Abstract
Introduction
Limited changes in serotype 3 invasive pneumococcal disease (IPD) incidence rates after a decade of 13-valent pneumococcal conjugate vaccine (PCV13) introduction into several national immunization programs (NIP) have raised questions about PCV13's effectiveness against this serotype.
Methods
We analyzed the impact of pediatric PCV programs on serotype 3 IPD with two approaches. First, we reviewed the publicly available surveillance data from countries identified in two recently published reviews to describe the population impact of pediatric PCV13 or PCV10 vaccination programs on serotype 3 IPD. We then compared the observed trends in PCV10 and PCV13 countries to a previously described dynamic transmission model that simulates the spread of pneumococcal carriage and development of IPD in a population over time.
Results
When serotype 3 disease rates are compared from countries that have introduced either a 10-valent (PCV10) vaccine that does not contain serotype 3 in its formulation or PCV13 in their pediatric NIP, over time, serotype 3 incidence rate trends are markedly different. Countries with a PCV10 NIP showed a substantial linear increase in serotype 3 pneumococcal disease among all age groups since the time of PCV10 introduction, whereas countries with a PCV13 NIP experienced a modest decline during the 3–4 years after vaccine introduction followed by an inflection upward in subsequent years.
Conclusion
These data suggest that PCV13 provides a certain degree of direct and indirect protection against serotype 3 at the population level and direct adult vaccination with a serotype 3-containing vaccine is likely to provide substantial benefit in the context of a pediatric PCV NIP. Further research around serotype 3 transmission patterns and epidemiology is nonetheless warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Limited changes in serotype 3 invasive pneumococcal disease (IPD) incidence rates after a decade of 13-valent pneumococcal conjugate vaccine (PCV13) introduction into several national immunization programs (NIP) have raised questions about PCV13's effectiveness against this serotype. |
It is possible to compare settings in which the pediatric NIP uses PCV13 (containing serotype 3) versus PCV10 (which does not contain serotype 3)—the null hypothesis being that if PCV13 provides no direct or indirect protection, PCV13 and PCV10 countries should have similar trends in serotype 3 disease occurrence post vaccine introduction in children. |
What was learned from the study? |
Countries with a PCV10 NIP showed a substantial linear increase in serotype 3 pneumococcal disease among all age groups since the time of PCV10 introduction, whereas countries with a PCV13 NIP experienced a modest decline during the 3–4 years after vaccine introduction followed by an inflection upward in subsequent years. |
These data suggest that PCV13 provides a certain degree of direct and indirect protection against serotype 3 at the population level and direct adult vaccination with a serotype 3-containing vaccine is likely to provide substantial benefit in the context of a pediatric PCV NIP. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13615010.
Introduction
The 13-valent pneumococcal conjugate vaccine (PCV13) is the only currently licensed PCV that contains serotype 3 polysaccharide in its formulation. It was first approved for use in children in 2009 and is now used in infant immunization programs in approximately 120 countries. In addition, PCV13 is recommended for use in adults (age-based or risk-based recommendations) in approximately 50 countries, with or without public reimbursement [1]. Consequently, there is now a large body of evidence regarding PCV13 direct vaccine efficacy/effectiveness against serotype 3 disease from both randomized controlled and observational trials in both infants and adults, though not all reaching statistical significance (Table 1) [2,3,4,5,6]. A recent study in Spain was one of the first to compare PCV13 to the only other serotype 3-containing adult vaccine—the plain (unconjugated) polysaccharide vaccine (PPV23)—against serotype 3 invasive pneumococcal disease (IPD). This study showed that regions using PCV13 for adults had a greater reduction of IPD cases by PCV13 serotypes than regions using PPV23 for adults (incidence rate ratio [IRR] 0.73 vs. 0.86) including a decreasing trend of serotype 3 (IRR 0.82 vs. 1.04) [7].
