Abstract
Introduction
Fracture-related infections (FRIs) are challenging for orthopedic surgeons, as conventional surgical treatment and systemic antimicrobial therapy cannot completely control local infections. Continuous local antibiotic perfusion (CLAP) is a novel and innovative therapy for bone and soft-tissue infections, and is expected to eradicate biofilms by maintaining a sustained high concentration of antimicrobial agents at the infected site. If CLAP therapy can eradicate infection even in cases with implants while preserving the implants, it would be an ideal and effective treatment for local refractory infections. This study aimed to evaluate the usefulness of novel CLAP therapy for FRIs.
Methods
Nine patients treated with CLAP therapy were retrospectively analyzed. The mean age was 65.9 (43–82) years, and the mean follow-up period was 14.9 (6–45) months. In all cases, the infected sites were related to the lower extremities (tibia, n = 6; fibula, n = 1; hip joint, n = 1; foot, n = 1). All patients underwent similar procedures for this therapy combined with negative-pressure wound therapy after thorough irrigation and debridement of infected tissues.
Results
The pathogens identified were Staphylococcus aureus (methicillin-resistant S. aureus, n = 5; methicillin-susceptible S. aureus, n = 1), Pseudomonas aeruginosa (n = 3), Enterococcus faecalis (n = 2), Corynebacterium (n = 1), and Enterobacter (n = 1); pathogens were not detected in one case. The mean duration of CLAP was 17.0 (7–35) days. In all cases, implants were preserved until bone union was achieved. Five cases relapsed; however, infection was finally suppressed in all cases by repeating this method. No side effects were observed.
Conclusion
This novel case series presents treatment outcomes using CLAP therapy for FRIs. This method has the potential to control the infection without removing the implants, because of the sustained high concentration of antimicrobial agents at the infected site, and could be a valuable treatment option for refractory FRIs with implants, in which bone union has not been achieved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Fracture-related infection (FRI) is one of the more challenging complications of musculoskeletal injuries, and the infection cannot be completely controlled in some cases. |
Continuous local antibiotic perfusion (CLAP) is a novel, innovative therapy that can maintain a high concentration of antimicrobials at the infected site for a sufficiently long duration to eradicate biofilms by continuous local administration through the bone marrow needle and dual-lumen tube using a syringe pump. |
The present study aimed to evaluate the usefulness of novel CLAP therapy for FRIs with implants, in which bone union has not been achieved. |
What was learned from the study? |
In all cases, implants were preserved until bone union was achieved. Five out of nine cases relapsed; however, infection was finally suppressed in all cases by repeating this method. No side effects were observed. |
This novel technique is not perfect, but it may be useful as a first choice for refractory FRIs with implants, in which bone union has not been achieved, as it is less invasive than conventional therapy. |
Digital Features
This article is published with digital features, including a video, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.19672842.
Introduction
Fracture-related infection (FRI), such as soft-tissue infection, nonunion, osteomyelitis, and pyogenic arthritis, is a major complication of musculoskeletal trauma and is one of the more challenging complications for orthopedic surgeons [1, 2]. Surgeons generally perform debridement of infected tissues through irrigation, incisional drainage, and systemic antimicrobial therapy [3, 4]; however, in refractory cases, additional repeated bone and soft-tissue debridement and implant removal are thus obligatory, leading to severe, irreversible functional loss, or even amputation of the affected limb, resulting in a significant decrease in patients’ quality of life [5, 6]. Even in early infection cases, the success rate of treatment for FRIs with implant-retention has been reported to be about 90% [7]. A major cause of FRI is the formation of biofilms, communities of antibiotic-tolerant bacteria encased within a matrix on non-living surfaces such as implants or dead bone fragments [8,9,10]. These localized, grouped bacteria resist antimicrobial therapy, and concentrations 100–1000 times higher than the minimum inhibitory concentration (MIC) of antimicrobial agents are required to eradicate biofilms [11, 12].
The concentrations achieved through systemic antimicrobial administration do not reach the minimum biofilm eradication concentration (MBEC) [13, 14], and the high systemic concentration necessary to suppress biofilms may cause side effects such as acute renal function and toxicity. Therefore, a high local antimicrobial concentration, exceeding the MIC, is necessary to control refractory bone and soft-tissue infections. In previous reports, antimicrobial powders [15, 16] and antibiotic-loaded, nonresorbable, polymethylmethacrylate (PMMA) bone cement [17,18,19] were often used for local antimicrobial therapy; however, these methods only afforded a temporary effect, and the concentration did not reach the MBEC [20].
