-eligible untreated controls, with benefits that were generally maintained across subgroups, including vaccinated patients. Given the emergence of new variants of concern, continual monitoring and reassessment of real-world effectiveness are integral to updating management strategies and identifying factors that can further improve patient outcomes and reduce pandemic transmission.
References
Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2020;384(3):238–51.
Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385(23):e81.
Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–44.
Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–50.
O’Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med. 2021;385(13):1184–95.
O’Brien MP, Forleo-Neto E, Sarkar N, et al. Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial. JAMA. 2022;327(5):432–41.
U.S. Food & Drug Administration. Fact sheet for health care providers emergency use authorization (EUA) for EVUSHELD™ (tixagevimab co-packaged with cilgavimab). Revised February 2022. https://www.fda.gov/media/154701/download. Accessed 6 Mar 2022.
U.S. Food & Drug Administration. Fact sheet for health care providers emergency use authorization (EUA) of casirivimab and imdevimab. Revised January 2022. https://www.fda.gov/media/145611/download. Accessed 6 Mar 2022.
Cavazzoni P. Coronavirus (COVID-19) update: FDA limits use of certain monoclonal antibodies to treat COVID-19 due to the Omicron variant. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-covid-19-due-omicron. Accessed 1 Feb 2022.
Cooper MH, Christensen PA, Salazar E, et al. Real-world assessment of 2,879 COVID-19 patients treated with monoclonal antibody therapy: a propensity score-matched cohort study. Open Forum Infect Dis. 2021;8(11):ofab512.
Bierle DM, Ganesh R, Tulledge-Scheitel S, et al. Monoclonal antibody treatment of breakthrough COVID-19 in fully vaccinated individuals with high-risk comorbidities. J Infect Dis. 2022;225(4):598–602.
Ganesh R, Philpot LM, Bierle DM, et al. Real-world clinical outcomes of bamlanivimab and casirivimab–imdevimab among high-risk patients with mild to moderate coronavirus disease 2019. J Infect Dis. 2021;224(8):1278–86.
Razonable RR, Aloia NCE, Anderson RJ, et al. A framework for outpatient infusion of antispike monoclonal antibodies to high-risk patients with mild-to-moderate coronavirus disease-19: the Mayo Clinic Model. Mayo Clin Proc. 2021;96(5):1250–61.
Verderese JP, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate coronavirus disease 2019 (COVID-19): a real-world experience. Clin Infect Dis. 2022;74(6):1063–9.
Webb BJ, Buckel W, Vento T, et al. Real-world effectiveness and tolerability of monoclonal antibody therapy for ambulatory patients with early COVID-19. Open Forum Infect Dis. 2021;8(7):ofab331.
Chilimuri S, Mantri N, Gurjar H, et al. Implementation and outcomes of monoclonal antibody infusion for COVID-19 in an inner-city safety net hospital: a South–Bronx experience. J Natl Med Assoc. 2021;113(6):701–5.
Close RM, Jones TS, Jentoft C, McAuley JB. Outcome comparison of high-risk Native American patients who did or did not receive monoclonal antibody treatment for COVID-19. JAMA Netw Open. 2021;4(9):e2125866.
Kakinoki Y, Yamada K, Tanino Y, et al. Impact of antibody cocktail therapy combined with casirivimab and imdevimab on clinical outcome for Covid-19 patients in a real-life setting: a single institute analysis. Int J Infect Dis. 2022;117:189–94.
Piccicacco N, Zeitler K, Montero J, et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 monoclonal antibody infusions in high-risk outpatients. Open Forum Infect Dis. 2021;8(7):ofab292.
Falcone M, Tiseo G, Valoriani B, et al. Efficacy of bamlanivimab/etesevimab and casirivimab/imdevimab in preventing progression to severe COVID-19 and role of variants of concern. Infect Dis Ther. 2021;10(4):2479–88.
Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315.
Wynia MK, Beaty LE, Bennett TD, et al. Real world evidence of neutralizing monoclonal antibodies for preventing hospitalization and mortality in COVID-19 outpatients. medRxiv. 2022. https://doi.org/10.1101/2022.01.09.22268963.
Razonable RR, Pawlowski C, O’Horo JC, et al. Casirivimab–imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine. 2021;40:101102.
McCreary EK, Bariola JR, Wadas RJ, et al. Association of subcutaneous or intravenous route of administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in COVID-19. JAMA Netw Open. 2022;5(4):e226920.
Hussein M, Wei W, Mastey V, et al. Real-world effectiveness of casirivimab/imdevimab among patients diagnosed with COVID-19 in the ambulatory setting: a retrospective analysis using a large claims database. medRxiv. 2022. https://doi.org/10.1101/2022.05.19.22272842.
Datavant. Overview of Datavant’s de-identification and linking technology for structured data. https://datavant.com/wp-content/uploads/dlm_uploads/2018/09/WhitePaper_-De-Identifying-and-Linking-Structured-Data.pdf. Accessed 3 Mar 2022.
Wadman M. What does the Delta variant have in store for the United States? We asked coronavirus experts. ScienceInsider. August 4, 2021. https://www.science.org/content/article/what-does-delta-variant-have-store-us-we-asked-coronavirus-experts. Accessed 6 Mar 2022.
Centers for Disease Control and Prevention. Science Brief: Omicron (B.1.1.529) variant. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-omicron-variant.html. Accessed 3 Mar 2022.
