Abstract
Introduction
Invasive meningococcal disease (IMD) is an uncommon but serious infectious disease. Its economic burden is known to be high but is poorly characterised. The objective of this study was to determine costs, as captured in the healthcare claims database, incurred by all patients hospitalised for IMD in France over a 6-year period.
Methods
This case–control study was performed using the French national public health insurance database (SNDS). Cases comprised all individuals hospitalised with acute IMD in France between 2012 and 2017 inclusive. For each case, three controls were identified, matched for age, gender and region of residence. All healthcare resource consumption by cases and controls during the follow-up period was documented. Costs were analysed for the index hospitalisation in cases, 1 year following the index date and then for 5 years following the index date. Costs were assigned from national tariffs. The analysis was performed from a societal perspective. IMD sequelae were identified from hospital discharge summaries.
Results
A total of 3532 cases and 10,590 controls were evaluated. The mean per capita cost of the index IMD hospitalisation was €11,256, and increased with age and with the presence of sequelae. In the year following the index date, mean per capita direct medical costs were €6564 in cases and €2890 in controls. Annual costs were €4254 in cases without sequelae, €10,799 in cases with one sequela and €20,096 in cases with more than one sequela. In the fifth year of follow-up, mean per capita costs were €2646 in cases and €1478 in controls. The excess cost in cases was principally due to the management of sequelae. Amputation, skin scarring and mental retardation generated per capita costs in excess of €20,000 in the first year and in excess of €10,000 for subsequent years.
Conclusion
The economic burden of IMD in France is high and, over the long-term, is driven by sequelae management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Information on the cost of invasive meningococcal disease (IMD), an uncommon but potentially life-threatening infectious disease, is limited and often inconsistent. |
This study aimed at determining the healthcare costs of all patients hospitalised for IMD in France between 2012 and 2017 using the national health insurance database (SNDS). |
Mean per capita costs were €11,256 for the initial hospitalisation related to an IMD acute episode and €6564 for the year following the hospitalisation. For the cohort of patients whose index hospitalisation occurred in 2012, the mean annual cost for years 2–5 was €2660. |
One-quarter of cases presented at least one sequela and incurred a disproportionate amount of the cost. |
The economic burden of IMD is high, and public health policies are needed to limit the number of cases and reduce the cost to society. |
Introduction
Invasive meningococcal disease (IMD) is an unpredictable complication of Neisseria meningitidis infections, which arises when the pathogen gains access to the systemic circulation [1, 2]. In Europe and North America, IMD is now an infrequent disease, although still relatively common in sub-Saharan Africa. The disease principally affects infants and young children under 3 years of age, as well as adolescents and young adults [3]. In Europe, the overall incidence of IMD in the general population in 2014 was 0.88 cases/100,000, but was nearly 20-fold higher in infants under 1 year of age, rising to 16 cases/100,000 [3]. However, the incidence of IMD also increases again in older adults. Meningococcaemia stimulates a rapid and powerful immune inflammatory response which may lead to life-threatening sepsis [1, 2]. Severe acute neurological complications and persistent sequelae are frequent in all age groups [4, 5]. Pulmonary complications are rare in childhood, but may be relatively common in patients aged 65 years or more [4,5,6].
The case fatality rate in Europe is currently around 8% [3, 7]. In patients who survive, there is a high risk of developing persistent severe sequelae, most commonly relating to neurological or auditory impairment [4, 8,9,10,11,12]. For patients experiencing IMD episodes in childhood, survivors present an elevated risk of mental disorders such as anxiety and depression, and other psychological and behavioural problems with significant functional impact [10]. In addition, limb amputation may be necessary in up to 15% of cases [12]. Up to half of patients who survive the acute infection may present some form of sequela, and approximately 20% will require continued treatment for sequelae after the primary infection is resolved [7, 10].
Cost of illness studies of IMD are scarce and, for this reason, the economic burden of IMD is poorly characterised [13]. A major reason for this is that IMD is an uncommon disease with a heterogenous prognosis, and so it is challenging to assemble large representative patient cohorts that enable these costs to be determined with precision. Moreover, many of the available cost estimates come from modelling studies rather than actual data collected in patients with IMD. A health insurance claims database study performed in 2005 in the USA reported a per capita cost of acute hospitalisation of $19,526 [14]. In an analysis of data from the Italian Hospital Discharge Dataset used to inform an economic model of IMD, Tirani et al. reported a per capita cost of an acute stay for IMD management in 2013 of €6800 for children and €8250 for adults [15]. The same authors estimated the annual per capita cost of management of sequelae to be €4148 [15]. A microcosting study performed in the UK estimated costs in the first year for two hypothetical cases of severe IMD with long-term sequelae to be £160,000–200,000 and lifetime costs to be £590,000–1,090,000 [16]. This study was subsequently reiterated in the French setting and estimated first-year costs to be €160,000 and lifetime costs to be between €770,000 and €2,267,000 depending on the nature and severity of the sequelae [17]. Most recently, a study from Germany estimated that the lifetime per capita direct medical costs of IMD amounted to €54,300, with management of sequelae accounting for around 80% of the total cost [18]. Finally, an Australian study estimated the lifetime societal per capita cost of IMD to be US $319,897, including direct healthcare costs of US $65,035 [19].