Recently, the usefulness of continuous local antibiotic perfusion (CLAP) therapy for bone and soft-tissue infections has been reported [21]. CLAP is a novel, innovative therapy that can maintain a high concentration of antimicrobials at the infected site for a sufficiently long duration to eradicate biofilms. Antimicrobials are continuously infused via the bone marrow needle (intramedullary antibiotic perfusion, iMAP) and dual-lumen tube using a syringe pump (intra-soft-tissue antibiotic perfusion, iSAP). Furthermore, the drainage can be reliably recovered by applying negative pressure to the main circuit, and the pressure gradients enable efficient transfer of antimicrobials to the infected site.
If CLAP therapy can eradicate infection even in cases with implants while preserving the implants, it would be an ideal and effective treatment for local refractory infections with minimal invasiveness. The present study aimed to evaluate the usefulness of novel CLAP therapy for FRIs with implants, in which bone union has not been achieved.
Case Presentations
Aim
We conducted a retrospective case series of patients with implants, in which bone union has not been achieved, who underwent CLAP therapy for the treatment of FRI to assess the effectiveness of this novel therapy.
Design
We retrospectively collected data regarding 12 patients with a diagnosis of FRI who were treated using CLAP therapy in our hospital between July 2017 and June 2021. The exclusion criteria were as follows: patients who did not meet the definition of FRI [1], patients who could not be followed up for more than 6 months, and patients with a history of multiple lifesaving surgical interventions. Finally, nine patients (men, n = 8; women, n = 1) were included in this study, and the data were collected from medical records. This study was performed in accordance with the Declaration of Helsinki, and ethical approval was obtained from the Institutional Review Board (IRB) (approval no. 2019-029) of the University of Occupational and Environmental Health, Japan. The study participants provided written informed consent for participation and the use of their clinical data in the study. All patients underwent CLAP therapy according to the treatment strategies described below. The following data were collected: patient age, sex, infection site, classification of FRI, pathogens, duration of CLAP, implant survival, relapse, and complications.
Setting of the Study
Diagnosis
If FRI was suspected on the basis of clinical signs—including local redness and heat, swelling, tenderness, fever, the presence of a fistula, sinus, wound breakdown, and pus—a blood test (including leukocyte and neutrophil count, and C-reactive protein concentration) and diagnostic imaging (including radiography, computed tomography [CT], and magnetic imaging resonance, if necessary) were performed. In patients with pyrexia, blood cultures and infection-site cultures were also performed before antimicrobial administration. FRI was classified into three groups based on the time of onset: early infection (less than 2 weeks), delayed infection (2–10 weeks), and late infection (more than 10 weeks) [22].
Surgical Procedures
Under general or regional anesthesia, an incision was made in the skin over the infected area. We regarded tenderness of the area as the most important clinical finding. Only obviously infected and biologically inactive tissues were removed, and thorough irrigation was performed. Surgical tissue samples were collected for microbiological examination and analysis. In cases with implants after osteosynthesis, if there were no signs of mechanically unstable fixation and loosening associated with the infection, we preserved the implant during the first-look surgery.
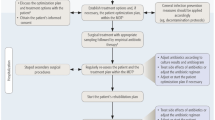
Bone infections were treated using iMAP; two bone marrow needles with a diameter of 3 mm were inserted above and below the sites of suspected infection. A 2.4-mm Kirschner wire was used to make a hole in the cortical bone, and a bone marrow needle (Tohoku University bone marrow puncture needle; Senko Medical Instrument Manufacturing Co., Tokyo, Japan) or iMAP pin (Cubex Medical, Tokyo, Japan) was inserted. Soft-tissue infections were treated with iSAP by inserting dual-lumen tubes (Salum Sump Tube™, Cardinal, unknown) into the dead space where infection was suspected. The tip of the tube was deeply inserted, far away from the suspected area of infection, and the antimicrobials were infused to fill the entire space. The wound was closed as much as possible and was covered using negative-pressure wound therapy (NPWT). The NPWT suction tube (RENASYS; Smith & Nephew Medical, Kingston upon Hull, UK) was branched, connecting to the suction port of the dual-lumen tube (Fig. 1).