Despite this increasing body of evidence, serotype 3 direct protection is still questioned, primarily based on surveillance data showing limited or no declines in serotype 3 IPD in all age groups following PCV13 introduction into national pediatric immunization programs [8]. This is in contrast to other vaccine serotypes, where substantial declines have occurred in disease incidence among persons of all ages following PCV13 introduction in children [8]. However, surveillance data represent the combined contribution of biological factors such as direct protection against disease and direct protection against carriage (acquisition, transmission, duration, density); epidemiologic factors such as population characteristics and mixing, population risk factors, and serotype distribution; and programmatic factors such as surveillance system characteristics. Differences in serotype 3 post PCV introduction could also reflect differences in serotype 3 transmission patterns by country, including the possibility of adult to adult transmission. Consequently, data from surveillance systems showing limited population-based impact could be due to the combination of PCV13 providing substantial direct protection against serotype 3 disease among vaccinated persons, while providing less or no indirect protection through reductions in carriage. This hypothesis has biological plausibility since a randomized controlled trial (RCT) in children showed no difference in serotype 3 carriage in PCV7 vs. PCV13 vaccinated children, although wide confidence intervals allowed for some degree of efficacy [9].
Methodologically, use of surveillance data to compare rates of vaccine-type disease pre- versus post-introduction has limitations, as there is no control group to determine what the disease epidemiology would have looked like in the absence of a vaccination program within a certain country or setting. In addition, with respect to adults, the plain polysaccharide vaccine (PPV23) has been used in adults for several decades with ~70% coverage (i.e., in the USA and the UK) [1]; thus, the use of PPV23 may also influence surveillance data. Furthermore, most surveillance systems do not stratify individuals by vaccination status. However, it is possible to compare settings in which the pediatric immunization program uses PCV13 (containing serotype 3) versus PCV10 (which does not contain serotype 3)—the null hypothesis being that if PCV13 provides no direct or indirect protection against serotype 3, PCV13 and PCV10 countries should have similar trends in serotype 3 disease occurrence post vaccine introduction in children. This is especially likely to be a valid comparison if other potential confounding factors such as dosing schedule, vaccine uptake, PPV23 use, or serotype 3 epidemiology before PCV introduction are comparable.
To test this hypothesis, we analyzed the impact of pediatric PCV programs on serotype 3 IPD. In doing so, we took two approaches. First, we reviewed the publicly available surveillance data from countries identified in two recently published reviews [10, 11] to describe the population impact of pediatric PCV13 or PCV10 vaccination programs on serotype 3 IPD (Analysis 1). We then compared the observed trends in PCV10 and PCV13 countries to a previously described dynamic transmission model that simulates the spread of pneumococcal carriage and development of IPD in a population over time (Analysis 2) [12, 13].
Methods
Analysis 1
To compare trends in serotype 3 IPD incidence since the introduction of PCV10 and PCV13, we derived serotype 3 IPD incidence from several different countries or settings with PCV13 or PCV10 childhood national immunization programs (NIP) that maintain robust pneumococcal surveillance systems, as identified in a recently published systematic review (Table 2) [11]. We included high-income countries with high-quality surveillance data available for at least 2 years before PCV program initiation and 3 years after higher-valent PCV implementation. Though there are other countries such as Germany, France, and Sweden that all have high-quality surveillance data, there are no publicly available longitudinal data for serotype 3 incidence from these countries, as far as the authors are aware. In addition, we included Colombia, with serotype 3 IPD distributions reported from the SIREVA database and then weighted against the all cause IPD incidence derived from the Individual Registration of Health Services (RIPS) database [14, 15]. For each country, we stratified the incidence of serotype 3 IPD by age (< 5 years, ≥ 65 years, and all age groups combined). If the source publication did not provide data for a specific age group, we contacted the authors directly to see if updated data were available. For example, incidence data for Israel were provided by Prof. Ron Dagan and incidence data for The Netherlands (2016–2018) were provided by Prof. Arie van der Ende (personal communications).
To compare across settings, serotype 3 incidence data were converted into IRR for years where data were available (Table 3). The IRRs were then evaluated and compared in two ways. First, we generated IRRs comparing serotype 3 incidence for each year with 2009, which was the last year before PCV13 or PCV10 was introduced in the selected countries (Analysis 1A). Second, we generated IRRs comparing serotype 3 incidence each year to the average serotype 3 incidence for all years before higher valent PCV introduction (Analysis 1B). This was done to capture and mitigate for any changes in serotype 3 incidence that may have been due to serotype changes resulting from PCV7 use, non-vaccine factors such as antibiotic use, changes in serotype 3 antibiotic resistance, or natural fluctuations. The first analysis was similar to that conducted by the Streptococcus pneumoniae Invasive Disease Network (SpIDnet), which recently reported on the effect of childhood PCV vaccination programs in older adults [10, 16].
