Abstract
Burkholderia pseudomallei (B. pseudomallei), a causative agent of an emerging infectious disease melioidosis, is endemic in the tropical regions of the world. Due to increased international travel, the infection is now also seen outside of the tropics. The majority of patients with identified risk factors such as diabetes mellitus, heavy alcohol use, malignancy, chronic lung and kidney disease, corticosteroid use, thalassemia, rheumatic heart disease, systemic lupus erythematosus and cardiac failure acquire this organism through percutaneous inoculation or inhalation. The clinical manifestations are variable, ranging from localized abscess formation to septicemia. Melioidotic bone and joint infections are rarely reported but are an established entity. The knee joint is the most commonly affected joint in melioidosis, followed by the ankle, hip and shoulder joints. Melioidosis should be in the differential diagnosis of bone and joint infections in residents or returning travelers from the endemic area. Melioidosis diagnosis is missed in many parts of the world due to the lack of awareness of this infection and limited laboratory training and diagnostic techniques. It also mimics other diseases such as tuberculosis. Delay in the diagnosis, or the initiation of appropriate and effective treatment against melioidosis, could worsen the outcome. Initial therapy with ceftazidime, or carbapenem with or without cotrimoxazole is recommended, followed by the oral eradication therapy (based on the antimicrobial susceptibility) with amoxicillin/clavulanic acid or cotrimoxazole. Surgical intervention remains important. This paper reviews current literature on the epidemiology, clinical features, diagnosis, and management of melioidotic bone and joint infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Burkholderia pseudomallei (B. pseudomallei) is a causative agent of a severe and fatal infectious disease which is called melioidosis. B. pseudomallei, a Gram-negative bacterium, is a water and soil pathogen in Eastern Asia and Northern Australia. Melioidosis has emerged as an important cause of morbidity and mortality for the last several decades in the Southeast hemisphere. Melioidosis has expanded its occurrence from the tropics to other parts of the world such as other Asian regions, South America and the Caribbean [1–5]. B. pseudomallei can infect healthy individuals, but it is seen more commonly in patients with co-morbidities such as diabetes mellitus, malignancy and immunosuppression. Clinical manifestations of melioidosis range from latent infection, localized cutaneous lesions, sub-acute pneumonia, bone and joint infections, abscesses in body organs and cranial abscesses to life-threatening septicemia [1, 3, 6, 7].
The clinical presentations of bone and joint infections due to B. pseudomallei are indistinguishable from other infectious causes. Melioidosis should be considered in the differential diagnosis in patients from the disease endemic area, or who are returning from these areas. Early diagnosis can be made using the laboratory and clinical parameters to prompt administration of appropriate antimicrobial therapy, and achieve better prognostic outcome [5]. Mortality in acute severe melioidosis even with appropriate treatment still remains considerably high, ranging from 30% to 47% [8]. This article reviews the microbiology, epidemiology, clinical features, diagnosis, management and aspects of melioidosis particularly associated with bone and joint infections. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Microbiology
Burkholderia pseudomallei, previously known as Pseudomonas pseudomallei, is an aerobic, motile, non-spore forming, intracellular, soil saprophyte which can be found in wet soil and surface water [9, 10]. It is an oxidase-positive, Gram-negative bacillus with bipolar staining, (appears as a safety pin in the Gram stain film) which grows easily on commonly used media in the microbiology laboratory at 37 °C. Variation in the colonial morphology can be noted; smooth colonies appear in young culture, while wrinkled and dry colonies appear in old culture [11, 12]. B. pseudomallei is able to resist hostile conditions such as extreme temperature, nutrient deficiency, acidic and alkaline conditions, dehydration and antiseptics and disinfectants [11, 12]. B. pseudomallei is resistant to different groups of antibiotics, for example, aminoglycosides, penicillins, cephalosporins (first and second generations), and rifamycins.
