Abstract
Introduction
The objective of the study was to evaluate the prevalence of Clostridium difficile-associated diarrhoea (CDAD) among hospitalised patients with antibiotic-associated diarrhoea (AAD) in general and by specific types of medical care and hospital units.
Methods
A prospective, cross-sectional, non-interventional, multicentre study. The main inclusion criteria were: patient age ≥ 18 years, hospital stay of at least 48 h, current antibiotic therapy or antibiotic therapy within the previous 30 days, loose stools (Bristol stool types 5–7 and stool frequency ≥ 3 within ≤ 24 consecutive hours or exceeding normal for the patient) and signed informed consent form. The stool sample was taken to the local (study site) microbiology laboratory for detection of glutamate dehydrogenase (GDH) and toxins A/B using enzyme immunoassay (EIA) stool test.
Results
From April 2016 to April 2017, a total of 1245 patients from 12 large hospitals were enrolled in the study. Data on 81 patients were excluded from the analysis for different reasons. Data on 1164 patients (45.2% males and 54.8% females) with a mean age of 54.9 years (range 18–95 years) were analysed. Length of hospitalisation was 2–188 days (median, 8 days). The EIA stool test showed CDAD-positive results in 21.7% (253/1164) patients. The patients were from surgery units (546/1164), internal medicine units (510/1164) and intensive care units (108/1164). The prevalence of CDAD among patients from surgery, internal medicine and intensive care units was 26.2, 17.8 and 17.6%, respectively. Oncology, gastroenterology, septic surgery, oncohaematology and general medical hospital units accounted for more than 75% of all patients included; the prevalence of CDAD by those hospital units was 11.3, 15.0, 39.2, 17.6, and 27.2%, respectively. The proportion of GDH-positive and toxin A/B-negative patients by the rapid stool test result was 16.8% (196/1164). The prevalence of CDAD varied widely between the hospitals (from 0 to 44.3%).
Conclusions
The prevalence of CDAD among hospitalised patients with AAD in this study was 21.7% (95% confidence interval: 14.8 and 28.7%). The percentage of CDAD varied widely between hospitals and by specific types of medical care and hospital units.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of antibiotics is associated with disorders of gastrointestinal tract function and diarrhoea in 5–39% patients [1, 2]. Clostridium difficile (C. difficile) is a common infectious cause of antibiotic-associated diarrhoea (AAD) [1,2,3,4]. C. difficile is capable of producing an enterotoxin (toxin A) and a cytotoxin (toxin B). Express laboratory tests for glutamate dehydrogenase (GDH) and free C. difficile toxins in stool are used for the diagnosis of C. difficile-associated diarrhoea (CDAD) [5].
Reports from other countries indicate that CDAD is becoming more common and requires special attention due to the risk of serious complications, including fatalities [6,7,8,9]. Russian clinical data on the epidemiology of CDAD are limited to individual retrospective–prospective single-centre studies [10,11,12,13]. Due to the limited number of Russian studies dedicated to CDAD, further research on this issue within a Russian prospective multicentre epidemiological study is of scientific and clinical interest.
The objective of the study was to determine the prevalence of CDAD in hospitalised patients with antibiotic-associated diarrhoea (AAD) in general and in subgroups of patients from wards with different types of care and specialties.
Methods
Patients aged ≥ 18 years and hospitalised for at least 48 h, who developed diarrhoea symptoms while in the hospital and while taking antibiotics, or within 30 days of starting antibiotic therapy, and who agreed to participate in the study, were included in the prospective non-interventional multicentre epidemiological study.
Diarrhoea is defined as liquid stool corresponding to types 5–7 on the Bristol Stool Form Scale, with a frequency of ≥ 3 defecations in succession within ≤ 24 h or with defecation frequency that is higher than normal for a given patient. Under the exclusion criteria, patients could not participate in the study more than once. A stool sample was placed in a sterile container and transported to the local (on-site) microbiology laboratory for an enzyme immunoassay express test (CoproStrip™; SavyonDiagnostics, Israel) for the presence of GDH, toxin A and toxin B of C. difficile. The express test interpretation protocol is shown in Table 1.
The following patient data were included in the individual electronic registration card: date of birth, sex, main diagnosis leading to hospitalisation according to the International Classification of Diseases, revision 10 (ICD-10), hospitalisation date, characteristics of diarrhoea, information about the date and results of the express test for the presence of GDH, toxin A and toxin B in stool, the type of care provided (surgery, therapy, intensive care) and the medical specialty (gastroenterology, oncohaematology, oncology, etc.).