Confidence intervals for individual country incidence rates were not included given that individual case counts were not available in source material for all countries. We calculated 95% confidence intervals for average IRRs for PCV10 and PCV13 for each year; however, these data should be interpreted with caution, given the small number of countries included in the analysis and that the surveillance systems inherently capture full population incidence for the respective country. Finally, we completed a sensitivity analysis weighting results based on country-specific coverage data, which can be found in Appendix 1.
Analysis 2
We updated a recently published transmission dynamic model to ascertain whether PCV13 provides direct or indirect protection for serotype 3 [12, 13]. Briefly, the model used the UK surveillance system and stratified individuals by the presence or absence of pneumococcal carriage, vaccine status, and age group. PCV13 vaccination with the 2 + 1 schedule in the UK (at 2, 4, and 12 months of age) was captured by transitioning eligible age groups through vaccine dose compartments based on dosing schedule and adherence.
In the model, individuals who acquire carriage may subsequently develop IPD. Entering a vaccine dose compartment protects against developing IPD by reducing the probability of developing IPD or reducing the probability of acquiring carriage. Using this model, several scenarios can be evaluated testing different vaccine effectiveness assumptions surrounding serotype 3, specifically in the UK. To validate the results in Analysis 1, we compared modeled serotype 3 IRRs in the UK assuming PCV13 provided 0% protection against serotype 3 carriage and IPD in children to test the hypothesis as to whether these predicted trends would match the observed incidence in the UK or be higher than actual observed incidence rates. We then compared this scenario of null direct and indirect protection against serotype 3 with the data reported by SpIDnet [10, 16] in countries using PCV13 or PCV10, as described in Table 2.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Analysis 1
Figures 1, 2, and 3 show serotype 3 IPD IRRs in individuals < 5 years of age (Fig. 1), adults aged ≥ 65 years (Fig. 2), and all ages (Fig. 3) since the introduction of PCV10 or PCV13 in seven countries. In each Figure, the IRRs are shown for each PCV10/13 year (2010–2018) compared with 2009 (Analysis 1A) or the pre-PCV10/13 years combined (Analysis 1B). When analyzing the averaged IRRs for serotype 3 IPD, we excluded Australia, as this country has a 3 + 0 infant program, which may constitute a confounder (data for Australia are however provided in Table 3).
Serotype 3 incidence and differences in PCV10 vs. PCV13 countries for individuals < 5 years of age. Complete data were available for all countries through 5 years post PCV implementation in the pediatric program. Panel A: Serotype 3 incidences for each year were compared with 2009, the last year before PCV13 or PCV10 was introduced in the infant immunization program (Analysis 1A). Panel B: Serotype 3 incidence each year was compared to the average serotype 3 incidence for all years before higher valent PCV introduction (Analysis 1B)
Serotype 3 incidence and differences in PCV10 vs. PCV13 countries for individuals ≥ 65 years of age. Complete data were available for all countries through 5 years post PCV implementation in the pediatric program. Panel A: Serotype 3 incidences for each year were compared with 2009, the last year before PCV13 or PCV10 was introduced into the infant immunization program (Analysis 1A). Panel B: Serotype 3 incidence each year was compared to the average serotype 3 incidence for all years before higher valent PCV introduction (Analysis 1B)
Serotype 3 incidence and differences in PCV10 vs. PCV13 countries for all ages. Complete data were available for all countries through 5 years post PCV implementation in the pediatric program. Panel A: Serotype 3 incidence for each year were compared with 2009, the last year before PCV13 or PCV10 was introduced in the infant immunization program (Analysis 1A). Panel B: Serotype 3 incidence each year was compared to the average serotype 3 incidence for all years before higher valent PCV introduction (Analysis 1B)
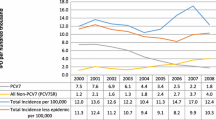
Figure 1a, b illustrates the yearly fluctuation observed for serotype 3 by individual country as well as the low incidence of serotype 3 IPD in children < 5 years of age. For example, in Finland, the incidence of serotype 3 IPD was six times higher in 2017 compared to 2015; however, the actual number of cases was one in 2015 and six in 2017. Despite the overall low incidence, there was a difference in the IRRs between PCV10 and PCV13 countries—with a 204% (Fig. 1a) and 113% (Fig. 1b) difference in the averaged IRRs 5 years after the introduction of each vaccine (2015), the last year for which complete data were available for all countries. Of note, of the seven countries shown in Fig. 1, only Colombia did not have incidence data before 2009.