The variability in the estimates obtained reflects differences in the costs evaluated (acute hospitalisation, first-year costs and lifetime costs), in the approaches used (microcosting, modelling or database analysis) and in the nature of the sequelae considered. In order to take a more comprehensive approach to the costs of IMD, the present study used the healthcare delivery and reimbursement database of the French national public health insurance system (SNDS; Système National d’Information Inter-régimes de l’Assurance Maladie) to identify costs incurred by all patients hospitalised for IMD over a 6-year period. This approach offers a rare opportunity to gain access to data on pluriannual healthcare resource consumption in the real-world setting at a national level and thus to have a sufficient and exhaustive sample in which to determine the cost of illness with precision. Costs of acute hospitalisation, first-year costs and 5-year costs were estimated. A case–control approach was taken to estimate costs specific to IMD.
Methods
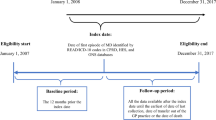
This observational cost-of-illness study was conducted in the SNDS database in France. A case–control approach was taken, in which total costs incurred by a group of cases with IMD and a matched control group without IMD were measured. The differences between costs in the two groups are considered to be costs associated with IMD. Non-specific costs related to other health conditions are expected to be distributed similarly between cases and controls and thus will not contribute to a difference in costs between the two groups. The design and methodology of the study have been described in detail elsewhere [20] and are summarised below. Cases comprised all individuals hospitalised with a diagnosis of acute IMD between 1 January 2012 and 31 December 2017. The date of hospitalisation admission was taken as the index date for the case. Each case was matched to three controls without IMD, and not necessarily hospitalised, randomly selected in the SNDS database on the basis of age, gender and administrative district of residence. The index date for the controls was identical to the calendar date of hospitalisation for the matched case. Cases and controls were followed until 31 December 2017 or death, if this occurred prior to this date.
The perspective of the cost analysis was principally a societal one, in order to facilitate international comparisons. This took into account direct medical costs, incurred through hospitalisation or community care, costed according to national tariffs and charged to the patient. These included costs reimbursed either by public health insurance or by complementary private insurance, as well as out-of-pocket expenses. It was not possible to evaluate indirect costs related to sick leave and invalidity pensions from a societal perspective since only payments from national health insurance are available in the database. Components such as loss of earnings and private insurance payments thus cannot be estimated. For this reason, only a portion of indirect costs were evaluated, corresponding to the indirect cost burden applicable to the French payer (i.e. payer perspective).
Figure 1 illustrates the time lines of the study and the different periods of cost assessment.
Data Collection
Data on demographics and health resource consumption were extracted from the SNDS database. Demographic information is limited to age, gender and municipality of residence.
All hospitalisations during the follow-up period were extracted. The type of hospital, the duration of hospitalisation and any procedure performed during the stay were identified. The reason for hospitalisation was identified from the hospital discharge summary in the form of a diagnostic code based on the International Classification of Diseases Version 10 (ICD-10). Information was extracted from the SNDS on all healthcare consumption in the community that was reimbursed during the study period. These items include all consultations (general practitioners, specialists practising in the community and outpatient visits for hospital consultations), paraclinical care (nurse visits, physiotherapy, speech therapy), laboratory tests, medications delivered in pharmacies and transportation (ambulance or medical taxi).
Potential long-term sequelae of IMD, selected from previous studies [21, 22], were identified from hospital discharge summaries by the corresponding ICD-10 or procedure code, or from medication delivery records, using a classification system developed in 2015 by the French national general health insurance fund (see Table S1 in the electronic supplementary material for detail) [23] and a previously described algorithm for identifying IMD-specific sequelae [24]. These included immediate and irreversible sequelae, such as amputation, that were identified from hospital discharge records and sequelae appearing and identified after discharge. In order for the latter to qualify as sequelae, they were required to be documented in the SNDS for the first time at a date after the index hospitalisation. This date was in general required to fall within 3 months of the index hospitalisation, except for motor deficits (6 months), epilepsy and mental retardation (18 months), or bilateral hearing loss, severe hearing loss requiring a cochlear implant and attention-deficit hyperactivity disorder (36 months), for which a broader time window was permitted. In the absence of formal definitions for these time windows, these were based on expert opinion. These conditions were also identified in the control group if they were documented for the first time in the specified time window following the matched index date.