The primary endpoint, reflecting the presence of CDAD, was the percentage of patients with CDAD among the hospitalised patients with AAD who had an express stool test for GDH and free toxins of C. difficile. The secondary endpoints were the percentages of hospitalised patients with CDAD in subgroups classified according to specialty (gastroenterology, oncohaematology, oncology, etc.) and the type of care (surgery, therapy, intensive care). An additional endpoint was the percentage of hospitalised patients with CDAD who had positive express test results for GDH, but negative results for C. difficile toxins.
It was planned to include 1230 patients in the study. The sample size, which would ensure accuracy of the primary endpoint was calculated based on the following conditions: a simple random sampling model, width of the 95% confidence interval (CI) not exceeding 5% (2.5% error), risk of obtaining incomplete data below 20%. Statistical analysis was performed using SPSS v22 for Windows operating system. The results were represented as standard variables of descriptive statistics. The study design was taken into account in the statistical analysis by the adjustment of the 95% CI using the methods proposed by the World Health Organization.
The study was conducted according to the principles of the Declaration of Helsinki as well as Good Epidemiological Practice (Guidelines of the International Society for Pharmaceutical Engineering and Good Pharmacy Practice, 2007).
Results
Characteristics of Patients
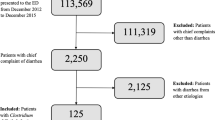
From April 2016 to April 2017, a total of 1245 patients from 12 large inpatient clinics located in Moscow and St Petersburg were enrolled in the study. Data from 81 patients (6.5%) were excluded from the statistical analysis and the description for the following reasons: invalid express test results, including negative results for GDH coinciding with positive results for C. difficile toxins (n = 60), admission to hospital for less than 48 h (n = 10), discrepancy between the express test dates and the study protocol (n = 5), violation of the exclusion criteria (n = 3), no full-time hospitalisation (day care facilities, n = 2), and incomplete patient data (n = 1) (Fig. 1).
The analysed data of the 1164 patients [526 males (45.2%) and 638 females (54.8%)] are presented. The age ranged from 18 to 95 years (average age, 54.9 years). The distribution of the patients by primary diagnosis is shown in Table 2.
The diagnoses chiefly responsible for hospitalisation of 72.3% of the patients included neoplasms, diseases of the circulatory system, and diseases of the digestive system. The length of hospitalisation varied from 2 to 188 days, the median being 8 days (1st quartile 5 days, 3rd quartile 15 days). In the majority of cases (1131), the patients were hospitalised for between 2 and 50 days, with 7 patients for over 100 days.
Totals of 522 patients (44.9%) had type 7 diarrhoea on the Bristol Stool Form Scale, 475 patients (40.8%) had type 6 and 167 (14.3%) had type 5. A diarrhoea frequency of 3–8 defecations per day was observed in the majority of patients (944, 81.1%); 15 or more defecations were observed in 27 patients (2.3%).
Primary Endpoint
CDAD was diagnosed in 21.7% of patients with AAD (253/1164) according to the express test results (Table 3). The 95% CI for CDAD prevalence, calculated based on the simple random sampling model, was 19.4%, 24.1%. At the same time, the 95% CI adjusted for the study design was significantly wider (14.8%, 28.7%).
Secondary Endpoints
CDAD prevalence among the patients with AAD divided into groups by specialty of the provided care is shown in Table 4.
The majority of patients participating were from the departments of surgery (546/1164) and therapy (510/1164). The percentage of CDAD was 26.2% in the surgery patients and 17.8 and 17.6% in the therapy and intensive care patient groups, respectively. The surgical patients were predominantly in oncology (n = 225) and septic surgery (n = 204) departments. The percentages of CDAD were 12.0% among the [surgical] oncology patients and 39.2% in the septic surgical group. The majority of the medical patients were in the departments of gastroenterology (194/510), oncohaematology (142/510) and general medicine (47/510). CDAD prevalence in these departments were 12.4, 17.6 and 31.9%, respectively. The majority of the patients with CDAD in the intensive care units were transplant patients (n = 41). The percentage of CDAD in this group was 7.3%.
The prevalence of CDAD in the groups of patients with AAD by specialty is presented in Table 5.