For the ≥ 65 year age group (Fig. 2), serotype 3 IPD incidence before PCV13 introduction was not available for Israel. Of the six countries in this analysis, only the US had an age-based PCV13 program in adults, which was initiated in late 2014, so any impact of the adult recommendation would not be reflected in the data prior to 2015. Thus, any changes in the incidence of serotype 3 attributable to vaccination would be driven primarily by indirect effects of pediatric immunization programs. As shown in Fig. 2a, b, a 216% (Analysis 1A) to 202% (Analysis 1B) difference was observed for persons ≥ 65 years of age between PCV13 and PCV10 countries 5 years post PCV introduction in children. In the last year complete data were available (2015), 95% confidence intervals between PCV10 and PCV13 countries did not cross, and average PCV10 incidence was statistically increasing in both Analysis 1A and 1B (Table 3). A similar magnitude in the differences was observed for all age groups combined; however, only five countries had data available (Fig. 3).
Sensitivity analysis weighting incidence rates based on local coverage rates can be found in Appendix 1. This analysis was consistent with equal weighting and did not impact the direction or interpretation of the results.
Analysis 2
Figure 4 shows data published by SpIDnet that used methodology similar to Analysis 1A. The six sites with universal PCV13 programs showed a general decrease in serotype 3 IPD from 2011 to 2014, followed by a plateau and slight increase of 12% in 2017. In contrast, data from four sites with universal PCV10 programs showed a general increase in serotype 3 IPD IRRs starting at the time of PCV10 introduction, with an increase of 56% in 2017. The UK modeled IPD IRRs in adults ≥ 65 years of age, assuming 0% PCV efficacy against serotype 3 disease or carriage in children, are aligned more closely with the observed data from PCV10 sites, although serotype 3 increases were significantly higher in PCV10 sites than those predicted by the model. Two PCV10 countries (Czech Republic and Sweden) included regions that used PCV13, which could potentially underestimate the increases of serotype 3.
Discussion
Since PCV13's introduction into routine infant immunization programs globally, the incidence of disease caused by PCV13 serotypes dramatically decreased among vaccinated children and among unvaccinated individuals, including adults ≥ 65 years of age who are at high risk of pneumococcal disease [17, 18]. The notable exception has been serotype 3 disease. A recent SAGE report suggests there is no difference in disease burden due to serotype 3 in countries that use PCV13 or PCV10 in the infant NIP [19]; however, surveillance data were not included in their assessment in the same manner as reported here. RCTs and observational studies have shown that PCV13 provides direct protection against serotype 3 IPD in children and IPD and CAP in adults [2,3,4,5,6]. By contrast, fewer data exist for PCV13's impact on serotype 3 carriage with the only RCT conducted to date showing no efficacy, although with wide confidence intervals [9]. To better interpret the direct and potential indirect effect, if any, of PCV13 on serotype 3, we examined surveillance data from several countries. We found that countries that have introduced PCV10 in routine infant vaccination programs showed a substantial linear increase in serotype 3 pneumococcal disease among all age groups since the time of PCV10 introduction. By contrast, countries with PCV13 in their routine pediatric programs experienced a modest decline during the 3–4 years after introduction followed by an inflection upward in subsequent years, which was most pronounced in the ≥ 65 year age group. It is also possible that as every year new infants are vaccinated according to the NIP, this population could represent the reservoir and the source of transmission to the adults. However, numerous studies have shown that older children (1 to 4 years of age) are the main reservoir for PCV transmission [20,21,22]. We also found that, using a model based on 0% vaccine effectiveness against serotype 3 IPD and carriage in children, the projected trajectory of serotype 3 disease in adults more closely resembled the epidemiology in PCV10 countries (although underestimating incidence rates of serotype 3 disease observed in PCV10 countries) and differed significantly from PCV13 countries.