Overview of CLAP therapy. Continuous local antibiotic perfusion combining intramedullary antibiotic perfusion with a bone marrow needle and intra-soft-tissue antibiotic perfusion with a double lumen tube (https://www.ismap-clap.com/information; with permission to publish)
Video abstract: Water flow test to ensure that an effective delivery circuit for CLAP has been constructed (MP4 2161 KB)
Postoperative Management
The suction pressure of the NPWT was set at a low continuous negative pressure between − 40 and − 80 mmHg, according to the condition of the wound and soft tissues. Gentamicin (60–120 mg/50 mL) was continuously administered at a dosage of 2 mL/h using a syringe pump through the bone marrow needles and/or dual-lumen tubes. The dose of gentamicin administered through one route was adjusted at the discretion of the surgeon depending on the degree of infection. In principle, cefazolin was administered intravenously; however, when the pathogen was identified as MRSA (methicillin-resistant Staphylococcus aureus), and the effect of CLAP was judged to be insufficient, the use of anti-MRSA agents was considered. The blood levels of gentamicin were measured 3 days after surgery to assess the systemic effect of CLAP therapy. If the blood concentration is greater than 1 μg/mL, the antibiotic concentration is reduced to 1200 μg/mL. We prioritized clinical findings such as the presence of exudate, local redness and heat, spontaneous pain, tenderness, fever, and C-reactive protein when assessing treatment effects; if there were signs of infection or wound healing problems, additional surgery was performed until there were no signs of infection.
Results
The clinical data of all patients are summarized in Table 1. The patients’ mean age was 65.9 years (range 43–82 years), and the mean follow-up period was 14.9 months (6–45 months). In all cases, the infected sites were related to the lower extremities (tibia, n = 6; fibula, n = 1; hip joint, n = 1; foot, n = 1). The classification of FRI was early infection in two cases, delayed infection in one case, and late infection in six cases. There were some cases of superinfection in which two or more pathogens were identified. Including the cases of superinfection, the pathogens identified were S. aureus (MRSA, n = 5; methicillin-susceptible S. aureus [MSSA], n = 1), Pseudomonas aeruginosa (n = 3), Enterococcus faecalis (n = 2), Corynebacterium (n = 1), and Enterobacter (n = 1); pathogens were not detected in one case. The mean CLAP duration was 17.0 days (7–35 days). Including two cases in which intramedullary nails were inserted in our hospital, we were able to preserve the implant until bone union in eight cases. Five cases relapsed; however, we finally suppressed infection in all patients through repeated CLAP therapy. The mean number of surgical interventions required to control infection was 2.5 (1–5 times). There were no side effects of CLAP therapy, including renal dysfunction or auditory disorders. Blood concentrations (gentamicin levels) were measured in four cases, and all were less than 2.0 μg/mL (0.6–1.3 μg/mL).
Representative Cases
Case 1 (Patient 2 in Table 1)
A 59-year-old man underwent open reduction and fixation with Ender nails and local pedicled flap (reverse sural artery flap) for left open tibial and fibular fracture (Gustilo grade IIIB) at a different hospital. The patient experienced a relapse of knee pain 3 months after surgery, and a postoperative infection was suspected. Irrigation and debridement were performed, and MRSA was detected from bacterial culture test of the abscesses. Postoperative wound breakdown, surrounding redness, heat, and pus were observed, and a diagnosis of tibial infectious pseudoarthrosis was made. The patient was transferred to our hospital 10 months after the first surgery (Fig. 2a). Bone union was not completely achieved, and there were signs suggestive of osteomyelitis on CT. The fracture was unstable owing to the infection; therefore, the infected tissue and Ender nails were removed. Bone marrow needles were placed in the proximal and distal metaphysis to the pseudoarthrosis, and after reaming the intramedullary cavity with a reamer, the bone marrow was thoroughly washed with saline using a bone marrow needle (Movie 1). Next, a dual-lumen tube was placed near the pseudoarthrosis (Fig. 2b, c), and incisional NPWT was administered to the open wound. Gentamicin (240 mg/day) was continuously administered using a syringe pump through the bone marrow needles and dual-lumen tube. After 1 week, a second-look surgery and intramedullary nailing were performed, and bone marrow needles and a dual-lumen tube were placed again (Fig. 3). All bone marrow needles and the dual-lumen tube were removed 35 days post-surgery, and the wound was closed because of improved clinical findings and negative inflammatory reactions. At the final follow-up 9 months post-surgery, no recurrence was observed, and bone union was achieved (Fig. 4).
