Abstract
Introduction
Balancing the benefits and risks of antimicrobials in health care requires an understanding of their effects on antimicrobial resistance at the population scale. Therefore, we aimed to investigate the association between the population antibiotics use and resistance rates and further identify their critical thresholds.
Methods
Data for monthly consumption of six antibiotics (daily defined doses [DDDs]/1000 inpatient-days) and the number of cases caused by five common drug-resistant bacteria (occupied bed days [OBDs]/10,000 inpatient-days) from inpatients during 2009–2020 were retrieved from the electronic prescription system at Nanjing Drum Tower Hospital, a tertiary hospital in Jiangsu Province, China. Then, a nonlinear time series analysis method, named generalized additive models (GAM), was applied to analyze the pairwise relationships and thresholds of these antibiotic consumption and resistance.
Results
The incidence densities of carbapenem-resistant Acinetobacter baumannii (CRAB), carbapenem-resistant Klebsiella pneumoniae (CRKP), and aminoglycoside-resistant Pseudomonas aeruginosa were all strongly synchronized with recent hospital use of carbapenems and glycopeptides. Besides, the prevalence of carbapenem-resistant Escherichia coli was also highly connected the consumption of carbapenems and fluoroquinolones. To lessen resistance, we determined a threshold for carbapenem and glycopeptide usage, where the maximum consumption should not exceed 31.042 and 25.152 DDDs per 1000 OBDs, respectively; however, the thresholds of fluoroquinolones, third-generation cephalosporin, aminoglycosides, and β-lactams have not been identified.
Conclusions
The inappropriate usage of carbapenems and glycopeptides was proved to drive the incidence of common drug-resistant bacteria in hospitals. Nonlinear time series analysis provided an efficient and simple way to determine the thresholds of these antibiotics, which could provide population-specific quantitative targets for antibiotic stewardship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Antimicrobial resistance leads to increased drug costs, adverse drug events, and high patient morbidity and mortality |
Nonlinear time series can describe the relationships of drug-resistance bacteria to antibiotics and identify certain thresholds for specific antibiotics |
What was learned from the study? |
Improper use of carbapenems and glycopeptides can lead to the occurrence of drug-resistant bacteria commonly found in hospitals |
Nonlinear time series analysis provided a way to determine the thresholds of antibiotics, which could provide population specific quantitative targets for antibiotic stewardship |
Introduction
Antimicrobial resistance affects all areas of public health worldwide and generates increased pharmaceutical fees, adverse drug events, as well as high morbidity and mortality of patients [1, 2]. Some drug-resistant bacteria, such as carbapenem-resistant Acinetobacter baumannii (CRAB), carbapenem-resistant Escherichia coli (E. coli), aminoglycoside-resistant Pseudomonas aeruginosa (P. aeruginosa), carbapenem-resistant Klebsiella pneumoniae (CRKP), and methicillin-resistant Staphylococcus aureus (MRSA), have exhibited high prevalence and accounted for the majority of antibiotic-resistant infections worldwide [3,4,5,6]. From several multi-center studies and reports estimated by the China Antimicrobial Resistance Surveillance System (CARSS), the prevalence of CRAB, carbapenem-resistant E. coli, CRKP, and MRSA in inpatients with bacterial infections were 8–55.4%, 1–6%, 5–15%, and 10–30.9% [5, 7,8,9], respectively. Moreover, controlling the growth of drug-resistant bacteria is urgent worldwide. About 25,000 people die each year from antibiotic-resistant bacteria in Europe [10]. In the USA, the proportion of Enterobacteriaceae bacteria resistant to carbapenems increased from 0% in 2001 to 1.4% in 2010, with the largest increase in Klebsiella spp. [11]. In Pakistan, 50–60% of community-acquired gram-negative pathogens have developed resistance to common oral antibiotics, and in South Africa, 52% of 1147 S. aureus isolates from patients with bacteremia in intensive care units were MRSA [12]. Recently, excessive or inappropriate consumption of antibiotics was considered to accelerate the prevalence and resistance spectrum of drug-resistant bacteria, for instance, the improper application of carbapenem antibiotics has promoted the rapid spread of CRKP, E. coli can be induced by a variety of antibiotics to develop resistance genes, and the hospital densities of MRSA were highly related to the fluoroquinolones and third-generation cephalosporins [13,14,15]. Therefore, given the current irregularity of antibiotic prescribing and its impact on bacterial resistance worldwide [16], identifying a balance between effective therapies and control of resistance is urgently needed for antibiotic management.