Costing
Direct medical costs were assigned to all documented healthcare resource consumption by cases and controls. Costs of hospitalisation were valued using the diagnostic code listed on the hospital discharge summary. This assigns the stay to a diagnosis-related group which has a specific associated unitary tariff. The relevant tariffs for each year between 2012 and 2017 were used. This included the cost of the hospital stay itself, remuneration of physicians and paramedical staff, medications and medical devices delivered in hospital and routine tests. Specific procedures (e.g. dialysis) and stays in an intensive care unit are identified by a procedure code, for which a specific tariff is applied. When an individual was hospitalised sequentially in more than one hospital department, or if the hospital stay was followed by a stay in another care facility, for example in a rehabilitation centre, without returning home between the two, then the costs of the different stays were aggregated to yield a single overall cost for the hospitalisation event.
Costs of healthcare delivered in the community were valued using French national tariffs. These community costs included physician visits, visits by nurses and other paramedical healthcare professionals, delivery of medication and medical devices in pharmacies, clinical laboratory tests and medical transportation.
Indirect costs evaluated were sick leave and invalidity pensions. Since no information is available on potential private insurance benefits for these items, indirect costs are presented from the payer perspective.
Three different types of cost were analysed in two study populations (Fig. 1). The first population (total population) consisted of all patients identified in the database during the study period (2012–2017 inclusive). In this population, we determined costs related to the index acute phase hospitalisation for IMD, as well as all costs incurred during the year following the index date. Hospitalisation costs are only presented for cases since controls were not necessarily hospitalised. The second population (2012 population) consisted of all patients first documented in the database in 2012 and was used to document costs generated over the 5 years following the index hospitalisation. These 5-year costs are broken down by year of follow-up. All hospital and community costs were presented by type and as aggregate costs and compared between cases and controls for all individuals.
Subgroup analyses were performed to evaluate costs as a function of age (under 25 years, 25–59 years, and 60 years and over) and as a function of the number of long-term sequelae (none, one or more than one). In addition, a specific analysis was performed on the costs of management of these sequelae. All costs are expressed per capita and per annum in 2019 euros. Typical unit costs for selected items of healthcare resource consumption are given in Table S2 in the electronic supplementary material.
Statistical Analysis
Continuous variables are presented as mean values with their standard deviations or 95% confidence intervals (95% CI) or median values with their interquartile range or full range. Categorical variables are presented as frequency counts and percentages. Health resource consumption was identified as the number of consumers and compared between cases and controls in the form of hazard ratios (HR) with their 95% CI. Certain healthcare consumption variables (number of visits and length of stay) are presented as continuous variables and compared using the Wilcoxon test. Certain categorical variables were compared with these χ2 test. Annual per capita costs are presented as mean values with their 95% CI. A probability value (p value) of 0.05 was taken as statistically significant. All statistical analyses were performed using SAS® software, Version 9.5 (Cary, USA).
Ethics
The study was conducted in accordance with the Helsinki Declaration of 1964, and its later amendments, as well as with relevant international and French regulatory requirements. Patient data in the database is anonymised using an irreversible double encryption. Access to the SNDS is regulated by a Committee of Expertise for Research, Studies and Evaluations in the field of Health, to which the present study protocol was submitted for approval. Since this was a retrospective study of an anonymised database and had no influence on patient care, ethics committee approval was not required. Use of the SNDS database for this type of study is regulated by the French national data protection agency (Commission Nationale de l'Informatique et des Libertés), to which the protocol was submitted for approval.
Results
Study Population
The cases corresponded to a total of 3532 individuals who were hospitalised for IMD between 2012 and 2017 and for whom at least 1 day of follow-up data was available. These cases were followed up for a median duration of 2.8 years [range 0–6.0 years]. The mean age of the cases was 29.7 years [95% CI 28.8–30.6], and 1970 cases (55.8%) were aged under 25 years, 622 (17.6%) aged between 25 and 49 years and 940 (26.6%) aged 50 years or more. A total of 778 cases were infants aged up to 24 months (22.0%). Of the 3532 cases, 1849 (52.3%) were male. For the majority of cases (N = 2709; 76.7%), no long-term sequelae were identified. A single sequela was identified in 525 cases (14.9%) and multiple sequelae in 298 cases (8.4%).
Overall, the 3530 cases were matched in a 1:3 ratio to 10,590 controls. Two cases could not be matched but were kept in the analysis. The index date for the controls was identical to the date of the index hospitalisation of the corresponding cases. The median follow-up duration for the controls was 3.0 years [range 0–6.0 years]. These 3530 cases and 10,590 controls constituted the total population.
Five-year costs could only be determined for 574 cases for whom the index hospitalisation occurred in 2012 and who survived throughout the 5-year period. These 574 cases were matched with 1722 controls and constituted the 2012 population. Some (less than 10%) of these patients died or were lost to follow-up over the 5-year period, and 526 cases and 1578 controls were available for analysis in the fifth year following the index hospitalisation.
Healthcare Resource Utilisation
Index Hospitalisation in IMD Cases
By definition, all cases underwent an initial hospitalisation which defined the index IMD episode. The mean duration of the index hospitalisation was 14.8 days [95% CI 14.0–15.6 days]. This included the acute hospital stay and any stay in relay residential care such as rehabilitation centres, without an intervening return home.