According to the results, more than 75% of all enrolled patients were in the departments of oncology, gastroenterology, septic surgery, oncohaematology, and general medicine. The percentages of patients with CDAD in these departments amounted to 11.3, 15.0, 39.2, 17.6, and 27.2%, respectively.
Other Endpoints
Positive GDH express test results were observed in 38.6% of the patients (449/1164), while C. difficile did not produce A and B toxins in 16.8% of the cases (196/1164). It should also be noted that the percentage of patients with a CDAD varied among hospitals, from 0 to 44.3% (Table 6).
A higher percentage of CDAD (26.7–42.9%) was observed in patients ≥ 65 years compared with patients < 65 years (14.1–17.2%) (Table 7).
Discussion
The use of antibiotics is a proven risk factor for diarrhoea. According to McFarland, CDAD occurs in 5–39% of patients [1]. The research of Bartlett and Gilbert published in the 1990s demonstrated that diarrhoea developed in 5–10% of patients receiving ampicillin; 10–25%, amoxicillin/clavulanate; 15–20%, cefixime; and 2–5%, cephalosporins, fluoroquinolones, azithromycin, clarithromycin, erythromycin and tetracycline [2, 3].
C. difficile predominates among the infectious causes of AAD [1,2,3,4]. The role of C. difficile in the development of CDAD and pseudomembranous colitis was established in 1978. In the majority of cases, the development of pathological conditions was associated with use of clindamycin [4]. Other pathogens of AAD include Clostridium perfringens [14], Staphylococcus aureus [15], Klebsiella oxytoca [16], and possibly Candida albicans [17].
C. difficile is able to produce enterotoxin (toxin A) and cytotoxin (toxin B), which disrupt the function of and damage mucous membranes and, therefore, play a leading role in the pathogenesis of the infectious diseases [18, 19]. The qualitative and quantitative parameters of toxin production are associated with severity of the disease [20, 21]. Non-toxin-producing strains of C. difficile are capable of colonisation, but do not usually damage the mucous membranes of the gastrointestinal tract [22].
Infectious diseases caused by C. difficile have different manifestations, from the mild form of the disease with few symptoms to a fulminant course of pseudomembranous colitis (toxic megacolon, bowel perforation) [6, 23,24,25,26]. Relapse is observed in approximately 10–25% of patients 30 days after successful treatment [27, 28]. According to a European study, the attributive mortality was 2% in patients with diarrhoea that developed after ≥ 3 days after their hospital stay or in patients with suspected C. difficile infection. In another 7% of cases, the infection played a significant role in fatal outcomes [6].
The few Russian data on CDAD prevalence in patients with AAD obtained in single-centre studies differ markedly. For instance, 28.7% (154/563) and 51.5% (67/130) of cases were reported in general hospitals [10, 11]. An increase of CDAD prevalence among cancer patients from 15.6% (7/45) in 2011 to 24.2% (57/236) in 2013 was reported [12]. A high CDAD prevalence was observed in gastroenterology patients, 39.1% (93/238) [13].
The present study was conducted due to the scarcity of information on CDAD prevalence in the Russian Federation and the lack of relevant Russian multicentre studies. According to the results of the conducted express tests, CDAD was diagnosed in 21.7% of patients with AAD, which corresponds to the international data (15–25%) [29]. The study also demonstrated pronounced differences in CDAD prevalence between different inpatient facilities (from 0 to 44.3%) and between patient groups by type of care (surgery, 26.2%; therapy, 17.6%; intensive care, 17.8%) and by specialty (septic surgery, 39.2%; general medicine, 27.2%; oncohaematology, 17.2%; gastroenterology, 15.0%; oncology, 11.3%; transplantation, 6.8%). These differences may reflect the local characteristics of CDAD epidemiology. In particular, the researchers at the site with the highest CDAD prevalence (44.3%) also reported a particularly severe contingent of patients and an outbreak of the disease during the study.
The percentage of patients with GDH-positive express test results, but negative results for toxins, was 16.8%, while the total percentage of GDH-positive patients was 38.6%. Thus, about 39% of the patients with AAD participating in the study were colonised with C. difficile, whereas about half of the C. difficile colonisation cases were accompanied by toxin production and the onset of infection.
Patients age ≥ 65 years demonstrated higher CDAD prevalence (26.7–42.9%) in comparison with the patients < 65 years (14.1–7.2%). This was expected, since advanced age (≥ 65 years) is one of the known risk factors for the development of CDAD [30].