Radiographic images. a Bone union was not completely achieved owing to tibial infectious pseudoarthrosis at the first visit, 10 months after primary surgery. b, c Postoperative radiographic images. After removal of Ender nails and thorough irrigation and debridement, bone marrow needles were placed in the proximal and distal metaphysis to the pseudoarthrosis, and a dual-lumen tube was placed near the pseudoarthrosis
Case 2 (Patient 5 in Table 1)
A 55-year-old man was injured following a motorcycle crush, resulting in a Gustilo grade IIIB fracture of the leg (AO 43A3.2, 4F2B). Thorough irrigation and debridement were performed on the day of injury, and an external fixator was attached. The next day, the first stage of the Masquelet technique was executed; the soft-tissue defect was covered by a primary free anterolateral thigh (ALT) flap 6 days after injury; however, ALT flap was unsuccessful. Therefore, secondary flap reconstruction was performed using a free latissimus dorsi flap 2 days after ALT flap reconstruction.
Two months after the first stage, the second stage was initiated; however, 2 weeks after initiation of the second stage, a fistula, as well as pus from the proximal part of the flap, was observed. We performed CLAP therapy (iSAP), and MRSA was detected via bacterial culture tests of the abscesses. Gentamicin (80 mg/day) was continuously administered for 11 days and although the infection subsided, recurrence was observed 8 months later. A second round of CLAP therapy (iMAP + iSAP) was performed, and gentamicin (120 mg/day) was continuously administered for 7 days; however, recurrence of infection was observed 13 months later. A fistula with pus from the medial distal wound (Fig. 5a) was observed. Bone union was not completely achieved because of infection (Fig. 6). Therefore, the first stage of the Masquelet technique was planned, in addition to CLAP therapy. Infected bone fragments and the medial support plate were removed, and the tibial bone defect was filled with antibiotic-loaded bone cement (Fig. 5b). A third round of CLAP therapy (iMAP + iSAP) was performed (Fig. 5c), and P. aeruginosa was detected using bacterial culture tests of the abscesses; gentamicin (120 mg/day) was continuously administered for 8 days. Two months later, the second stage was performed (Fig. 7). At the final follow-up visit 45 months after injury, there was no recurrence, and bone union was achieved (Fig. 8).
Discussion
FRI is a severe complication and is often further complicated by the development of biofilms. Surgical management is often unavoidable, and sometimes implant removal is required. Successful eradication of infection requires thorough debridement of infected tissues, dead space management, a stable fracture environment, and appropriate antimicrobial therapy. In patients with implants, the timing of implant removal should be carefully determined on the basis of the clinical course of the FRI; additionally, the extent of infection and stability of the implant should be considered [22, 23]. Any sign of instability or loosening associated will force implant removal. Gentamicin–PMMA bone beads are generally used; however, the local concentration is as low as 200–300 μg/mL, and the gentamicin stops being released within 2 weeks [24]. The Lautenbach method has been also reported as a local antibiotic perfusion therapy for chronic osteomyelitis. However, the local concentration is presumed to be low because the solution is mixed with antimicrobial agents and irrigated at a volume of 1 L per day. Even if the infection subsides, thorough debridement is unavoidable, and the problem of reconstructing bone and soft-tissue defects remains [25]. On the other hand, CLAP is a method that can deliver a sufficient local concentration of antimicrobials in a small amount of saline at low speed. Therefore, CLAP may be able to control infection even with minimal debridement. Our priority goal is to control infection with implant preservation, unless there are no signs of mechanically unstable fixation or loosening associated with infections. In this case series, we were able to control the infection in all patients through repeated CLAP therapy without removing the implants until bone union was achieved.