Resource Consumption in the Year Following the Index Date in the Total Population
During the follow-up period, 1448 cases (41.0%) had at least one overnight stay in a general hospital (Table 1). Although this proportion was similar to that of controls, individual cases were rehospitalised around two times more often than controls (mean 4.9 [95% CI 4.2–5.6] hospitalisations per case and 2.8 [2.6–3.0] per control; p < 0.0001) and for a longer total duration (mean 15.0 days [95% CI 12.3–17.7 days] for cases and 7.7 days [7.1–8.3 days] for controls; p < 0.0001). In addition, a higher percentage of cases were admitted to rehabilitation facilities and required home care (Table 1).
In the year following the index IMD event, 2770 cases (78.4%) consulted a general practitioner and 1924 (54.5%) consulted a specialist physician practising in the community setting (Table 1). Although differences in consultation rates for community physicians between cases and controls were statistically significant, absolute differences were small (less than 5%). In contrast, nearly two times the proportion of cases compared to controls consulted a hospital-based specialist as an outpatient (Table 1). Cases also more frequently received nursing care, physiotherapy and speech therapy compared to controls (Table 1).
Direct Medical Costs
Index Hospitalisation in IMD Cases
The mean per capita cost of the index hospitalisation for IMD was €11,256 [95% CI €10,869–11,643]. The mean costs of the index stay increased with age (p < 0.001), from €9637 [€9202–10,072] for cases under 25 years of age to €12,635 [€11,526–13,744] for cases aged 25–49 years and €14,165 [€13,365–14,965] for cases 60 years of age or older.
The cost of the index stay was also greater (p < 0.001) for cases who subsequently presented sequelae than in those who did not. The mean cost was €9393 [95% CI €9029–9757] in cases without sequelae, €14,469 [€13,352–15,586] in cases with a single sequela and €22,537 [€20,564–24,510] in 298 cases with multiple sequelae.
Costs Accrued During the Year Following the Index Date in the Total Population
Costs accrued in the year following the index date (date of hospitalisation for IMD for the cases and matched date for the controls) for cases and controls are presented in Table 2. For the cases, these costs exclude those of the index hospitalisations. Total mean per capita costs were more than twice as high for cases (€6564) than for controls (€2890). Moreover, all individual cost elements were higher for cases than for controls, although the difference was not significant for psychiatric unit stays.
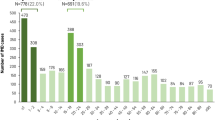
Mean total per capita costs increased with age in both cases and controls, for both hospitalisation and community costs. In cases, hospitalisation costs and community care costs each represented around half of the total cost in all age groups (Fig. 2). Total costs also increased with the number of sequelae from €4254 in cases who recovered from the index IMD without any sequelae to €20,096 in those with multiple sequelae, an over five-fold difference in cost.
Mean per capita costs in the year following the index hospitalisation in cases and controls as a function of age and of sequelae of IMD. The analysis is performed in the total population. The columns and the costs above represent total costs (hospitalisation and community care costs). IMD invasive meningococcal disease
Costs Accrued During the 5 Years Following the Index Date in the 2012 Population
The mean cost of the index hospitalisation in the subgroup of cases followed for 5 years was €9912, somewhat lower than for the total cohort of cases. Total mean per capita costs during the first year after the index event were €17,358. After the first year following the index event, total mean per capita costs in cases descended to a plateau between €2000 and €3500 per year (mean annual cost for years 2–5 was €2660 for cases and €1641 for controls) (Fig. 3). However, costs in cases remained significantly higher than costs incurred by controls throughout the follow-up period. In the fifth year after the index date, mean per capita costs were €2646 [95% CI 1835–3457] in cases and €1478 [95% CI 1222–1734] in controls.
Yearly mean per capita costs in IMD cases and controls in the 5 years following the index hospitalisation. The analysis is performed in the 2012 population. Total costs accrued are presented by year of follow-up as mean values with their 95% confidence intervals. The numbers below the data points indicate the number of cases or controls available for analysis each year. Cases and controls were compared with the Wilcoxon test (p). IMD invasive meningococcal disease
Cases with sequelae incurred significantly higher costs than cases without sequelae at each time point evaluated (Table 3). In the fifth year after the index date, mean per capita costs were €8674 [95% CI 4449–12,899] in cases with multiple sequelae, €5033 [95% CI 2871–7195] in cases with a single sequela and €1494 [95% CI 1010–1494] in cases without sequelae. The mean costs incurred by this last group were close to those incurred by controls in the fifth year (€1478; n = 1578; Fig. 4).