Wei W, Murdock D, Jalbert JJ, et al. Real-world effectiveness of casirivimab and imdevimab in patients with COVID-19 in the ambulatory setting: an analysis of two large US national claims databases. medRxiv. 2022. https://doi.org/10.1101/2022.02.28.22270796.
Karim ME, Gustafson P, Petkau J, Tremlett H, Long-Term Benefits and Adverse Effects of Beta-Interferon for Multiple Sclerosis (BeAMS) Study Group. Comparison of statistical approaches for dealing with immortal time bias in drug effectiveness studies. Am J Epidemiol. 2016;184(4):325–35.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Centers for Disease Control and Prevention. Clinical growth charts. https://www.cdc.gov/growthcharts/clinical_charts.htm. Accessed 23 Nov 2021.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–61.
Stuart EA, Lee BK, Leacy FP. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66(8 Suppl):S84.e1–S90.e1.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Hall WJ, Wellner JA. Confidence bands for a survival curve from censored data. Biometrika. 1980;67:133–43.
Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–8.
Jones JM, Faruqi AJ, Sullivan JK, Calabrese C, Calabrese LH. COVID-19 outcomes in patients undergoing B cell depletion therapy and those with humoral immunodeficiency states: a scoping review. Pathog Immun. 2021;6(1):76–103.
Shields AM, Burns SO, Savic S, Richter AG, Consortium UPC. COVID-19 in patients with primary and secondary immunodeficiency: the United Kingdom experience. J Allergy Clin Immunol. 2021;147(3):870 e1-875 e1.
Fajnzylber J, Regan J, Coxen K, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11(1):5493.
Trunfio M, Venuti F, Alladio F, et al. Diagnostic SARS-CoV-2 cycle threshold value predicts disease severity, survival, and six-month sequelae in COVID-19 symptomatic patients. Viruses. 2021;13(2):281.
Boyapati A, Wipperman MF, Ehmann PJ, et al. Baseline severe acute respiratory syndrome viral load is associated with coronavirus disease 2019 severity and clinical outcomes: post hoc analyses of a phase 2/3 trial. J Infect Dis. 2021;224(11):1830–8.
Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71(15):896–7.
Singh S, Bilal M, Pakhchanian H, Raiker R, Kochhar GS, Thompson CC. Impact of obesity on outcomes of patients with COVID-19 in United States: a multicenter electronic health records network study. Gastroenterology. 2020;159(6):2221-5.e6.
Tamara A, Tahapary DL. Obesity as a predictor for a poor prognosis of COVID-19: a systematic review. Diabetes Metab Syndr. 2020;14(4):655–9.
Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020;173(10):773–81.
Lambrou AS, Shirk P, Steele MK, et al. Genomic surveillance for SARS-CoV-2 variants: predominance of the delta (B.1.617.2) and omicron (B.1.1.529) variants—United States, June 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(6):206–11.
Acknowledgements
Funding
This study was funded by Regeneron Pharmaceuticals, Inc. Regeneron also funded the journal’s Rapid Service Fee.
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by E. Jay Bienen, PhD, and editorial assistance was provided by Prime, Knutsford, United Kingdom. The study was funded by Regeneron Pharmaceuticals, Inc. Data programming and analytics support was provided by Wenqin Qiang and Dehua Kan from KMK Consulting Inc. and was funded by Regeneron Pharmaceuticals, Inc.
Author Contributions
Jessica J. Jalbert and Wenhui Wei had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Jessica J. Jalbert, Mohamed Hussein, Vera Mastey, Robert J. Sanchez, Dana Murdock, Wenhui Wei. Acquisition, analysis, or interpretation of data: Jessica J. Jalbert, Mohamed Hussein, Vera Mastey, Robert J. Sanchez, Dana Murdock, Degang Wang, Laura Farinas, Jonathan Bussey, Carlos Duart, Wenhui Wei. Statistical analysis: Degang Wang, Wenhui Wei. Administrative, technical, or material support: Vera Mastey, David M. Weinreich, Boaz Hirshberg. All authors critically revised the manuscript and gave final approval for publication.
Disclosures
Jessica J. Jalbert, Mohamed Hussein, Vera Mastey, Robert J. Sanchez, Degang Wang, Dana Murdock, Boaz Hirshberg, David M. Weinreich, and Wenhui Wei are employees and stockholders of Regeneron Pharmaceuticals, Inc. Laura Farinas, Jonathan Bussey, and Carlos Duart have nothing to disclose.
Compliance with Ethics Guidelines
The authors conducted secondary research using de-identified data licensed from a third party, Komodo, in compliance with 45 CFR 164.514(a)-(c). The data had identifying patient information removed and were coded in such a way that the data could not be linked back to subjects from whom they were originally collected prior to the authors gaining access to it. This research, which used the de-identified licensed data described above, does not require institutional review board or ethics review, as analyses with these data do not meet the definition of ‘research involving human subjects’ as defined within 45 CFR 46.102(f), which stipulates human subjects as living individuals about whom an investigator obtains identifiable private information for research purposes.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available from Komodo Health due to licensing restrictions. Data are however available from the authors upon reasonable request and with permission of Komodo Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jalbert, J.J., Hussein, M., Mastey, V. et al. Effectiveness of Subcutaneous Casirivimab and Imdevimab in Ambulatory Patients with COVID-19. Infect Dis Ther 11, 2125–2139 (2022). https://doi.org/10.1007/s40121-022-00691-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00691-z