CLAP is a relatively new drug delivery system that has recently been reported to achieve a high concentration of antimicrobials at the infected site for a sufficiently long duration to eradicate biofilms [21]. By combining iMAP and/or iSAP, antimicrobials can be effectively guided and spread to the infected sites. Some of the antimicrobials in the bone (inserted via bone marrow needles) spread throughout the body via the bloodstream, whereas some antimicrobials leak into the hematoma and dead space around the infected site. Thus, by inserting dual-lumen tubes into the dead space created by infection or surgery and by applying continuous negative pressure, the antimicrobials can be guided to the drain according to the pressure gradient; this mechanism reduces the amount of antimicrobial agent that enters the blood, reducing systemic side effects. In cases with diaphyseal fractures, bone marrow needles are placed proximal and distal to the fracture sites, whereas dual-lumen tubes are placed near the fracture sites. In cases with an intramedullary nail, the needles are placed within the vicinity of the intramedullary nail, as the fluid spreads along the reaming mantle. Thus, it is important to build an effective delivery circuit that considers the range of spread of infection, using both diagnostic imaging and surgical findings.
Local administration of antimicrobial agents should be concentration dependent and bactericidal; aminoglycosides and quinolones fulfill these criteria [26]. Aminoglycosides, which have poor tissue penetration, are unsuitable for intravenous administration. However, it has been reported that local aminoglycoside administration in combination with systemic antibiotics may be effective in lowering infection rates in open fractures [27]. However, local administration is advantageous because aminoglycosides are not easily transferred to the whole body; gentamicin is particularly useful for treating bacteria resistant to other antimicrobials. Many previous reports have studied the efficacy and safety of aminoglycosides [27,28,29,30], and the blood and local concentrations of the administered antimicrobials can be measured; therefore, gentamicin is mainly used for CLAP therapy. A previous study on antibiotic susceptibility tests targeting biofilm-producing S. aureus, including MRSA, showed that the MIC of gentamicin ranged from 0.06 to 0.64 μg/mL, whereas the MBEC ranged from 1 to > 256 μg/mL, and gentamicin was effective in eradicating biofilms. Conversely, the MIC of linezolid and vancomycin ranged from 1 to 2 μg/mL and from 0.25 to 1 μg/mL, respectively, whereas the MBEC ranged from 4 to > 1024 μg/mL and from 8 to > 1024 μg/mL, respectively, indicating that these antimicrobial agents lacked activity against S. aureus grown in biofilm [31]. Thus, S. aureus is prone to forming biofilms, whereas gentamicin, which is effective against biofilms including antibiotic-resistant bacteria, seems to be suitable for local administration. Renal dysfunction and ototoxicity are representative side effects of aminoglycosides [32]. The trough concentration of gentamicin was 2.0 μg/mL, and the dose was adjusted by monitoring blood levels to avoid exceeding trough levels for CLAP therapy.
This study presents some limitations. First, this was a retrospective study that only assessed nine cases. Additionally, there were no controls to clarify the effectiveness of CLAP therapy compared with that of conventional therapy; therefore, more cases should be included in future studies. Second, gentamicin blood levels were only measured in four cases. In this case series, there were no side effects, such as renal dysfunction. However, blood levels should be measured periodically in all patients undergoing CLAP therapy, and it is necessary to pay attention to changes in renal function.
In bone and soft-tissue infections such as FRI, it is difficult for antimicrobials to reach local infected sites with poor blood flow; additionally, a high concentration of the antimicrobial is required. It is considered that CLAP therapy may be able to reduce infection while preserving the tissue, avoiding the need for resection of infected tissue, which was normally undertaken before the use of CLAP therapy. However, in our case series, five cases relapsed, and the mean number of surgical interventions required to control infection was 2.5. As shown by these results, CLAP therapy for FRI is not perfect. Even when combined with CLAP therapy, FRI is often refractory, and multiple surgical interventions may be required. It is important not only to correctly assess the infection focus, and perform definitive irrigation and debridement, but also to set up an effective delivery circuit that considers the range of spread of infection.
Conclusion
We report the clinical results of nine cases of FRI treated via CLAP therapy. We were able to control the infection while preserving the implants until bone union with repeated CLAP therapy. CLAP therapy is a non-invasive strategy that has the potential to control local infection. Although CLAP therapy is not a perfect treatment for FRI, it may be a valuable treatment option for refractory FRI with implants in which bone union is not achieved, particularly when there are no signs of mechanically unstable fixation or loosening associated with infections.
References
Metsemakers WJ, Morgenstern M, McNally MA, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury. 2018;49:505–10. https://doi.org/10.1016/j.injury.2017.08.040.