Since the antibiotic stewardship can reduce antimicrobial resistance [7, 17], over-restriction may be counterproductive. In 1994, Stuart Levy first proposed the non-linear relationship between antibiotics and resistance and proposed the definition of thresholds, which represent the maximum consumption of one antibiotic [18]. When the consumption of a specific antibiotic surpasses its threshold, the selection pressure will exceed the fitness costs, leading to a dramatic increase of infection [18]. To determine these relationships and thresholds, linear time series analyses were first considered and applied empirically, including unary linear regression and piecewise linear regression [7, 19, 20]. However, emerging evidence has suggested that nonlinear relationships, such as curvilinear regression, spline regression, and generalized additive models (GAMs), are more authentic and efficient to describe the relationships; these can also determine the thresholds of specific antibiotics [8, 15, 21].
Herein, using five statistical methods, we first investigated the correlations between antibiotic consumption and drug-resistant infections among inpatients in a tertiary hospital in Nanjing, China, from 2009 to 2020. Then, by choosing the best-fit statistical method and its calculated regressions, we determined the critical thresholds for β-lactam-β-lactamase inhibitor combinations, fluoroquinolones, carbapenems, third-generation cephalosporins, aminoglycosides, and glycopeptides.
Methods
Study Design and Ethics Statement
A retrospective observational study of patients with infections caused by five kinds of crucial drug-resistant bacteria were conducted in Nanjing Drum Tower Hospital in Jiangsu Province, China. These drug-resistant bacteria were carbapenem-resistant Acinetobacter baumannii (CRAB), carbapenem-resistant Escherichia coli (E. coli), aminoglycoside-resistant Pseudomonas aeruginosa (P. aeruginosa), carbapenem-resistant Klebsiella pneumoniae (CRKP), and methicillin-resistant Staphylococcus aureus (MRSA), where the carbapenem-resistant E. coli and CRKP are parts of carbapenem-resistant Enterobacterales (CRE), which are defined as members of the Enterobacterales order resistant to at least one carbapenem antibiotic or producing a carbapenemase enzyme [22]. The hospital under study is a tertiary medical center with > 3000 beds, admitting approximately 100,000 admissions per year. All inpatients admitted from January 1, 2009, to March 31, 2020, who were treated with antibiotics were included (Fig. 1).
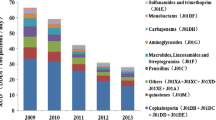
A Antibiotics consumption (defined daily doses [DDDs]/1000 inpatient-days) and percentage of resistant isolates at Nanjing Drum Tower Hospital (January 2009–March 2020). B Cases caused by multidrug resistant bacteria (occupied bed days [OBDs]/10,000 inpatient days) at Nanjing Drum Tower Hospital (January 2009–March 2020)
Multi-drug-resistant bacteria and antibiotic use. A Charts illustrating the relationship between carbapenem use and CRAB incidence density. B Charts illustrating the relationship between glycopeptide use and CRAB incidence density. C Charts illustrating the relationship between carbapenem use and E. coli incidence density. D Charts illustrating the relationship between fluoroquinolones use and E. coli incidence density
Bacterial Identification and Antimicrobial Susceptibility Testing
Data on antibiotic-resistant bacteria from non-repetitive isolates were retrieved from electronic medical records and duplicated isolates from the excluded inpatients. Bacterial identifications were made by the hospital clinical laboratory; the authors did not perform bacterial identification. The automated Vitek-2 system (bioMérieux, l’Étoile, France), supplemented by Kirby-Bauer Disk Diffusion Agar, was used to identify strains to the species level and test antimicrobial drug susceptibility. Results were interpreted according to guidelines from the Clinical and Laboratory Standards Institute. Piperacillin/tazobactam, ciprofloxacin, imipenem, ceftazidime, amikacin, and vancomycin were used for the susceptibility test representing β-lactam-β-lactamase inhibitor combinations (BLBLIS), fluoroquinolones, carbapenems, third-generation cephalosporins, aminoglycosides, and glycopeptides, respectively. Intermediate isolates were also defined as resistant isolates in the study.