Epidemiology
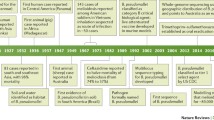
Melioidosis was first diagnosed in intravenous morphine users with septicemia in Burma (now Myanmar) in 1912 by Whitmore and Krishnaswami [13]. The majority of cases are reported from Southeast Asia and Northern Australia, corresponding to tropical latitudes between 20°N and 20°S, and sporadic cases have been reported from Malaysia, Pakistan, Indonesia, Japan, Bangladesh, India, Sri Lanka and Indonesia [3, 14–16]. It is thought that B. pseudomallei may be brought up from a clay layer and distributed in the environment during the rainfall, as it resides in the clay layer 25–30 cm underneath the soil surface [17–19]. Between 75% and 85% melioidosis cases from northeast Thailand and northern Australia occur during the wet season [18]. One study from Malaysia reported that 57% of melioidosis cases occurred during the rainy season, which usually takes place every year between October and April [20]. It is thought that there may be a shift from inoculation to inhalation of B. pseudomallei during the monsoon stormy weather, hurricanes, typhoons and cyclones, as pneumonia is a predominant presenting feature in melioidosis [21].
Bone and joint infections due to B. pseudomallei are a well-recognized entity of this disease. The majority of the melioidotic bone and joint infections are found in the case reports from different regions of the world [5]. One study [22] from an endemic area in Northern Australia reported a 7.6% incidence of bone and joint infections in melioidosis patients, which was much less than a melioidosis case series of bone and joint infections from Thailand (14–27% incidence) [4, 23, 24]. A total of 29% patients with melioidosis in Brunei had bone and joint infections [5].
Modes of Transmission
Hematogenous spread after percutaneous inoculation is thought to be an important mode of transmission in bone and joint infections. The organism may also reach directly from other organs, or from soft tissue infection over bones or joints. Septic arthritis and osteomyelitis, one or both, can be the primary manifestation in patients with melioidosis. Other important routes of spread of melioidosis are ingestion and inhalation especially during heavy rainfall and cyclones [4, 25].
Risk Factors
A number of environmental and patient-related host factors have been defined in several studies from the endemic areas. Many cases have been linked to occupational and recreational exposure to surface water, for example, in rice paddy farmers in Thailand, [26] and outdoor work, landscaping and gardening in Australia [22]. Melioidosis usually affects patients with underlying illnesses such as diabetes mellitus, heavy alcohol consumption, chronic lung disease, renal disease, malignancy, corticosteroid use, thalassemia, previous trauma, rheumatic heart disease and/or cardiac failure, and surgery [4, 22, 27–29]. Other risk factors include splenectomy, aplastic anemia, cystic fibrosis, glucose 6 phosphate dehydrogenase deficiency, and systemic lupus erythematosus (SLE) [1]. Diabetes mellitus is the most important predisposing risk factor, and it increases the risk of melioidosis by 100-fold [19, 22, 23, 29]. The underlying comorbidities lead to immune dysfunction such as impaired polymorphonuclear phagocyte functions, so impaired phagocytic cells fail to clear this organism. It is thought that the immune deficiency increases the risk of melioidosis [30, 31]. One study reported that there is a 5.7 times higher risk of septic arthritis in melioidotic patients with diabetes mellitus, SLE and chronic renal failure [24]. Melioidosis in bone and joint infection is more common in males [4, 23]. This may be the case because males are exposed to B. pseudomallei while working in the rice paddies and they may be more involved in other outdoor activities.
Clinical Features
Melioidotic bone and joint infections remain uncommon and are usually difficult to differentiate from other causative agents such as Staphylococcus, Streptococcus and others, unless microbiologically proven in cultures. Acquisition in bone and joint infections is usually via direct spread through small skin abrasions, wound infections and abscess or hematogenous spread in patients who presented with another primary diagnosis, such as pneumonia or septicemia. There is no specific clinical presentation, and it mimics different forms of osteomyelitis, septic arthritis infections and rheumatoid disorders. Prominent features in septic arthritis are swelling, tenderness, redness, and heat around joints [21, 24]. In melioidotic bone and joint infections, the presentation picture is usually chronic in nature, such as persistent fever rather than shock or respiratory failure, and overall mortality is low in this group [23]. Either single or multiple bone or joint involvement is observed in the past in musculoskeletal infections due to melioidosis. B. pseudomallei mainly causes infections in knee and hip joints (Table 1). It also affects the shoulder and other joints. Increased vascular supply in the metaphyseal regions of long bones helps it spread easily to bones and joints.