Yearly mean per capita costs in IMD cases and controls in the 5 years following the index hospitalisation, displayed as a function of the number of sequelae. The analysis is performed in the 2012 population. Costs accrued are presented by year of follow-up as mean values with their 95% confidence intervals. IMD invasive meningococcal disease
Cost of Management of Long-Term Sequelae
The costs of management of long-term sequelae of IMD are presented in Table 3. During the year following the index hospitalisation, the most expensive of these sequelae were amputation, skin scarring, mental retardation and bilateral hearing loss, all of which cost over €20,000 in the first year. For the first three of these, annual costs in excess of €10,000 persisted over the entire follow-up period.
Indirect Medical Costs
Sick Leave
Of the 3532 cases, 1567 were of working age (18–65 years). Of these, 442 (28.2%) took sick leave in the year following their index hospitalisation. This compares to 846 out of 4701 controls of working age (18.0%). The proportion of cases taking sick leave was significantly elevated compared to controls (p < 0.0001). The mean cost to the health insurance of this sick leave was €4719 [95% CI €3969–5469] per case taking sick leave, compared to €2921 [€2883–2960] for the controls taking sick leave.
In the second and third years after the index event, the proportion of cases taking sick leave was similar to that of the controls (year 2: 10.1% vs 9.9%, p = 0.008; year 3: 7.3% vs 7.7%, p = 0.13). However, the mean costs of this sick leave remained significantly higher (p < 0.05) in cases than in controls in year 2 (€5008 [95% CI €3460–6556] versus €2430 [€2021–2839]; p < 0.05), but not in year 3 (€3711 [€1745–5677] versus €2445 [€2018–2872]).
Invalidity Pensions
In the year following the index event, 49 cases (1.4%) took an invalidity pension, compared to 54 controls (0.5%) (p < 0.0001). The mean annual cost to the health insurance was €7101 [€5477–8725] per case taking a pension, compared to €7688 [€6448–8928] for the controls (p > 0.05).
Discussion
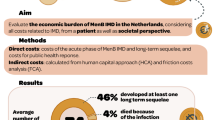
This study demonstrates that IMD, despite of being an uncommon disease in European countries, generates significant direct medical costs to society, as well as important indirect costs to the payer, in the French healthcare system. Mean per capita costs were €11,256 for the index hospitalisation and €6564 for the year following the index event. For the cohort of patients whose index hospitalisation occurred in 2012, the mean annual cost of years 2–5 was €2660. Assuming an average of 586 incident cases of IMD in France each year, this corresponds to an annual total direct medical cost for all individuals hospitalised for IMD in France of €6.6 million for the index hospitalisation, €3.9 million for the first year and €1.5 million each year for the following years (years 2–5).
One-quarter of cases presented at least one sequela and these individuals generated a disproportionate amount of the cost, both for the initial hospitalisation and for costs accrued over the following years. Compared to cases who did not present sequelae, the index hospitalisation cost 1.5 times as much for cases with a single sequela and 2.4 times as much for those with multiple sequelae. These cases with sequelae accounted for the additional direct medical costs compared to controls from the second year post-IMD onwards. The most frequent of these sequelae were severe neurological deficits, epilepsy and anxiety. This is consistent with previous studies (reviewed by Olbrich et al. [10]), which suggest that, as well as the better characterised physical and neurological sequelae, psychological and behavioural consequences also contribute to the disease burden of IMD.
In the year following the IMD event, the most expensive sequelae to manage on a per capita basis were amputation, skin scarring, bilateral hearing loss and mental retardation. These findings are generally consistent with previous data [21, 22]. However, it should be noted that hearing deficits in our study were distributed across three different diagnoses (unilateral hearing loss, bilateral hearing loss and hearing loss requiring a cochlear implant), and the combined cost of hearing deficits will be higher. The high cost of skin scarring is probably because that care includes a number of surgical procedures with high unit costs, such as skin grafts, deep tissue repair, and scar excision. On the other hand, the total cost of certain sequelae may not be fully reflected in these direct medical costs. For example, severe neurological deficits incur costs for special educational needs, home care and adaptions of the home environment, which are very expensive but which are supported by social services rather than health insurance.
For certain sequelae, such as hearing loss, the costs were principally generated during the first year; for others, notably mental retardation, skin scarring and the consequences of amputation, considerable cost continued to accrue over the following years. Nonetheless, these findings emphasise the need for more research to identify and pursue health policies to limit the risk of long-term sequelae in patients hospitalised with IMD.
It is difficult to compare the actual costs obtained in the present study with those estimated in the French microcosting study of two hypothetical cases [17] because of differences in the type of costs analysed and the time horizon of the costing. Direct per capita medical costs of the acute management phase of IMD determined in the present study are nonetheless close to those used in the recent German modelling study, which were derived from a federal hospital database [18].