Antibiotic Consumption
We extracted antibiotic consumptions in the form of defined daily doses (DDDs) per 1000 occupied bed days (OBDs) from the database of the Department of Pharmacy, according to the 2012 World Health Organization (WHO) anatomic therapeutic chemical classification system [23]. All above-mentioned antibiotics were extracted, including β-lactam (penicillin G, amoxicillin/clavulanate potassium, benzathine benzylpenicillin, and piperacillin/tazobactam), aminoglycosides (amikacin and gentamicin), fluoroquinolones (ciprofloxacin, moxifloxacin, levofloxacin, and norfloxacin), carbapenems (biapenem, imipenem, and meropenem), glycopeptides (vancomycin and teicoplanin), and third-generation cephalosporins (ceftriaxone, ceftizoxime, cefminox, cefodizime, cefoperazone/sulbactam, cefdinir, and ceftazidime). Antibiotics that achieve systemic therapeutic concentrations (including oral and injection) were included. Antibiotics used as topical reagents were excluded.
Statistical Analysis
To adopt a more appropriate statistical method to evaluate the associations between antimicrobial consumption and rates of resistance, three linear and two non-linear regression analyses were performed, including unary linear regression, piecewise linear regression, curvilinear regression, spline regression, and generalized additive models (GAM), respectively. The residual standard error (RSE) and adjusted R-squared (R-sq) were compared to illustrate the degree of model fitting. The RSE is the average error of the model using independent variables to predict the dependent variable, and a smaller value represented a better model fitting, while the R-sq represents how well the model interprets the data set, and a larger value represents a better fit. In all five statistical methods, GAM showed the minimum RSE value (Table 1). The models with adjusted R2 > 0.3 were considered statistically significant [20, 24] (Table 2).
After comparison, the GAM relationship exhibited the prominent data fit (Table 1) and thus was applied to evaluate the trends of monthly consumptions of six antibiotic prescriptions and five antimicrobial resistances during this study. P values < 0.05 with the R-squared values > 0.3 were considered statistically significant [8, 20, 25]. Furthermore, a cross-correlation function test published previously was used to identify the temporal correlation between the quarterly isolation proportion of antibiotic susceptibility [7, 26], and the lag value stands for the delay (in months) between changes in antimicrobial consumption and associated change in rates of resistance. While the GAM relationship exhibited the prominent data fit, it could not provide coefficients or explain the intrinsic relationship between variables, so we implemented a search algorithm based on piecewise regression to filter out the potential thresholds of each regression model. All statistical analyses were performed by R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Compliance with Ethics Guidelines
Our study protocol was submitted to the ethics committee at Nanjing Drum Tower Hospital, and it was exempt from ethical approval from the Institutional Review Board of Nanjing Drum Tower Hospital. Because the study was a retrospective study that analyzed the use of antibiotics and drug-resistant bacteria in the entire hospital, it did not involve personal information or samples of patients.
Results
Evaluation of Different Time Series Analysis Methods
After statistical analysis, RSE values (representing the average fitting errors) of the GAM method were the smallest among five regression models, which were 0.099–0.738, 0.917–2.683, 0.123–0.451, 0.044–0.652, 0.006–0.142, and 0.512–2.474 in the resistant models of carbapenems, aminoglycosides, fluoroquinolones, glycopeptides, third-generation cephalosporin, and β-lactams, respectively (Table 1). Therefore, the GAM method performed best and could perform various nonparametric fittings between dependent variables and multiple independent variables, thus finding the curve that best fit reality in this study. In our study, the independent variable was the consumption of the six antibiotics, and the dependent variable was the number of cases caused by the five drug-resistance bacteria.