The initial declines in serotype 3 IPD among unvaccinated older adults in all countries implementing public PCV13 programs suggest that at least some indirect effect occurs. In theory, this seems at odds with the RCT of PCV13 vaccine efficacy against carriage, which showed no impact on serotype 3. However, confidence intervals in this study were wide (rate ratio, 0.99; 95% CI 0.48–2.06) [9], and the study did not assess PCV13's impact against carriage density or duration, the latter being important, as a human challenge RCT for serotype 6B has shown that the primary effect of PCV13 vs. unconjugated 23-valent pneumococcal polysaccharide vaccine was against density and not acquisition [23, 24]. In addition, although the aggregate correlate of protection of 0·35 μg/ml is used in the licensing of new PCVs, at least one study has suggested serotype-specific correlates of protection vary widely, with a very high serum IgG concentration of 2·83 μg/ml needed for protection against serotype 3—a concentration that is rarely attained from vaccination [25]. In addition, serum IgG concentrations may not be the correct correlate for protection, but rather memory B cells, or other aspects of the immune systems such as mucosal IgA, may play a role.
If there is an indirect effect of PCV13 against serotype 3 carriage, it appears to be incomplete and possibly of relatively short duration, based on gradual increases in serotype 3 IPD that occur in unvaccinated persons within approximately 4 years. Several explanations exist. PCV13 immune responses (e.g., antibody levels) against serotype 3 may be sufficient to provide direct protection but insufficient for sustained protection against carriage, which may be mediated by additional immunological mechanisms such as memory B cells [25, 26]. This in combination with increased global circulation of serotype 3 strains with reduced antibiotic susceptibility could lead to increases in serotype 3 over time [27]. Aging within the elderly population, including increasing prevalence of co-morbidities, could increase the percent of the population susceptible to serotype 3 diseases. Lastly, serotype 3 could consist of multiple variants. For example, it could be a serogroup with multiple serotypes (similar to the earlier experience with serotypes 6A/6C and 19A/19F) with PCV13 reducing phenotypes that are more similar to the polysaccharide used in vaccine construction. Alternatively, the polysaccharide could be consistent across different serotype 3 genotypes, but subcapsular proteins mediating immunological effects such as complement binding could differ [28]. Regardless, a holistic analysis of surveillance data has shown that countries using PCV10 experience a substantially different population-based evolution of serotype 3 IPD than countries using PCV13, which in turn supports some degree of PCV13-induced indirect protection in addition to the previously documented direct protection against IPD and pneumonia.
A secondary conundrum is why PCV7 did not lead to the increases in serotype 3 that appear to have occurred following PCV10 introduction. While no definitive answer exists, this likely points to the multifactorial nature of serotype 3 dynamics. For example, serotype 3 has historically been antibiotic sensitive, while during the PCV10/PCV13 periods a much more antibiotic resistant clade has emerged from Asia to become the dominant strain in some locations [27]. It is possible that PCV7 did not create the optimal circumstances for serotype 3, possibly because of more aggressive replacement by serotype 19A, which is not present in PCV7. As above, population aging and increases in co-morbidities predisposing to serotype 3 disease may have reached a critical level.
The current study relied on surveillance data, which can have numerous limitations. For example, the number of IPD cases was not available for each country; thus, we could not calculate confidence intervals. Instead, we relied on a standardized reporting of incidence per 100,000, allowing for comparisons between countries. Though we used the most robust publicly available information, including studies that used similar methodologies across multiple countries, we excluded some countries from the analysis, such as: (1) Australia, which uses a 3 + 0 schedule; (2) New Zealand, which had multiple switches between PCV10 and PCV13; (3) countries in Latin America such as Brazil, where incidence data are not available. In addition, South Africa did not meet the inclusion criteria as it is a middle income country that introduced a novel three-dose schedule, with two primary doses given to infants at 6 and 14 weeks of age and a booster given at 9 months of age [29]. Nonetheless, in South Africa, data collected in the 1–2 years following PCV13 introduction in children showed a decrease of serotype 3 IPD in those < 2 years of age compared to the baseline years (i.e., 2005–2008 vs. 2012). Surveillance data also showed a decrease in the number of serotype 3 IPD isolates in the first 4 years following PCV13 introduction, followed by an increase [29, 41]. The trends in these countries show the epidemiology of serotype 3 IPD post PCV13 introduction is similar to that of the other countries in our analysis.