The results of this study and previously published Russian epidemiological studies suggest that CDAD is a common complication occurring in Russian hospitals. This makes it more relevant to carefully examine a patient with unexplained diarrhoea when the patient is on antibiotic treatment or within 30 days following such treatment. Significant differences in CDAD prevalence among hospitals and hospital departments may be associated with specific characteristics of the hospitalised patients, the environmental risk factors of C. difficile-associated disease and the local approaches to the monitoring of nosocomial infections.
Limitations of the Study
Only large inpatient treatment facilities in the Russian cities with a high population density (Moscow and St Petersburg) participated in the study. This limits the potential for extrapolating the data at the country-wide level.
The pronounced differences in CDAD prevalence rates between the research sites impacted the accuracy of primary endpoint assessment: the 95% CI adjusted for the study design turned out to be wider than the 95% CI based on the simple random sampling model, which was used for sample size calculation during the study planning process.
The express test used in the study has known sensitivity and specificity limitations reducing the accuracy of CDAD diagnosis. In particular, negative results of this laboratory test did not entirely rule out CDAD.
Conclusion
The prevalence of CDAD among hospitalised patients with AAD amounted to 21.7% (95% CI adjusted for the study design: 14.8%, 28.7%). There were pronounced differences in CDAD prevalence among inpatient care facilities (from 0% to 44.3%) and patient groups by the type of care and specialty.
References
McFarland L. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis. 1998;16(5):292–307.
Bartlett J. Antibiotic-associated diarrhea. Clin Infect Dis. 1992;15:573–81.
Gilbert D. Aspects of the safety profile of oral antimicrobial agents. Infect Dis Clin Pract. 1995;4(Suppl 2):S103–12.
Bartlett J, Chang T, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin producing Clostridia. New Engl J Med. 1978;298:531–4.
Crobach M, Planche T, Eckert C, et al. European society of clinical microbiology and infectious diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2016;22:63–81.
Bauer M, Notermans D, van Benthem B, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377:63–73.
Lyytikäinen O, Turunen H, Sund R, et al. Hospitalizations and deaths associated with Clostridium difficile infection, Finland, 1996-2004. Emerg Infect Dis. 2009;15:761–5.
Soler P, Nogareda F, Cano R. Rates of Clostridium difficile infection in patients discharged from Spanish hospitals, 1997-2005. Infect Control Hosp Epidemiol. 2008;29:887–9.
Vonberg R, Schwab F, Gastmeier P. Clostridium difficile in discharged inpatients. Germany. Emerg Infect Dis. 2007;13:179–80.
Mulyar N.F., Vereschagina S.A., Fadeeva T.V., Spasov G.P., Kanya O.V. Clostridium difficile associated diarrhea in multidisciplinary hospital. Bjulleten’ VSNC SO RAMN. 2012;5(87):72–75 (Russian).
Zakharova N.V., Fil T.S. Antibiotic-associated diarrhea: stratification of risk factors for development of Clostridium difficile infection in multidisciplinary hospital. Farmateka. 2016;s5:60–64 (Russian).
Shilnikova I., Petukhova I., Dmitrieva N., Grigoryevskaya Z., Bagirova N. Dynamics of Clostridium difficile-associated diarrhea in the Russian cancer research center. In: Proceedings of 24th ECCMID, 10–13 May 2014, Barcelona, Spain. Abstr R482.
Volchkova E.V., Belousova E.A., Makarchuk P.A., Rusanova E.V., Velikanov. E.V. Prevalence of Clostridium difficile infection in hospitalized patients. Al’manah Klinicheskoj Mediciny. 2014;33:72–76 (Russian).
Sparks S, Carman R, Sarker M, McClane B. Genotyping of enterotoxigenic Clostridium perfringens fecal isolates associated with antibiotic-associated diarrhea and food poisoning in North America. J Clin Microbiol. 2001;39:883–8.
Gravet A, Rondeau M, Harf-Monteil C, et al. Predominant Staphylococcus aureus isolated from antibiotic-associated diarrhea is clinically relevant and produces enterotoxin A and the bicomponent toxin LukE-lukD. J Clin Microbiol. 1999;37:4012–9.
Högenauer C, Langner C, Beubler E, et al. Klebsiella oxytoca as a causative organism of antibiotic-associated hemorrhagic colitis. N Engl J Med. 2006;355:2418–26.