Antimicrobial Consumption
Between 2009 and 2020, the consumption of third-generation cephalosporin was the highest in hospital with a 1-year average of 101.37 DDDs per 1000 OBDs, followed by fluoroquinolones (DDDs per 1000 OBDs) and β-lactams (DDDs per 1000 OBDs). However, in contrast to the declining use of fluoroquinolones, the consumptions of carbapenems and glycopeptides were gradually increasing, reaching an average of 100 and 86 DDDs per 1000 OBDs in 2020, respectively. Besides, usage of aminoglycosides was the lowest and stable in recent years with a 1-year average of usage of 3.2 DDDs per 1000 OBDs (Fig. 1A).
Carbapenem-resistant Acinetobacter baumannii
During the study, the incidence density of CRAB escalated from a 1-year average of 3.94–9.43 cases per 10,000 occupied bed days (OBDs) from 2009 to 2020 (Fig. 1B). Among the six bactericides included in this study, the incidence density of CRAB was only significantly synchronized with current use of glycopeptides (lag = 0, adjusted R2 = 0.644), and carbapenems (lag = 0, adjusted R2 = 0.647) in the hospital (Table 2); no other independent associations were identified. By further analysis, an initial decrease in the density of CRAB could be observed where the usages of glycopeptides and carbapenems were below the thresholds of 31.647 DDDs per 1000 OBDs and 41.613 DDDs per 1000 OBDs, respectively (Fig. 2A, B).
Carbapenem-resistant Klebsiella pneumoniae
In CRKP, the incidence density between 2009 and 2017 was basically stable at 1.24 cases per 1000 OBDs, but then began to gradually increase, reaching a maximum of 14.23 cases per 1000 OBDs in January 2021 (Fig. 1B). In the best-fit model by nonlinear time series analysis, its incidence density was strongly synchronized with recent hospital use of glycopeptides (lag = 0, adjusted R2 = 0.652) and carbapenems (lag = 0, adjusted R2 = 0.738) (Table 2), no independent associations with other antibiotics uses were identified. To prevent its density from increasing, a threshold was determined in glycopeptides and carbapenems use, where their maximum consumption should not exceed 31.042 DDDs per 1000 OBDs, respectively (Fig. 2C, D).
Aminoglycosides-Resistant Pseudomonas aeruginosa
In P. aeruginosa, the incidence density was highest (approximately 2.82 DDDs per 1000 OBDs) in 2012 and slightly decreased from a 1-year average of 1.80–0.93 cases per 10,000 OBDs from 2013 to 2020. Its incidence density was synchronized with recent hospital use of carbapenems (lag = 1, adjusted R2 = 0.632) and glycopeptides (lag = 1, adjusted R2 = 0.348) (Table 2), and no independent association with other antibiotic use was identified. However, the incidence density of P. aeruginosa decreased gradually when the effects of carbapenem uses were above a threshold of 19.946 DDDs per 1000 OBDs; no threshold value was identified for the usage of glycopeptides in this study (Fig. 3A, B).
Multi-drug-resistant bacteria and antibiotic use. A Charts illustrating the relationship between carbapenem use and P. aeruginosa incidence density. B Charts illustrating the relationship between glycopeptides use and P. aeruginosa incidence density. C Charts illustrating the relationship between carbapenem use and CRKP incidence density. D Charts illustrating the relationship between glycopeptide use and CRKP incidence density
Carbapenem-resistant Escherichia coli
As for carbapenem-resistant E. coli, its prevalence initially decreased from a 1-year average of 1.84–0.38 cases per 10,000 OBDs from 2009 to 2012 and then remained a stable up to the present. The time series analysis revealed that its density can be predicted by the current use of carbapenems (lag = 2, adjusted R2 = 0.387) in the hospital; it was also highly related to the recent use of fluoroquinolones with the lag value of 0 and adjusted R2 of 0.490 (Table 2), indicating resistance mainly occurred after 2 months of medication. No independent associations were identified between the incidence of carbapenem-resistant E. coli and other antibiotics in this study. According to further analysis, the trend of E. coli incidence density decreased when the effect of carbapenem uses was below a threshold of 19.449 DDDs per 1000 OBDs and tended to increase slowly when gradually increasing the usage beyond the threshold (Fig. 3C). However, it was hard to find a threshold for the increasing prevalence of carbapenem-resistant E. coli. caused by fluoroquinolones (Fig. 3D).