To our knowledge, only one other study has analyzed surveillance data in a similar manner. The aforementioned study by SpIDnet reported the indirect effect of all PCV10/PCV13 serotypes through 2015 [10]. Among the sites with universal pediatric PCV13 programs, a general decrease in serotype 3 IPD was observed from 2011 to 2014, followed by an increase, resulting in an 11% decrease from baseline during the last surveillance year. In contrast, data from four sites with universal PCV10 programs showed a general increase in serotype 3 IPD IRRs starting at the time of PCV10 introduction, with an increase of 58% in 2015 (Fig. 5). As a control, the shared serotypes (1, 5, and 7F) showed a decrease in both sets of countries, whereas the other non-shared serotypes, 6A and 19A, also decreased in PCV13 countries and increased in PCV10 countries.
Ratio of IPD incidence in adults aged ≥ 65 years post PCV10/13 compared to 2009, by PCV serotype and vaccine policy adapted from reference [10]
Conclusions
Our data support the hypothesis that PCV13 provides some indirect protection against serotype 3. Additional research should focus on why the overall population-level impact remains less for serotype 3 than that for other PCV13 serotypes, the contribution of non-vaccine factors to serotype 3 epidemiology, assessment of potential variants (at either the capsular or subcapsular level) within the serotype 3 population, and development of a better serotype 3 vaccine. In the meantime, direct vaccination of groups at higher risk of serotype 3 disease, such as the elderly and persons with underlying co-morbidities, may help overcome the relatively limited ability of pediatric PCV13 programs to protect unvaccinated persons.
References
Sings HL. Pneumococcal conjugate vaccine use in adults—addressing an unmet medical need for non-bacteremic pneumococcal pneumonia. Vaccine. 2017;35(40):5406–17.
Sings HL, De Wals P, Gessner BD, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against invasive disease caused by serotype 3 in children: a systematic review and meta-analysis of observational studies. Clin Infect Dis. 2019;68(12):2135–43.
Bonten MJM, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Eng J Med. 2015;372:1114–25.
Gessner BD, Jiang Q, Van Werkhoven CH, et al. A post-hoc analysis of serotype-specific vaccine efficacy of 13-valent pneumococcal conjugate vaccine against clinical community acquired pneumonia from a randomized clinical trial in the Netherlands. Vaccine. 2019;37(30):4147–54.
McLaughlin JM, Jiang Q, Gessner BD, et al. Pneumococcal conjugate vaccine against serotype 3 pneumococcal pneumonia in adults: a systematic review and pooled analysis. Vaccine. 2019;37(43):6310–6.
Warren JL, Weinberger DM. Estimating serotype-specific efficacy of pneumococcal conjugate vaccines using hierarchical models. Epidemiology. 2020;31(2):259–62.
de Miguel S, Domenech M, González-Camacho F, et al. Nationwide trends of invasive pneumococcal disease in Spain (2009–2019) in children and adults during the pneumococcal conjugate vaccine era. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa1483.AccessedOctober26.
Ladhani SN, Collins S, Djennad A, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–51.
Dagan R, Patterson S, Juergens C, et al. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis. 2013;57(7):952–62.
Hanquet G, Krizova P, Valentiner-Branth P, on behalf of The SpIDnet/I-MOVE+ Pneumo Group, et al. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination. Thorax. 2019;74(5):473–82.
Izurieta P, Bahety P, Adegbola R, Clarke C, Hoet B. Public health impact of pneumococcal conjugate vaccine infant immunization programs: Assessment of invasive pneumococcal disease burden and serotype distribution. Expert Rev Vaccines. 2018;17(6):479–93.
Wasserman M, Lucas A, Jones D, et al. Dynamic transmission modelling to address infant pneumococcal conjugate vaccine schedule modifications in the UK. Epidemiol Infect. 2018;146(14):1797–806.