The estimated costs of IMD can be compared with those of other acute infectious diseases in the French setting, although it should be born in mind that the methods used, the date and the cost items considered will vary between studies. For example, in a study of pneumococcal community-acquired pneumonia in 2014, the average cost of the hospitalisation stay was €7293 and the average cost of follow-up was €1242 [25]. In a budget impact study of antipneumococcal vaccination in France, the cost of an episode of pneumococcal meningitis was estimated at €5636, and the cost of management of post-meningitis sequelae at €8000 per year (2013/2014 costs) [26]. Compared with the present findings, costs in these earlier studies were lower, although within the same order of magnitude. Another infantile infectious disease that can be prevented by vaccination is whooping cough. A Spanish burden of disease study reported mean per capita direct medical costs of whooping cough in 2012 to be €856, although this cost was highly age-dependent, ranging from €2988 in infants aged less than 1 year to €161 in individuals aged 65 years or older [27]. However, as with IMD in the present study, management of complications of whooping cough, such as pneumonia or encephalopathy, inflates costs considerably [28]. Moreover, unlike whooping cough, IMD case management is systematically in hospital settings while whooping cough can be managed under outpatient mode.
Compared with other rare diseases, direct medical costs of IMD are in the middle of the range. In 2015, the BURQOL-RD (Social Economic Burden and Health-Related Quality of Life in patients with Rare Diseases in Europe) research group reviewed the relatively sparse and inconsistent data available on cost of illness in ten rare diseases [29]. Annual per capita direct medical costs ranged from €1042 to €745,376 for haemophilia, €1983 for Duchenne muscular dystrophy, €2202 to €27,601 for juvenile idiopathic arthritis, €3858 to €4926 for scleroderma, €7108 to €51,551 for cystic fibrosis, rising to €130,451 to €474,885 for mucopolysaccharidosis [29].
The data generated in this study could be of use in economic modelling studies to determine the impact of different healthcare policies aimed at improving the prevention or treatment of IMD in France or elsewhere. Such an approach has been followed elsewhere to estimate the cost-effectiveness of anti-meningococcal B vaccination in Italy [15, 30] and in England [24], where this vaccine has been introduced in recent years (Italy, 2017; England, 2015). In particular, the English study also took explicit account of the major impact on costs of management of a broad range of long-term sequelae [24].
In this study, a case–control approach was adopted in which total costs incurred by a group of cases with IMD and a matched control group without IMD were measured. This approach has been widely used in outcomes research in general and in cost of illness studies in particular, including in infectious diseases [31, 32], and is well adapted to studies of health claims databases. One advantage of such a “top-down” approach is that no a priori assumptions are made about which cost items are specifically attributable to IMD. In addition, all healthcare resource consumption is captured. Disadvantages include the level of detail that can be obtained and the absence of information on the reasons motivating healthcare resource use. Alternative “bottom-up” approaches, such as microcosting studies, are not associated with these disadvantages but have their own limitations, notably in ensuring exhaustive data collection and in identifying which costs to include. Case–control studies and microcosting studies each have their advantages and disadvantages. The microcosting approach is most reliable when the data are collected prospectively, which is challenging in the case of rare, sporadic acute infectious diseases such as IMD.
The study has several strengths and limitations. The strengths include the relatively large number of individuals from whom the cost data were obtained, corresponding to all patients hospitalised for IMD in France over a recent 6-year period. In addition, all the costs represent actual costs identified directly from healthcare spending, rather than from indirect extrapolations from other sources, as in several previous cost of illness studies in IMD. The case–control approach used allows the specific economic burden of IMD to be estimated with precision. However, it should be noted that any supplementary charges made by healthcare professionals in the private sector, or any out-of-pocket expenses incurred by parents, will not have been captured, which may lead to underestimating the real costs. Concerning the limitations, exhaustive case identification cannot be guaranteed. However, individuals who develop IMD will generally require hospitalisation unless they die beforehand. Indeed, on a year-by-year basis, the number of cases identified in the SNDS database is close to that reported from public health surveillance of infectious diseases in France [33]. In this respect, it is noteworthy that the rate of under-reporting in the surveillance system in France has been evaluated by a capture-recapture study to be less than 10% [33]. Similarly, sequelae are identified from diagnostic or procedure codes, but any causal relationship with IMD is not documented in the SNDS database. These conditions have been considered to be sequelae of IMD on the basis of their temporal association with the index IMD event. However, it cannot be excluded that certain associations are fortuitous, nor that certain sequelae may not have been retrieved. In addition, the sequelae studied were prespecified on the basis of previous reports, and others may well exist, which contribute to long-term costs.
The costs identified in this study are limited to those captured in the healthcare claims database and to costs reimbursed for the patient only. With respect to indirect costs, the information that can be extracted from the SNDS database is limited to sick leave and invalidity pensions paid for by public health insurance. However, no information is available on spending by private insurance or other payers, so the full societal cost cannot be determined using the present approach. Moreover, half the cases occurred in individuals aged under 25 years, who may not be of working age, and any financial impact on the parents of these cases has not been captured, such as loss of earnings, non-medical costs and dealing with the psychological burden of care. With respect to cases who develop sequelae, indirect costs are likely to evolve over time as care modalities change, e.g. from full-time home care to institutionalisation. Finally, out-of-pocket expenses incurred by the families of children with sequelae, e.g. modifications to the home environment to facilitate mobility or special educational needs, are not documented in the present study. Further work is required to quantify this segment of the economic burden of IMD, which may be substantial.