Bartlett J. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–9.
Brito G, Sullivan G, Ciesla W Jr, et al. Clostridium difficile toxin A alters in vitro-adherent neutrophil morphology and function. J Infect Dis. 2002;185:1297–306.
Hecht G, Koutsouris A, Pothoulakis C, et al. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology. 1992;102:416–23.
Akerlund T, Svenungsson B, Lagergren A, Burman L. Correlation of disease severity with fecal toxin levels in patients with Clostridium difficile-associated diarrhea and distribution of PCR ribotypes and toxin yields in vitro of corresponding isolates. J Clin Microbiol. 2006;44:353–8.
Lyras D, O’Connor J, Howarth P, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–9.
McFarland L, Elmer G, Stamm W, Mulligan M. Correlation of immunoblot type, enterotoxin production, and cytotoxin production with clinical manifestations of Clostridium difficile infection in a cohort of hospitalized patients. Infect Immun. 1991;59:2456–62.
Triadafilopoulos G, Hallstone A. Acute abdomen as the first presentation of pseudomembranous colitis. Gastroenterology. 1991;101:685–91.
Kelly C, LaMont J. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932–40.
Bagdasarian N, Rao K, Malani P. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398–408.
Rubin M, Bodenstein L, Kent K. Severe Clostridium difficile colitis. Dis Colon Rectum. 1995;38:350–4.
Bouza E, Dryden M, Mohammed R, et al. Results of a phase III trial comparing tolevamer, vancomycin and metronidazole in patients with Clostridium difficile-associated diarrhoea. Clin Microbiol Infect. 2008;14(Suppl 7):S103–4.
Louie T, Miller M, Mullane K, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31.
Bartlett J, Gerding D. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S12–8.
Lessa F, Gould C, McDonald L. Current Status of Clostridium difficile Infection Epidemiology. Clin Infect Dis. 2012;55(Suppl 2):S65–70.
Acknowledgements
This study was originally published in Russian in Clinical Microbiology and Antimicrobial Chemotherapy 2017; 19(4): 268-74. The study has been reproduced here in English with kind permission of the publisher, CMAC (www.antibiotic.ru/cmac). The acknowledgements section has been altered from the original publication in accordance with Infectious Diseases and Therapy’s editorial requirements. The authors express their gratitude to the following for the participation in the organisation and conduct of the study: E.V. Volchkova, Z.V.Grigoryevskaya, S.N. Ignatyeva, S.A. Karaylova, I.A. Klyuchnikova, N.K. Kuznetsova, O.S. Lyashenko, T.P. Makedonskaya, N.V. Elionshkaya, E.A. Poluektova, A.Kh. Khubieva, E.P. Yakovenko.
Funding
The study and its publication were funded by JSC (joint stock company) Astellas Pharma. All the authors had copies of the Clinical Study and Statistical Report during preparation of the manuscript and had full access to the clinical study database, related to their own site (investigation centre). The data management (analysis) was performed by CRO, including certified statisticians.
Editorial Assistance
No writing support was provided. Conversis provided support for the translation, which was reviewed and approved by the corresponding author. This support was funded by Astellas.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Stanislav V. Kazakov is an employee of the Medical Department of Astellas Pharma. Natalia V. Dmitrieva, Galina A. Klyasova, Natalia V. Bakulina, Marina A. Sukhina, Sergey V. Zhuravel, Elena A. Belousova, Vladimir T. Ivashkin, Sergey V. Goryunov, Elena A. Prokhorovich, Tatyana R. Kameneva, Aleksey A. Samsonov and Andrey V. Yakovenko received compensation as investigators for the DESCRIPTOR study.
Compliance with Ethics Guidelines
The manuscript included the statement ‘The study was conducted according to the principles of the Declaration of Helsinki as well as Good Epidemiological Practice [Guidelines of the International Society for Pharmaceutical Engineering and Good Pharmacy Practice, 2007], which is a translation from the original Russian publication.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6974294.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Dmitrieva, N.V., Klyasova, G.A., Bakulina, N.V. et al. Prevalence of Clostridium Difficile-Associated Diarrhoea in Hospitalised Patients (Results of a Russian Prospective Multicentre Study). Infect Dis Ther 7, 523–534 (2018). https://doi.org/10.1007/s40121-018-0209-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-018-0209-y