Discussion
This study analyzed the popular density of five common drug-resistant bacteria and six kinds of antimicrobial consumption in recent years and investigated their potential non-linear associations. From 2009 to 2020, the third-generation cephalosporins and fluoroquinolones were the most widely used anti-infective drugs in clinical practice, similar to results in some previous studies. However, the consumptions in this study were higher than those in Europe [8]. Recently, due to the gradual increase in resistance to fluoroquinolones in China, the use of fluoroquinolones was seen to gradually decrease in this study, and the carbapenem, as an effective broad-spectrum antibiotic recommended by Clinical and Laboratory Standards Institute (CLSI), has been increasingly applied in clinical practice [27]. In addition, the use of glycopeptides has gradually increased in recent years. However, this study suggested that the escalating or sustained high consumption of antibiotics was highly connected with the prevalence of common drug-resistant bacteria, including the CRAB, CRKP, aminoglycoside-resistant P. aeruginosa, and imipenem-resistant E. coli in hospitals.
Carbapenems were proved to drive the incidence of CRAB, CRKP, aminoglycoside-resistant P. aeruginosa, and imipenem-resistant E. coli in this study. First, there was obviously a large increase in the prevalence of CRAB/CRKP and significant positive associations between them and carbapenems, in line with some previous studies [28], but different lag effects (delay necessary to observe the effect in months) were found between this study (lag = 0) and other studies (lag = 2, lag = 4). To control rates of CRAB infections, the thresholds of carbapenem consumption determined in this study were 41.613 DDDs per 1000 OBDs, which was lower than the results of 102.4 and 86.0 DDDs per 1000 OBDs in two comprehensive studies, respectively [8, 29]. These differences may be caused by varied healthcare systems, volumes of antibiotic use, molecular epidemiology, and antibiotic resistance patterns in different regions, so tailored thresholds for antibiotic stewardship based on local data may be more valuable for preventing infection [29]. Apart from carbapenems, the relationships between CRAB/CRKP and other antibiotics, such as fluoroquinolones, along with thresholds have been reported previously, perhaps because of the different bacterial resistance mechanisms and medication habits [8, 20, 29]. Second, our findings suggested an inverse relationship without thresholds between the use of carbapenem and the incidence density of E. coli or P. aeruginosa, which might be caused by their low incidence density, leading to an insignificant change in trends. However, the use of ciprofloxacin and moxifloxacin was proved to be statistically related with the incidence density of antibiotic-resistant E. coli [30], so confirming their relationships in a multi-center and large-scale study is expected in the future.
For the first time to our knowledge, synchronized correlations between the incidence of CRAB, CRKP and aminoglycoside-resistant P. aeruginosa and glycopeptides usages were found in this study. As the antimicrobial spectrum of glycopeptides contains mostly gram-positive bacteria, we speculate this inhibition provided gram-negative bacteria, such as CRAB and CRKP, with a superior survival advantage. For CRAB and CRKP prevention, we demonstrated the glycopeptide consumption should not exceed 31.647 and 26.152 DDDs per 1000 OBDs, respectively, but no threshold was identified for P. aeruginosa prevention because of its low incidence density. However, MRSA, as the main target of glycopeptides, has not been found to be significantly related to any antibiotic usages in this study; this result was inconsistent with other studies, which found that the use of fluoroquinolones, co-amoxiclav, third-generation cephalosporins, and macrolides may drive the prevalence of MRSA in Antrim [15]. These discrepancies may be partly explained by different medication use in hospital; for example, the MRSA-infected cases decreased following the restriction of fluoroquinolones in Antrim, but remained stable in this study. In addition, no thresholds for fluoroquinolones, third-generation cephalosporin, aminoglycosides, and β-lactams were identified; this might arise from momentous selection pressure of other antibiotics in this study. For instance, the carbapenem usage in studies by Guo et al. were less than in our study (10 vs. 32.68 DDDs per 1000 OBDs), indicating that the selection pressure from other antibiotics was much higher in our study [7, 31]. Therefore, the establishment of the model of multiple antibiotics with multi-drug-resistant (MDR) and extensively drug-resistant (XDR) bacteria has become the focus of further research.