Conclusion
This study using data collected from all patients hospitalised for IMD in France demonstrates the high economic burden of this disease, which, over the long term, is driven by the management of sequelae. Vaccination coverage for IMD in France is limited and better coverage and extended vaccination programmes should be considered in order to limit the number of cases of IMD, and thus reduce the cost to society of this serious infectious disease. In high-income countries, the high case-fatality rate of IMD even when treated, the substantial cost of disease-related sequelae and the threat of outbreaks make vaccination an attractive target for preventive immunisation programmes.
References
Acevedo R, Bai X, Borrow R, et al. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines. 2019;18:15–30.
Harrison LH, Pelton SI, Wilder-Smith A, et al. The Global Meningococcal Initiative: recommendations for reducing the global burden of meningococcal disease. Vaccine. 2011;29:3363–71.
Whittaker R, Dias JG, Ramliden M, et al. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004–2014. Vaccine. 2017;35:2034–41.
Feldman C, Anderson R. Meningococcal pneumonia: a review. Pneumonia (Nathan). 2019;11:3.
Sall O, Stenmark B, Glimaker M, et al. Clinical presentation of invasive disease caused by Neisseria meningitidis serogroup Y in Sweden, 1995 to 2012. Epidemiol Infect. 2017;145:2137–43.
Vienne P, Ducos-Galand M, Guiyoule A, et al. The role of particular strains of Neisseria meningitidis in meningococcal arthritis, pericarditis, and pneumonia. Clin Infect Dis. 2003;37:1639–42.
Martinon-Torres F. Deciphering the burden of meningococcal disease: conventional and under-recognized elements. J Adolesc Health. 2016;59:S12-20.
Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:317–28.
Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51-63.
Olbrich KJ, Muller D, Schumacher S, Beck E, Meszaros K, Koerber F. Systematic review of invasive meningococcal disease: sequelae and quality of life impact on patients and their caregivers. Infect Dis Ther. 2018;7:421–38.
Rivero-Calle I, Vilanova-Trillo L, Pardo-Seco J, et al. The burden of pediatric invasive meningococcal disease in Spain (2008–2013). Pediatr Infect Dis J. 2016;35:407–13.
Vyse A, Anonychuk A, Jakel A, Wieffer H, Nadel S. The burden and impact of severe and long-term sequelae of meningococcal disease. Expert Rev Anti Infect Ther. 2013;11:597–604.
Wang B, Santoreneos R, Afzali H, Giles L, Marshall H. Costs of invasive meningococcal disease: a global systematic review. Pharmacoeconomics. 2018;36:1201–22.
Davis KL, Misurski D, Miller JM, Bell TJ, Bapat B. Cost of acute hospitalization and post-discharge follow-up care for meningococcal disease in the US. Hum Vaccin. 2011;7:96–101.
Tirani M, Meregaglia M, Melegaro A. Health and economic outcomes of introducing the new MenB vaccine (Bexsero) into the Italian routine infant immunisation programme. PLoS ONE. 2015;10:e0123383.
Wright C, Wordsworth R, Glennie L. Counting the cost of meningococcal disease: scenarios of severe meningitis and septicemia. Paediatr Drugs. 2013;15:49–58.
Benard S, Wright C, Voisine J, Olivier CW, Gaudelus J. Lifetime cost of meningococcal disease in France: scenarios of severe meningitis and septicemia with purpura fulminans. J Infect Public Health. 2016;9:339–47.
Scholz S, Koerber F, Meszaros K, et al. The cost-of-illness for invasive meningococcal disease caused by serogroup B Neisseria meningitidis (MenB) in Germany. Vaccine. 2019;37:1692–701.
Wang B, Afzali HHA, Giles L, Marshall H. Lifetime costs of invasive meningococcal disease: a Markov model approach. Vaccine. 2019;37:6885–93.
Taha MK, Weil-Olivier C, Bouee S, et al. Risk factors for invasive meningococcal disease: a retrospective analysis of the French national public health insurance database. Hum Vaccin Immunother. 2021:17(6):1858–66.
Bettinger JA, Scheifele DW, Le Saux N, et al. The disease burden of invasive meningococcal serogroup B disease in Canada. Pediatr Infect Dis J. 2013;32:e20–5.
Viner RM, Booy R, Johnson H, et al. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol. 2012;11:774–83.
Quantin C. Etude des algorithmes de définition de pathologies dans le système national d’information inter-régimes de l’assurance maladie (SNIIRAM). Paris: Caisse nationale d’Assurance maladie des travailleurs salariés; 2015.