Govaert GAM, Kuehl R, Atkins BL, et al. Diagnosing fracture-related infection: current concepts and recommendations. J Orthop Trauma. 2020;34:8–17. https://doi.org/10.1097/BOT.0000000000001614.
Metsemakers WJ, Kuehl R, Moriarty TF, et al. Infection after fracture fixation: current surgical and microbiological concepts. Injury. 2018;49:511–22. https://doi.org/10.1016/j.injury.2016.09.019.
Depypere M, Kuehl R, Metsemakers WJ, et al. Recommendations for systemic antimicrobial therapy in fracture-related infection: a consensus from an international expert group. J Orthop Trauma. 2020;34:30–41. https://doi.org/10.1097/BOT.0000000000001626.
Metsemakers WJ, Onsea J, Neutjens E, et al. Prevention of fracture-related infection: a multidisciplinary care package. Int Orthop. 2017;41:2457–69. https://doi.org/10.1007/s00264-017-3607-y.
Thakore RV, Greenberg SE, Shi H, et al. Surgical site infection in orthopedic trauma: a case–control study evaluating risk factors and cost. J Clin Orthop Trauma. 2015;6:220–6. https://doi.org/10.1016/j.jcot.2015.04.004.
Tschudin-Sutter S, Frei R, Dangel M, et al. Validation of a treatment algorithm for orthopaedic implant-related infections with device-retention-results from a prospective observational cohort study. Clin Microbiol Infect. 2016;22(457):e1-9. https://doi.org/10.1016/j.cmi.2016.01.004.
Saeed K, McLaren AC, Schwarz EM, et al. International consensus meeting on musculoskeletal infection: summary from the biofilm work group and consensus on biofilm related musculoskeletal infections. J Orthop Res. 2019;37:1007–17. https://doi.org/10.1002/jor.24229.
Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. https://doi.org/10.1126/science.284.5418.1318.
Arciola CR, Campoccia D, Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018;16:397–409. https://doi.org/10.1038/s41579-018-0019-y.
Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37:1771–6. https://doi.org/10.1128/JCM.37.6.1771-1776.1999.
Reiter KC, Villa B, Paim TGDS, de Oliveira CF, d’Azevedo PA. Inhibition of biofilm maturation by linezolid in meticillin-resistant Staphylococcus epidermidis clinical isolates: comparison with other drugs. J Med Microbiol. 2013;62:394–9. https://doi.org/10.1099/jmm.0.048678-0.
Badha V, Moore R, Heffernan J, Castaneda P, McLaren A, Overstreet D. Determination of tobramycin and vancomycin exposure required to eradicate biofilms on muscle and bone tissue in vitro. J Bone Jt Infect. 2019;4:1–9. https://doi.org/10.7150/jbji.29711.
Castaneda P, McLaren A, Tavaziva G, Overstreet D. Biofilm antimicrobial susceptibility increases with antimicrobial exposure time. Clin Orthop Relat Res. 2016;474:1659–64. https://doi.org/10.1007/s11999-016-4700-z.
Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976). 2011;36:2084–8. https://doi.org/10.1097/BRS.0b013e3181ff2cb1.
Li S, Rong H, Zhang X, et al. Meta-analysis of topical vancomycin powder for microbial profile in spinal surgical site infections. Eur Spine J. 2019;28:2972–80. https://doi.org/10.1007/s00586-019-06143-6.
Zalavras CG, Patzakis MJ, Holtom P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Orthop Relat Res. 2004;427:86–93. https://doi.org/10.1097/01.blo.0000143571.18892.8d.
Wininger DA, Fass RJ. Antibiotic-impregnated cement and beads for orthopedic infections. Antimicrob Agents Chemother. 1996;40:2675–9. https://doi.org/10.1128/AAC.40.12.2675.
Conway J, Mansour J, Kotze K, Specht S, Shabtai L. Antibiotic cement-coated rods: an effective treatment for infected long bones and prosthetic joint nonunions. Bone Joint J. 2014;96-B:1349–54. https://doi.org/10.1302/0301-620X.96B10.33799.
Anagnostakos K, Wilmes P, Schmitt E, Kelm J. Elution of gentamicin and vancomycin from polymethylmethacrylate beads and hip spacers in vivo. Acta Orthop. 2009;80:193–7. https://doi.org/10.3109/17453670902884700.