Lucas A, Wilson M, Sings HL, et al. Estimating the vaccine effectiveness against serotype 3 for the 13-valent pneumococcal conjugate vaccine: a dynamic modeling approach. Int J Infect Dis Ther. 2019;4(4):56–66.
Ministry of Health Colombia [Internet]. Individual registration of health services (RIPS) database. Available at: https://www.minsalud.gov.co/proteccionsocial/Paginas/rips.aspx. Accessed 16 June 2019.
Sanabria O, Valderrama C. Streptococcus pneumoniae distribución de los aislamientos invasores por año de vigilancia, departamento, grupos de edad, serotipos y sensibilidad antimicrobiana: Instituto Nacional de Salud. Grupo de Microbiología. Informe Nacional SIREVA II. Colombia 2006–2016 [Analysis in brief on the Internet]. Available at: http://www.paho.org/hq/index.php?option=com_content&view=article&id=5536%3A2011-sireva-ii&catid=1591%3Aabout&Itemid=3966&lang=es. Accessed 16 June 2019.
Pilishvili T, Advisory Committee on Immunization Practices. 13-valent pneumococcal conjugate vaccine (PCV13) effects on disease caused by serotype 3 [Analysis in brief on the Internet]. 2019 Feb 28. Available at https://www.cdc.gov/vaccines/acip/meetings/slides-2019-02.html/. Accessed 16 June 2019. Note that requests for slide presentation files prior to the ones currently posted on their website may be sent to acip@cdc.gov.
Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41.
Shiri T, Datta S, Madan J, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(1):e51–9.
World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, October 2020—conclusions and recommendations. Wkly Epidemiol Rec. 2020;95(48):585–608. Available at: https://apps.who.int/iris/bitstream/handle/10665/337100/WER9548-eng-fre.pdf?ua=1. Accessed 21 Dec 2020
Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018;16(6):355–67.
Siegel SJ, Weiser JN. Mechanisms of bacterial colonization of the respiratory tract. Annu Rev Microbiol. 2015;69:425–44.
Dagan R, Givon-Lavi N, Zamir O, et al. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J Infect Dis. 2002;185(7):927–36.
Collins AM, Wright AD, Mitsi E, et al. First human challenge testing of a pneumococcal vaccine. Double-blind randomized controlled trial. Am J Respir Crit Care Med. 2015;192(7):853–8.
German EL, Solorzano C, Sunny S, et al. Protective effect of PCV vaccine against experimental pneumococcal challenge in adults is primarily mediated by controlling colonization density. Vaccine. 2019;37(30):3953–6.
Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–46.
Pennington SH, Pojar S, Mitsi E, et al. Polysaccharide-specific memory B cells predict protection against experimental human pneumococcal carriage. Am J Respir Crit Care Med. 2016;194(12):1523–31.
Azarian T, Mitchell P, Georgieva M, et al. Global emergence and population dynamics of divergent serotype 3 CC180 pneumococci. PLoS Pathog. 2018;14(11):e11007438.
Groves N, Sheppard CL, Litt D, et al. Evolution of streptococcus pneumoniae serotype 3 in England and Wales: a major vaccine evader. Genes (Basel). 2019;10(11):845.
von Gottberg A, de Gouveia L, Tempia S, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. 2014;371:1889–99.
Australian Department of Health and Ageing [Internet]. National Notifiable Disease Surveillance System (NNDSS): pneumococcal disease invasive. Public dataset. 2016 [Accessed 2019 June 16]. Available at http://www9.health.gov.au/cda/source/cda-index.cfm and National Centre for Immunization Research and Surveillance [Internet]. FactSheet pneumococcal vaccines for Australians: information for immunisation providers. 2018 Jun [Accessed 2019 June 16]. Available at http://www.ncirs.org.au/sites/default/files/2018-12/pneumococcal-fact-sheet_September%202018_Final.pdf.
Toronto Invasive Bacterial Diseases Network [Internet]. Case definition and ascertainment. Mount Sinai Hospital; 2017. Available at: http://www.tibdn.ca/methodology/case-definition. Accessed 16 June 2019
Public Health Agency of Canada. National Advisory Committee on Immunization (NACI): Update on the use of pneumococcal vaccines in adults 65 years of age and older [Analysis in brief on the Internet]. 2018 Nov. Available at: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/healthy-living/update-on-the-use-of-pneumococcal-vaccines-in-adult/update-on-the-use-of-pneumococcal-vaccines-in-adult-eng.pdf. Accessed 16 June 2019.