Nevertheless, some limitations should be mentioned and improved in the future. First, our research is based on a single-center analysis, and confounders such as length of stay, multi-system disease, serious condition, and other factors were not considered; thus, studies from multiple medical centers and communities are needed to improve the situation. Second, it is still unclear whether the combined use of drugs has an impact on the selection pressure and whether any specific antibiotic could dominate in the combined selection pressure. Lastly, compared with previous studies, this study did not intervene in the antibiotic usage according to thresholds in the short term, which will be done in the future to determine the effectiveness of this measure in controlling the incidence of drug-resistant infections [17, 21, 32].
Conclusion
Our data revealed the impact of antibiotic consumption on bacterial resistance, highlighting that the risk of cases infected by drug-resistant bacteria is still high when antibiotic usage exceeds the threshold. We demonstrated that carbapenem and glycopeptide consumption should be kept below 31.042 and 26.152 DDDs per 1000 OBDs, respectively, thus informing hospital antibiotic policies through quantitative targets. More importantly, we established a simple and efficient tool for non-linear time series analysis to identify antibiotic usage thresholds for appropriate treatment strategies and effective control of antimicrobial resistance during the clinical practice.
References
Drug-resistant infections: a threat to our economic future (Discussion Draft) (World Bank, 2016). http://pubdocs.worldbank.org/en/689381474641399486/1701381-AMR-Lab-Report-Web.pdf. Accessed May 2021.
O’Neill, J. Review on antimicrobial resistance: tackling a crisis for the health and wealth of nations (HM Government, 2014). https://amr-review.org/. Accessed May 2021.
Gurjar M, Saigal S, Baronia AK, Rao BP, Azim A, Poddar B, Singh RK. Carbapenem-resistant Acinetobacter ventilator-associated pneumonia: clinical characteristics and outcome. Indian J Crit Care Med. 2013;17(3):129–34.
Shah PG, Shah SR. Treatment and outcome of carbapenem-resistant gram-negative bacilli blood-stream infections in a Tertiary Care Hospital. J Assoc Physicians India. 2015;63(7):14–8.
Stefani S, Chung DR, Lindsay JA, Friedrich AW, Kearns AM, Westh H, Mackenzie FM. Meticillin-resistant Staphylococcus aureus (MRSA): global epidemiology and harmonisation of typing methods. Int J Antimicrob Agents. 2012;39(4):273–82.
Jarlier V, Diaz Hogberg L, Heuer OE, Campos J, Eckmanns T, Giske CG, Grundmann H, Johnson AP, Kahlmeter G, Monen J, Pantosti A, Rossolini GM, van de Sande-Bruinsma N, Vatopoulos A, Zabicka D, Zemlickova H, Monnet DL, Simonsen GS, Ears-Net P. Strong correlation between the rates of intrinsically antibiotic-resistant species and the rates of acquired resistance in Gram-negative species causing bacteraemia, EU/EEA, 2016. Euro Surveill. 2019;24(33):1800538.
Guo W, He Q, Wang Z, Wei M, Yang Z, Du Y, Wu C, He J. Influence of antimicrobial consumption on gram-negative bacteria in inpatients receiving antimicrobial resistance therapy from 2008–2013 at a tertiary hospital in Shanghai, China. Am J Infect Control. 2015;43(4):358–64.
Lopez-Lozano JM, Lawes T, Nebot C, Beyaert A, Bertrand X, Hocquet D, Aldeyab M, Scott M, Conlon-Bingham G, Farren D, Kardos G, Fesus A, Rodriguez-Bano J, Retamar P, Gonzalo-Jimenez N, Gould IM, Group TS. A nonlinear time-series analysis approach to identify thresholds in associations between population antibiotic use and rates of resistance. Nat Microbiol. 2019;4(7):1160–72.