One recent study [32] from Australia revealed that 25.4% patients (16/63 episodes) and 31.7% patients with melioidosis (20/63 episodes) presented with septic arthritis and osteomyelitis as the primary illness, respectively. More than one focus of infection was present in more than half of the patients (32/50). In this study, knee (15), tibia (11) and ankle (11) joints were the most affected, followed by metatarsal (4), femur (3), and lumbar spine (3) (Table 1). Teparrakkul et al. [23] from Thailand reported that of 679 patients, 98 had musculoskeletal melioidosis. Among joints, knee joints (41 patients) were the most affected joint; followed by ankle (20), hip (15), and shoulder (10) joints. Among bones, the femur and tibia were more involved than upper limb bones. This study also showed three patients suffering with discitis. The high incidence of knee joint involvement was recorded in these two studies [23, 32]. On the contrary, Kosuwon et al. [24] reported the shoulder joint being more commonly infected by B. pseudomallei. Morse et al. [4] from Northern Australia presented a 20-year study of osteomyelitis and septic arthritis due to B. pseudomallei, in which 41/536 patients with melioidosis had suffered with osteomyelitis or septic arthritis. Of the 41 patients, primary melioidosis was identified in 13 patients with septic arthritis and 7 patients with osteomyelitis while 14 had melioidosis of the bone/joint secondary to primary melioidosis elsewhere. Seven patients showed signs of both bone and joint involvement. Saravu et al. [33] reported 25 culture-confirmed adult cases of melioidosis, of which 48% (12/25) had osteomyelitis or septic arthritis. This proportion was higher than the 14–33% reported in studies from Malaysia and Thailand [23, 24, 34].
Diagnosis
Lack of awareness about the disease, limited laboratory resources to isolate the organism, and confusion with other infectious diseases such as Mycobacterium tuberculosis, may lead to the misdiagnosis of melioidosis. Melioidosis is diagnosed on the basis of the clinical and laboratory parameters, and radiology. The majority of patients have increased or low white blood cell and neutrophil cell counts, increased C-reactive protein or procalcitonin or erythrocyte sedimentation rate, impaired renal or liver functions, and in some cases organ failure. Accurate and timely diagnosis remains crucial to initiate prompt definitive antimicrobial therapy, to reduce the mortality. Several factors play a pivotal role in the laboratory diagnosis of melioidosis: (1) collection of an appropriate sample; for example, needle aspiration from the abscess, such as a psoas abscess, or bone in the case of osteomyelitis, tissue or bone in discitis, tissue from ulcers or wounds, and blood cultures, (2) transportation of the samples in appropriate media, or in a sterile container, to the microbiology laboratory without any delay, (3) handling of the samples by trained and experienced laboratory staff, (4) processing using appropriate media, incubating and using the best methods to identify this organism, (5) use of specific antimicrobial discs which are used as intravenous or oral therapy for the treatment of melioidosis. Breach of any of the above-mentioned steps may lead to low chances of isolation of B. pseudomallei [1, 3, 35–37].
Isolation of B. pseudomallei from the clinical specimens is considered to be the gold standard in the diagnosis of melioidosis. Isolation of B. pseudomallei from clinical samples is regarded as a significant isolate. Use of selective media for processing samples from non-sterile sites is helpful in suppressing commensal organisms, which otherwise can overgrow and give a false-negative result. A modified Ashdown media which contains colistin is currently used in the laboratories for the isolation of this organism [1, 36, 37]. Serology may be helpful in cases of culture-negative results, or in the absence of clinical samples from patients with melioidosis. However, the serology results should be interpreted cautiously in endemic areas, where local populations have raised melioidosis antibody levels. A group of researchers from north eastern Thailand reported that the indirect hemagglutination assay has a 95% sensitivity but the specificity is low (59%). In this study, patients with bacterial infections had a high titer of 1:1280 [38]. One Malaysian group developed an indirect immunofluorescence test using whole-cell antigen for the detection of total antibodies to B. pseudomallei. They found this test quite rapid and reliable [39]. A raised B. pseudomallei antibody titer in healthy individuals is probably due to repeated natural exposure to this saprophytic organism during outdoor activities. Molecular methods, for instance Polymerase Chain Reaction, or Pulsed Field Gel Electrophoresis, are being employed in the clinical and research laboratories; these are less sensitive than gold-standard culture results [40].