Himeno D, Matsuura Y, Maruo A, Ohtori S. A novel treatment strategy using continuous local antibiotic perfusion: a case series study of a refractory infection caused by hypervirulent Klebsiella pneumoniae. J Orthop Sci. 2020;19(2658):S0949. https://doi.org/10.1016/j.jos.2020.11.010.
Trampuz A, Zimmerli W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury. 2006;37(Suppl 2):S59–66. https://doi.org/10.1016/j.injury.2006.04.010.
Berkes M, Obremskey WT, Scannell B, et al. Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am. 2010;92:823–8. https://doi.org/10.2106/JBJS.I.00470.
Walenkamp GH, Vree TB, van Rens TJ. Gentamicin-PMMA beads. Pharmacokinetic and nephrotoxicological study. Clin Orthop Relat Res. 1996;205:171–83.
Hashmi MA, Norman P, Saleh M. The management of chronic osteomyelitis using the Lautenbach method. J Bone Joint Surg Br. 2004;86:269–75. https://doi.org/10.1302/0301-629x.86b2.14011.
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. https://doi.org/10.1086/516284(quiz 11).
Lawing CR, Lin FC, Dahners LE. Local injection of aminoglycosides for prophylaxis against infection in open fractures. J Bone Joint Surg Am. 2015;97:1844–51. https://doi.org/10.2106/JBJS.O.00072.
Cavanaugh DL, Berry J, Yarboro SR, Dahners LE. Better prophylaxis against surgical site infection with local as well as systemic antibiotics. An in vivo study. J Bone Joint Surg Am. 2009;91:1907–12. https://doi.org/10.2106/JBJS.G.01237.
Lovallo J, Helming J, Jafari SM, et al. Intraoperative intra-articular injection of gentamicin: will it decrease the risk of infection in total shoulder arthroplasty? J Shoulder Elbow Surg. 2014;23:1272–6. https://doi.org/10.1016/j.jse.2013.12.016.
Yarboro SR, Baum EJ, Dahners LE. Locally administered antibiotics for prophylaxis against surgical wound infection. An in vivo study. J Bone Joint Surg Am. 2007;89:929–33. https://doi.org/10.2106/JBJS.F.00919.
Mottola C, Matias CS, Mendes JJ, et al. Susceptibility patterns of Staphylococcus aureus biofilms in diabetic foot infections. BMC Microbiol. 2016;16:119. https://doi.org/10.1186/s12866-016-0737-0.
Hayward RS, Harding J, Molloy R, et al. Adverse effects of a single dose of gentamicin in adults: a systematic review. Br J Clin Pharmacol. 2018;84:223–38. https://doi.org/10.1111/bcp.13439.
Acknowledgements
We thank the participants of the study.
Funding
The authors declare no financial support for the research, authorship, and/or publication of this article. The Rapid Service Fee was funded by the authors.
Medical Writing, Editorial, and Other Assistance
The authors thank Editage (http://www.editage.jp) for editing and reviewing the manuscript. This assistance was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
YZ conceived the original idea. All authors collected patient data. KK and YZ analyzed and interpreted the data. KK wrote the manuscript. YZ supervised and revised the manuscript throughout. All authors discussed the results and have read and approved the final version of the manuscript.
Disclosures
Kenji Kosugi, Yukichi Zenke, Naohito Sato, Daishi Hamada, Kohei Ando, Yasuaki Okada, Yoshiaki Yamanaka, and Akinori Sakai have nothing to disclose.
Compliance with Ethics Guidelines
This study was performed in accordance with the Declaration of Helsinki, and ethical approval was obtained from the Institutional Review Board (IRB) (approval no. 2019-029) of the University of Occupational and Environmental Health, Japan. The study participants provided written informed consent for participation and the use of their clinical data in the study. Written informed consent for publication of their clinical details (text and any pictures or video) was obtained from the patients or relatives of the patients; it will be treated confidentially.
Data Availability
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kosugi, K., Zenke, Y., Sato, N. et al. Potential of Continuous Local Antibiotic Perfusion Therapy for Fracture-Related Infections. Infect Dis Ther 11, 1741–1755 (2022). https://doi.org/10.1007/s40121-022-00653-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00653-5