China antimicrobial resistance surveillance system. http://www.carss.cn/Report/Details?aId=648. Accessed Oct 2021.
ECDC/EMEA The bacterial challenge: time to react. https://www.ecdc.europa.eu/en/publications-data/ecdcemea-joint-technical-report-bacterial-challenge-time-react. Accessed Jan 2022.
Centers for Disease, C Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62(9):165–70.
Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Woodhouse W, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta ZA, Coates A, Bergstrom R, Wright GD, Brown ED, Cars O. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–98.
Oz T, Guvenek A, Yildiz S, Karaboga E, Tamer YT, Mumcuyan N, Ozan VB, Senturk GH, Cokol M, Yeh P, Toprak E. Strength of selection pressure is an important parameter contributing to the complexity of antibiotic resistance evolution. Mol Biol Evol. 2014;31(9):2387–401.
Tamma PD, Rodriguez-Bano J. The use of noncarbapenem beta-lactams for the treatment of extended-spectrum beta-lactamase infections. Clin Infect Dis. 2017;64(7):972–80.
Lawes T, Lopez-Lozano JM, Nebot CA, Macartney G, Subbarao-Sharma R, Dare CR, Wares KD, Gould IM. Effects of national antibiotic stewardship and infection control strategies on hospital-associated and community-associated meticillin-resistant Staphylococcus aureus infections across a region of Scotland: a non-linear time-series study. Lancet Infect Dis. 2015;15(12):1438–49.
Zhao H, Wei L, Li H, Zhang M, Cao B, Bian J, Zhan S. Appropriateness of antibiotic prescriptions in ambulatory care in China: a nationwide descriptive database study. Lancet Infect Dis. 2021;21:847–57.
Hou D, Wang Q, Jiang C, Tian C, Li H, Ji B. Evaluation of the short-term effects of antimicrobial stewardship in the intensive care unit at a tertiary hospital in China. PLoS One. 2014;9(7):e101447.
Levy SB. Balancing the drug-resistance equation. Trends Microbiol. 1994;2(10):341–2.
Aldeyab MA, Harbarth S, Vernaz N, Kearney MP, Scott MG, Darwish Elhajji FW, Aldiab MA, McElnay JC. The impact of antibiotic use on the incidence and resistance pattern of extended-spectrum beta-lactamase-producing bacteria in primary and secondary healthcare settings. Br J Clin Pharmacol. 2012;74(1):171–9.
Ryu S, Klein EY, Chun BC. Temporal association between antibiotic use and resistance in Klebsiella pneumoniae at a tertiary care hospital. Antimicrob Resist Infect Control. 2018;7:83.
Haber M, Levin BR, Kramarz P. Antibiotic control of antibiotic resistance in hospitals: a simulation study. BMC Infect Dis. 2010;10:254.
Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America Guidance on the treatment of extended-spectrum beta-lactamase producing enterobacterales (ESBL-E), carbapenem-resistant enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. 2021;72(7):1109–16.
WHO, Guidelines for ATC Classification and DDD Assignment. WHO Collaborating Centre for Drug Statistics Methodology. Oslo, Norway: Norwegian Institute of Public Health; 2012.
Hsu LY, Tan TY, Tam VH, Kwa A, Fisher DA, Koh TH, Network for Antimicrobial Resistance, S. Surveillance and correlation of antibiotic prescription and resistance of gram-negative bacteria in Singaporean hospitals. Antimicrob Agents Chemother. 2010;54(3):1173–8.
Hsu LY, Tan TY, Tam VH, Kwa A, Fisher DA, Koh TH. Surveillance and correlation of antibiotic prescription and resistance of Gram-negative bacteria in Singaporean hospitals. Antimicrob Agents Chemother. 2010;54(3):1173–8.
Gilca R, Fortin E, Frenette C, Longtin Y, Gourdeau M. Seasonal variations in Clostridium difficile infections are associated with influenza and respiratory syncytial virus activity independently of antibiotic prescriptions: a time series analysis in Quebec, Canada. Antimicrob Agents Chemother. 2012;56(2):639–46.