Treatment
Normally systemic antimicrobial therapy active against B. pseudomallei, vigorous and repeated washouts, and several extensive debridements of infected bone are the cornerstones of treatment of bone and joint infections. Prompt administration of antibiotics remains important to reduce morbidity and mortality. Inflammatory markers and clinical signs of improvement are used to gauge the response to the specific therapy for melioidotic bone and joint infections. Intravenous antimicrobial therapy should be prolonged for deep-seated infection or complicated infections from four to eight weeks followed by the oral maintenance therapy for a minimum of 12 weeks (Tables 2, 3) [1, 4, 28, 31, 41]. Mortality was quite high before the antibiotic era. In an open-labeled randomized trial, the group compared the efficacy of ceftazidime (120 mg/kg/day) with “conventional therapy” (chloramphenicol 100 mg/kg/day, doxycycline 4 mg/kg/day, trimethoprim 10 mg/kg/day, and sulphamethoxazole 50 mg/kg/day) in the treatment of severe melioidosis. Ceftazidime was associated with 50% (74–37%) reduction in mortality rate than the conventional therapy [42]. Ceftazidime has become the drug of choice for intensive therapy after this study. Ceftazidime, or a member of the carbapenem group, is the drug of choice to treat this infection, followed by oral cotrimoxazole as a monotherapy or amoxicillin–clavulanic acid [41, 43, 44]. On the other hand, Inglis suggests the use of ceftazidime, meropenem or imipenem and trimethoprim–sulfamethoxazole and folic acid in the acute phase followed by any two antibiotics from the group of trimethoprim–sulfamethoxazole, doxycycline, and amoxicillin–clavulanic acid, as eradication therapy in deep-seated infections [11]. One study from Malaysia showed a low-level resistance to the commonly used antibiotics for the treatment of melioidosis. In this study, all isolates (170) of B. pseudomallei appeared sensitive to meropenem and piperacillin/tazobactam, while less than 1% resistance was recorded in ceftazidime, imipenem, and amoxicillin–clavulanic acid. Of 170 isolates, 98 were resistant to ciprofloxacillin [45]. Table 1 summarizes the antibiotic therapy. No specific vaccine is available for melioidosis.
Out-of-hospital intravenous antimicrobial treatment in clinically stable patients, or those who do not require intensive care support or hospitalization, has become popular in many parts of the world. This service saves not only hospital bed days, and healthcare costs, but also healthcare workers’ time. These patients are closely monitored by specialist staff during the treatment period [46, 47]. One study from Southeast Asia looked at outpatient antimicrobial therapy in 56 patients with confirmed melioidosis who were treated in two large tertiary care hospitals. Patients received ceftazidime 100–200 mg/kg/day via a peripherally inserted central catheter (PICC) and elastomeric infuser. Eighty-six percent of patients (47/56) completed the course. Of the nine patients who did not complete the therapy, four patients experienced adverse reactions, two needed surgical intervention, and in three patients the underlying illness worsened. The overall outcome was good [47].
Relapse
High relapse rates in B. pseudomallei infections are mentioned in the literature [4, 48–50]. It remains important to complete therapy (Table 1), including the maintenance therapy, to prevent relapse in melioidosis. Patients treated with appropriate antibiotics require long-term follow-up, as this organism remains latent for up to 26 years in the body. One recent study on melioidotic bone and joint infections reported relapse in 10 patients. Of the 10 patients, seven received less than 4 weeks of antibiotics [32]. The average time between discharge from the hospital and relapse is approximately 21 weeks [49]. A higher relapse rate (30%) has been noted if the overall duration of antimicrobial therapy is less than eight weeks [50]. Relapse should be considered and treated as a first episode [50].
Conclusion
In endemic areas, B. pseudomallei should be considered as one of the causative agents of bone and joint infections because of its rising incidence and high rate of morbidity and mortality if not diagnosed and treated early on. A high index of suspicion of melioidosis is required to make the diagnosis. Isolation of B. pseudomallei from clinical specimens is considered to be the gold standard; however, to achieve this, samples must be processed carefully and in the appropriate media. Treatment of melioidosis affecting bones and joints consists of antimicrobial therapy coupled with surgical management including washouts of joints and debridement of infected bone. Those patients with deep-seated or complicated infections require intravenous antibiotics for 4–8 weeks, followed by oral antibiotics for a minimum of 12 weeks. Ceftazidime is usually the intravenous antibiotic of choice, which is followed by oral therapy such as cotrimoxazole. Some countries are now using outpatient antimicrobial therapy for their clinically stable patients. Unfortunately no vaccine has yet been developed for this disease, which makes the awareness and understanding of melioidotic bone and joint infections, and the need for timely diagnosis and treatment, all the more relevant to microbiologists today.
References
Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416.
Gopalakrishnan R, Sureshkumar D, Thirunarayan MA, Ramasubramanian V. Melioidosis: an emerging infection in India. J Assoc Physicians India. 2013;61:612–4.