Finnish National Institute for Health and Welfare [Internet]. Incidence of invasive pneumococcal disease in Finland. Available at https://thl.fi/en/web/thlfi-en/research-and-expertwork/projects-and-programmes/monitoring-the-population-effectiveness-of-pneumococcal-conjugate-vaccination-in-the-finnish-national-vaccination-programme/incidence-of-invasive-pneumococcal-disease-in-finland. Accessed 16 June 2019.
Finnish National Institute for Health and Welfare [Internet]. Vaccination programme for adults. Available at https://thl.fi/en/web/vaccination/national-vaccination-programme/vaccination-programme-for-adults. Accessed 16 June 2019.
National Institute for Public Health and the Environment: Ministry of Health, Welfare and Sport [Internet]. Pneumococcal disease in the elderly. 2017 Dec. Available at https://www.rivm.nl/en/Documents_and_publications/Common_and_Present/Newsmessages/2017/Pneumococcal_disease_in_the_elderly. Accessed 16 June 2019.
NHS [Internet]. Pneumococcal Vaccine. 2019 Feb. Available at http://www.nhs.uk/Conditions/vaccinations/Pages/pneumococcal-vaccination.aspx. Accessed 16 June 2019.
Pilishvili T, Bennett NM. Pneumococcal disease prevention among adults: strategies for the use of pneumococcal vaccines. Vaccine. 2015;33(Suppl 4):D60–5.
Health Service Executive. Chapter 16: Pneumococcal infection [Analysis in brief on the Internet]. 2018 Jul. Available at http://www.hse.ie/eng/health/immunisation/hcpinfo/guidelines/chapter16.pdf. Accessed 16 June 2019.
Folkehelseinstittutet [Internet]. Pneumokokkvaksinasjon. 2008 Apr. Available at https://www.fhi.no/nettpub/vaksinasjonsveilederen/vaksiner-mot-de-enkelte-sykdommene/pneumokokkvaksinasjon---veileder-fo/. Accessed 16 June 2019.
Folkhälsomyndigheten. Pneumokockvaccination som särskilt vaccinationsprogram [Analysis in brief on the Internet]. 2016. Available at https://www.folkhalsomyndigheten.se/documents/smittskydd-sjukdomar/vaccinationer/vaccinationsprogram/e3-pneumokockvaccination-riskgrupper-beslutsunderlag-ru-s2013-240-fs-e3.pdf. Accessed 16 June 2019.
Meiring S, Cohen C, de Gouveia L, et al. Germs-sa annual surveillance report for laboratory confirmed invasive meningococcal, haemophilus Influenzae and pneumococcal disease, South Africa, 2017 [Analysis in brief on the Internet]. National Institute of Communicable Diseases. 2018 Sept [accessed 2019 Dec]. 16(2):78–90. Available at http://www.nicd.ac.za/wp-content/uploads/2018/09/GERMS-SA-annual-surveillance-report-for-laboratory-confirmed-invasive-meningococcal-Haemophilus-influenzae-and-pneumococcal-disease-South-Africa-2017.pdf.
Pilishvili T, Gierke R, Xing W, et al. Changes in invasive pneumococcal disease (IPD) among adults following 6 years of 13-valent pneumococcal conjugate vaccine use in the US. Presented at the 11th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD), 2018 April 15–19; Melbourne, Australia. Available at https://isppd.kenes.com/2018/scientific-program-(2)/e-poster#.XQe9alXD_ik.
Acknowledgements
Funding
This work was supported by Pfizer, Inc. The Journal’s Rapid Service Fee was paid by Pfizer.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Heather L. Sings, Bradford D. Gessner, Matt D. Wasserman, and Luis Jodar are employees and shareholders of Pfizer, Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sings, H.L., Gessner, B.D., Wasserman, M.D. et al. Pneumococcal Conjugate Vaccine Impact on Serotype 3: A Review of Surveillance Data. Infect Dis Ther 10, 521–539 (2021). https://doi.org/10.1007/s40121-021-00406-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-021-00406-w