Beck E, Klint J, Neine M, Garcia S, Meszaros K. Cost-effectiveness of 4CMenB infant vaccination in england: a comprehensive valuation considering the broad impact of serogroup B invasive meningococcal disease. Value Health. 2021;24:91–104.
Saba G, Andrade LF, Gaillat J, et al. Costs associated with community acquired pneumonia in France. Eur J Health Econ. 2018;19:533–44.
Jiang Y, Gervais F, Gauthier A, Baptiste C, Martinon P, Bresse X. A comparative public health and budget impact analysis of pneumococcal vaccines: the French case. Hum Vaccin Immunother. 2015;11:2188–97.
Plans P, Munoz-Almagro C, Godoy P, Jane M, Carmona G. Clinical characteristics and pertussis costs in cases reported to epidemiological services and cases detected in household contacts in Catalonia (Spain). Eur J Clin Microbiol Infect Dis. 2016;35:285–92.
Tormans G, Van Doorslaer E, van Damme P, Clara R, Schmitt HJ. Economic evaluation of pertussis prevention by whole-cell and acellular vaccine in Germany. Eur J Pediatr. 1998;157:395–401.
Angelis A, Tordrup D, Kanavos P. Socio-economic burden of rare diseases: a systematic review of cost of illness evidence. Health Policy. 2015;119:964–79.
Gasparini R, Landa P, Amicizia D, et al. Vaccinating Italian infants with a new multicomponent vaccine (Bexsero(R)) against meningococcal B disease: a cost-effectiveness analysis. Hum Vaccin Immunother. 2016;12:2148–61.
Amand C, Tong S, Kieffer A, Kyaw MH. Healthcare resource use and economic burden attributable to respiratory syncytial virus in the United States: a claims database analysis. BMC Health Serv Res. 2018;18:294.
Pourcher V, Gourmelen J, Bureau I, Bouee S. Comorbidities in people living with HIV: an epidemiologic and economic analysis using a claims database in France. PLoS ONE. 2020;15:e0243529.
du Chatelet IP, Deghmane AE, Antona D, et al. Characteristics and changes in invasive meningococcal disease epidemiology in France, 2006–2015. J Infect. 2017;74:564–74.
Acknowledgements
Funding
This work was supported by GlaxoSmithKline Biologicals SA funded this study (GSK identifier HO-18–19371). GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis, and financed the costs associated with the development and publishing of the present manuscript.
Medical Writing, Editorial, and Other Assistance
The authors would like to thank Valérie Grange (GSK) and Kinga Meszaros (GSK) for their participation in the scientific committee and their involvement in the discussions. Medical writing support was provided by Adam Doble (Foxymed on behalf of GSK). Business and Decision Life Sciences platform provided editorial support, on behalf of GSK, Lyes Derouiche coordinated manuscript development and editorial support.
Authorship
All authors participated in the design or implementation of the study and were involved in the analysis and interpretation of the results and the development of this manuscript. All authors had full access to the data and gave final approval before submission. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentations
Abstract and poster presentation at the International Society for Pharmacoeconomics and Outcomes Research–23rd Annual European Congress 2020.
Disclosures
Céline Pribil, Emmanuel Aris, Ekkehard Beck, Gaëlle Nachbaur and Véronique Loncle-Provot are employees of the GSK group of companies. Emmanuel Aris, Ekkehard Beck and Gaëlle Nachbaur reported restricted shares ownership of the GSK group of companies. Catherine Weil-Olivier received honoraria for lectures from the GSK group of companies and from AstraZeneca, MedImmune, Pfizer, Sanofi-Pasteur, and Seqirus outside of the presented work. Muhamed-Kheir Taha reported his institution received fees from the GSK group of companies for the work presented here and from GSK group of companies, Pfizer, Sanofi-Pasteur for activities outside the presented work. Muhamed-Kheir Taha reported a patent 630133 issued. Stéphane Bouée and Corinne Emery reported that their institution (CEMKA) received grants from the GSK group of companies to perform the study related to the present publication. All authors declare no other financial or non-financial relationships and activities.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Helsinki Declaration of 1964, and its later amendments, as well as with relevant international and French regulatory requirements. Patient data in the database is anonymised using an irreversible double encryption. Access to the SNDS is regulated by a Committee of Expertise for Research, Studies and Evaluations in the field of Health, to which the present study protocol was submitted for approval. Since this was a retrospective study of an anonymised database and had no influence on patient care, ethics committee approval was not required. Use of the SNDS database for this type of study is regulated by the French national data protection agency (Commission Nationale de l'Informatique et des Libertés), to which the protocol was submitted for approval.
Data Availability
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies that evaluate medicines upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an inquiry via the website.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Weil-Olivier, C., Taha, MK., Emery, C. et al. Healthcare Resource Consumption and Cost of Invasive Meningococcal Disease in France: A Study of the National Health Insurance Database. Infect Dis Ther 10, 1607–1623 (2021). https://doi.org/10.1007/s40121-021-00468-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-021-00468-w