Raja NS, Ahmed MZ, Singh NN. Melioidosis: an emerging infectious disease. J Postgrad Med. 2005;51:140–5.
Morse LP, Smith J, Mehta J, Ward L, Cheng AC, Currie BJ. Osteomyelitis and septic arthritis from infection with Burkholderia pseudomallei: a 20-year prospective melioidosis study from northern Australia. J Orthop. 2013;10:86–91.
Pande KC, Kadir KA. Melioidosis of the extremities in Brunei Darussalam. Singap Med J. 2011;52:346–50.
Ng W, Kwan M, Merican A. Melioidotic osteomyelitis treated with antibiotic-calcium hydroxyapatite composite: case report with four year follow up. Singap Med J. 2006;47:714.
Vestal M, Wong E, Milner D, Gormley W, Dunn I. cerebral melioidosis for the first time in the western hemisphere. J Neurosurg. 2013;119:1591–5.
Rajadhyaksha A, Sonawale A, Khare S, Kalal C, Jankar R. Disseminated melioidosis presenting as septic arthritis. J Assoc Physicians India. 2012;60:44–5.
White NJ. Melioidosis. Lancet. 2003;361:1715–22.
Raja NS, White NJ, Simpson AH. Burkholderia pseudomallei (Melioidosis) and B. mallei (Glanders). Chapter in book. Antimicrobe. http://www.antimicrobe.org/new/b89.asp Date accessed: July 2015.
Inglis T. The treatment of melioidosis. Pharmaceuticals. 2010;3:1296–303.
Chaowagul W, Simpson AJ, Suputtamongkol Y, White NJ. Empirical cephalosporin treatment in melioidosis. Clin Infect Dis. 1999;28:1328.
Whitmore A, Krishnaswami CS. An account of the discovery of a hitherto undescribed infective disease occurring among the population of Rangoon. Indian Med Gaz. 1912;47:e262–7.
Puthucheary SD, Parasakthi N, Lee MK. Septicaemic melioidosis: a review of 50 cases from Malaysia. Trans R Soc Trop Med Hyg. 1992;86:683–5.
Rodrigo K, Premaratna R, De Silva E. Melioidosis as a cause of femoral osteomyelitis and multifocal intramuscular abscess around the hip joint in a farmer: a case report. Sri Lankan J Infect Dis. 2013;3:50–4.
Raja NS. Localised melioidosis. J Pak Med Assoc. 2003;53:373–4.
Thomas AD, Forbes-Faulkner J, Parker M. Isolation of Pseudomonas pseudomallei from clay layers at defined depths. Am J Epidemiol. 1979;110:515–21.
Currie BJ, Jacups SP. Intensity of rainfall and severity of melioidosis, Australia. Emerg Infect Dis. 2003;9:1538–42.
Suputtamongkol Y, Hall AJ, Dance DA, Chaowagul W, Rajchanuvong A, Smith MD, et al. The epidemiology of melioidosis in Ubon Ratchatani, northeast Thailand. Int J Epidemiol. 1994;23:1082–90.
Raja NS. Cases of melioidosis in a university teaching hospital in Malaysia. J Microbiol Immunol Infect. 2008;41:174–9.
Currie B. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med. 2015;36:111–25.
Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:e900.
Teparrakkul P, Tsai JJ, Chierakul W, et al. Rheumatological manifestations in patients with melioidosis. Southeast Asian J Trop Med Public Health. 2008;39:e649–55.
Kosuwon W, Taimglang T, Sirichativapee W, Jeeravipoolvarn P. Melioidotic septic arthritis and its risk factors. J Bone Jt Surg [Am]. 2003;85:e1058–61.
Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18(2):383–416. (Erratum, Clin Microbiol Rev 2007;20:533).
Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035–44.
Raja NS, Singh NN. Fatal septicaemic melioidosis in young adult. Infect Dis J. 2005;14:137–8.
Pandey V, Rao SP, Rao S, Acharya KK, Chhabra SS. Burkholderia pseudomallei musculoskeletal infections (melioidosis) in India. Indian J Orthop. 2010;44:216–20.
Parameswaran U, Baird RW, Ward LM, Currie BJ. Melioidosis at Royal Darwin Hospital in the big 2009–2010 wet season: comparison with the preceding 20 years. Med J Aust. 2012;196:345–8.
Currie BJ. Melioidosis: an important cause of pneumonia in residents of and travellers returned from endemic regions. Eur Respir J. 2003;22:542–50.