Ceriotti F, Zakowski J, Sine H, Altaie S, Horowitz G, Pesce AJ, Boyd J, Horn P, Gard U, Horowitz G. Clinical and Laboratory Standards Institute (CLSI). 2012.
Yang P, Chen Y, Jiang S, Shen P, Lu X, Xiao Y. Association between antibiotic consumption and the rate of carbapenem-resistant Gram-negative bacteria from China based on 153 tertiary hospitals data in 2014. Antimicrob Resist Infect Control. 2018;7:137.
Hayajneh WA, Al-Azzam S, Yusef D, Lattyak WJ, Lattyak EA, Gould I, Lopez-Lozano JM, Conway BR, Conlon-Bingham G, Aldeyab MA. Identification of thresholds in relationships between specific antibiotic use and carbapenem-resistant Acinetobacter baumannii (CRAb) incidence rates in hospitalized patients in Jordan. J Antimicrob Chemother. 2021;76(2):524–30.
Vernaz N, Huttner B, Muscionico D, Salomon JL, Bonnabry P, Lopez-Lozano JM, Beyaert A, Schrenzel J, Harbarth S. Modelling the impact of antibiotic use on antibiotic-resistant Escherichia coli using population-based data from a large hospital and its surrounding community. J Antimicrob Chemother. 2011;66(4):928–35.
Hao Y, Chen S, Chang H, Yan X, Zhou W, Cao X, Huang R, Zhang Z, Zhang H, Jia B, Wu W, Xiong Y, Chen Y, Wu C. Temporal association between carbapenems usage and antimicrobial resistance in gram-negative bacteria at a tertiary hospital in Nanjing, China. Diagn Microbiol Infect Dis. 2020;98(2):115083.
Yusef D, Hayajneh WA, Bani Issa A, Haddad R, Al-Azzam S, Lattyak EA, Lattyak WJ, Gould I, Conway BR, Bond S, Conlon-Bingham G, Aldeyab MA. Impact of an antimicrobial stewardship programme on reducing broad-spectrum antibiotic use and its effect on carbapenem-resistant Acinetobacter baumannii (CRAb) in hospitals in Jordan. J Antimicrob Chemother. 2021;76(2):516–23.
Acknowledgements
Funding
The authors declare that they have no conflicts of interest. This study was supported by Nanjing Medical Science and Technique Development Foundation (QRX17141, YKK19056, YKK20058, and YKK20076), National Natural Science Foundation of China (82002133). The Rapid Service Fee was funded by Nanjing Medical Science and Technique Development Foundation (QRX17141).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
Shixing Chen conducted major data analysis and manuscript writing. Zepeng Li contributed to analysis and manuscript preparation. Jiping Shi contributed to the writing and editing of the article. Wanqing Zhou, Haixia Zhang, Guangmei Chen, and Yi Kang contributed to data collection, processing, and discussion. Haiyan Chang and Xiaoli Cao contributed to the conception of the study. Changgui Gu contributed to the establishment of the nonlinear time series Models. Yuxin Chen directed data analysis and manuscript writing. Chao Wu guided the writing of the article and the establishment of models.
Disclosures
Shixing Chen, Zepeng Li, Jiping Shi, Wanqing Zhou, Haixia Zhang, Haiyan Chang, Xiaoli Cao, Changgui Gu, Guangmei Chen, Yi Kang, Yuxin Chen, Chao Wu all have nothing to disclose.
Compliance with ethics guidelines
Our study protocol was submitted to the ethics committee at Nanjing Drum Tower Hospital, and it was exempt from ethical approval from the Institutional Review Board designated by Nanjing Drum Tower Hospital. Because the study was a retrospective study that analyzed the use of antibiotics and drug-resistant bacteria in the entire hospital, it did not involve personal information or samples of patients.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chen, S., Li, Z., Shi, J. et al. A Nonlinear Time-Series Analysis to Identify the Thresholds in Relationships Between Antimicrobial Consumption and Resistance in a Chinese Tertiary Hospital. Infect Dis Ther 11, 1019–1032 (2022). https://doi.org/10.1007/s40121-022-00608-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00608-w