Subhadrabandhu T, Prichasuk S, Sathapatayavongs. Localized melioidotic osteomyelitis. J Bone Joint Surg (Br). 1995;77:445–9.
Shetty R, Mathew M, Smith J, Morse LP, Mehta JA, Currie BJ. Management of melioidosis osteomyelitis and septic arthritis. Bone Joint J. 2015;97:277–82.
Saravu K, Mukhopadhyay C, Vishwanath S, Valsalan R, Docherla M, Vandana KE, Shastry BA, Bairy I, Rao SP. Melioidosis in southern India: epidemiological and clinical profile. Southeast Asian J Trop Med Public Health. 2010;41:401–9.
Ahmad S, Azura L, Duski S, Aziz MY. Melioidosis: a retrospective review of orthopaedic manifestation. Malaysia Orthopaedic J. 2009;3:53–4.
Inglis T, Rolim D, Rodriguez. Clinical guidelines for diagnosis and management of melioidosis. Rev Inst Med Trop S Paulo. 2006;48:1–4.
HowardK Inglis T. Novel selective medium for isolation of Burkholderia pseudomallei. J Clin Microbiol. 2003;41:3312–6.
Lipsitz R, Garges S, Aurigemma R, et al. Workshop on treatment of and postexposure prophylaxis for Burkholderia pseudomallei and B. mallei infection. Emerging Infect Dis. 2010;2012(18):e2.
Chaowagul W, White NJ, Dance DA, Wattanagoon Y, Naigowit P, Davis TM, Looareesuwan S, Pitakwatchara N. Melioidosis: a major cause of community-acquired septicaemia in northeastern Thailand. J Infect Dis. 1989;159:890–9.
Vadivelu J, Puthucheary SD. Diagnostic and prognostic value of an immunofluorescent assay for melioidosis. Am J Trop Med Hyg. 2000;62:297–300.
Richardson LJ, Kaestli M, Mayo M, et al. Towards a rapid molecular diagnostic for melioidosis: comparison of DNA extraction methods from clinical specimens. J Microbiol Methods. 2012;88:179–81.
Dance D. Treatment and prophylaxis of melioidosis. Int J Antimicrob Agents. 2014;43:310–8.
White NJ, Dance DA, Chaowagul W, Wattanagoon Y, Wuthiekanun V, Pitakwatchara N. Halving of mortality of severe melioidosis by ceftazidime. Lancet. 1989;1989(2):697–701.
Kamal M, Rhani SA, Norbaya M. Melioidosis septic arthritis: report of two cases. Rawal Med J. 2011;36:62–5.
Limmathurotsakul D, Peacock S. Melioidosis: a clinical review. Br Med Bull. 2011;99:125–39.
Norazah A, Rohaidah H, Azura M. The in vitro antibiotic susceptibility of Malaysian isolates of Burkholderia pseudomallei. Int J Microbiol. 2013;2013:121845.
White HA, Davis JS, Kittler P, Currie BJ. Outpatient parenteral antimicrobial therapy-treated bone and joint infections in a tropical setting. Intern Med J. 2011;41:668–73.
Seetoh T, Lye DC, Cook AR, Archuleta S, Chan M, Sulaiman Z, Zhong L, Llorin RM, Balm M, Fisher D. An outcomes analysis of outpatient parenteral antibiotic therapy (OPAT) in a large Asian cohort. Int J Antimicrob Agents. 2013;41:569–73.
Currie BJ, Fisher DA, Anstey NM, Jacups SP. Melioidosis: acute and chronic disease, relapse and re-activation. Trans R Soc Trop Med Hyg. 2000;94:301–4.
Mays EE, Ricketts EA. Melioidosis: recrudescence associated with bronchogenic carcinoma twenty-six year following initial geographic exposure. Chest. 1975;68:261–3.
Chaowagul W, Suputtamongkol Y, Dance DA, Rajchanuvong A, Pattara-arechachai J, White NJ. Relapse in melioidosis: incidence and risk factors. J Infect Dis. 1993;168:1181–5.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
N. S. Raja and C. Scarsbrook have nothing to disclose.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Raja, N.S., Scarsbrook, C. Burkholderia Pseudomallei Causing Bone and Joint Infections: A Clinical Update. Infect Dis Ther 5, 17–29 (2016). https://doi.org/10.1007/s40121-015-0098-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-015-0098-2