Abstract
Spinal muscular atrophy (SMA) is a neuromuscular disease caused by deletions or mutations in the survival of motor neuron 1 (SMN1) gene resulting in reduced levels of SMN protein. SMN protein is produced by cells throughout the body, and evidence suggests that low SMN protein can have systemic implications, including in male reproductive organs. However, a paucity of research exists on this important topic. This article will discuss findings from non-clinical studies on the role of SMN in the male reproductive system; additionally, real-world observational reports of individuals with SMA will be examined. Furthermore, we will review the non-clinical reproductive findings of risdiplam, a small-molecule SMN2 splicing modifier approved for the treatment of SMA, which has widespread distribution in both the central nervous system and peripheral organs. Specifically, the available non-clinical evidence of the effect of risdiplam on male reproductive organs and spermatogenesis is examined. Lastly, the article will highlight available capabilities to assess male fertility as well as the advanced reproductive technologies utilized to treat male infertility. This article demonstrates the need for further research to better understand the impacts of SMA on male fertility and reproduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Animal studies have shown that survival of motor neuron (SMN) protein plays a key role in spermatogenesis; however, clinical data on the reproductive system of men with spinal muscular atrophy (SMA) are lacking |
Men with Type 1 and 2 SMA have a significant rate of unilateral and bilateral undescended testis |
Negative reproductive effects of SMN2 splicing modifiers on spermatogenesis were stage specific to the postpubertal event of meiosis 1 and were reversible in male animals |
These effects are expected to be translatable to men with SMA treated with SMN2-splicing modifiers |
Advancements in assisted reproductive technologies will aid men with SMA who also have male factor infertility in achieving parenthood |
Introduction
Spinal muscular atrophy (SMA) is a progressive neuromuscular disease that affects individuals with a broad age range and spectrum of disease severity [1]. SMA is caused by reduced levels of survival of motor neuron (SMN) protein due to deletions and/or loss of function mutations in the SMN1 gene [1, 2]. Most humans carry a second gene, SMN2, which has a single-base substitution that can cause exclusion of exon 7 during splicing, leading to the production of SMNΔ7 [3], a shortened version of SMN protein which is unstable and rapidly degrades [4]. Approved disease-modifying therapies (DMTs) for SMA aim to increase the level of SMN protein by either increasing the amount of functional SMN protein produced by SMN2 or delivering a copy of SMN1 [5,6,7].
SMN protein is ubiquitously produced by human cells, and reduced levels of SMN protein throughout the body are thought to play a vital role in the disease pathophysiology of SMA [8, 9]. While the effect of reduced SMN protein on motor neurons is well established, SMA is considered to be a systemic disease with widespread implications [10, 11]. Non-neuromuscular phenotypes have been observed in individuals with SMA, including those specific to the cardiovascular, gastrointestinal, metabolic, and reproductive systems [11, 12]. It is important to note that high levels of SMN protein are produced in the male reproductive system of mice [13,14,15] and humans [16, 17].
With more patients living longer because of the availability of DMTs for SMA, the number of individuals considering family-building options will likely increase [18]. How SMA may affect fertility, particularly in male individuals, is not well understood, nor has it been thoroughly investigated. A variety of genetic and acquired variables, as well as lifestyle and environmental risk factors, may impact male fertility [19]. A recent meta-analysis reported the worldwide prevalence of infertility in individuals of reproductive potential across the general population as ranging from 12.6 to 17.5% [20]. The limited data on the fertility status of men with SMA make it difficult to understand the impact that DMTs may have on the male reproductive system.
The authors assess the available published literature on the potential effects of SMA on male fertility, the non-clinical effects of selected oral SMN2 splicing modifiers on male fertility, and the tools and technologies available for fertility assessment and treatment.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
SMA and Male Fertility
Experimental Studies
Although the relationship between SMA and male fertility in humans is not well established, animal models suggest that SMA does negatively impact male reproductive competency. In experimental mouse models that exhibit a deficiency in SMN protein, a broad range of negative reproductive consequences have been observed [13, 14, 21]. In the most severe animal models, low SMN expression was linked to impairments in male reproductive organ development (reduced testis size), defective sperm maturation, degenerated seminiferous tubules, and a reduction in sperm count [15]. Reduced male fecundity was observed, with only two out of eight male SMA mice able to successfully sire a litter compared with all wild-type male mice mated [15]. It has been suggested that high expression of SMN protein in the testis is due to its critical role in the development and maintenance of male germ cells, in particular for spermatogonia and the initial stages of sperm development [14, 15].
Experiments conducted in a mouse model of SMA in which mice have a knockout of the smn gene but express a human SMN2 transgene reported higher expression of full-length SMN2 mRNA in testis compared with other tissues, indicating there may be a specific mechanism facilitating the inclusion of exon 7 in SMN2 mRNA in the testis [15, 22]; however, this has not been confirmed in men with SMA. The presence of a specific mechanism for SMN2 splicing in testes might indicate that the SMN2 gene and the SMN protein produced from SMN2 may have a unique and pivotal role in human spermatogenesis. This specific SMN gene is evolutionarily isolated to humans and shows high expression in the testes [16, 17].
In humans, an in vitro analysis of pluripotent stems cells derived from men with the most severe form of infertility, azoospermia (i.e., a sperm count of zero), found low expression of SMN1 compared with stem cells from healthy controls [21], identifying a link between SMN1 expression and azoospermia. Expression of SMN protein in pluripotent stem cells derived from patients with azoospermia resulted in upregulation of germ-cell markers and induced differentiation into primordial germ cell-like cells [21]. This in vitro human study provides additional evidence that SMN protein plays an important role in human spermatogenesis.
Observational Studies
An analysis of insurance claims in the US healthcare system has identified a higher prevalence of testicular hypofunction and male infertility in individuals with adult‑onset SMA compared with matched controls [23]. Lipnick et al. reported that males living with SMA who were diagnosed between the ages of 21 and 65 (N = 196) (suggestive of milder disease) were more likely to have had a diagnosis of testicular hypofunction (odds ratio = 2.4, p = 0.02) or male infertility (odds ratio = 5.1, p = 0.01) [23]. This analysis was based upon insurance claims covering a period (January 2008 to October 2015) primarily before the approval of DMTs.
In a recent retrospective study by Ribault et al. from the Lyon Neuromuscular Disease Center, patients with SMA were followed across a period covering the availability of DMTs (2012–2022) [24]. Among seven adult males who attempted to undergo sperm cryopreservation prior to initiating risdiplam therapy, three (43%) had a complete absence of sperm in the ejaculate, a condition termed azoospermia. This extraordinarily high prevalence rate of azoospermia (43%) is far beyond the incidence of 1% for the male general population or the rate of 10% observed in men with infertility [25].
Two real-world observational reports of individuals with SMA have reported high rates of cryptorchidism (undescended testes) in patients with Type 1 or 2 SMA [26, 27]. Brener et al. assessed 27 male patients with Types 1–3 SMA, reporting bilateral cryptorchidism in 60% of patients with Type 1 SMA (n = 10) and 30% in patients with Type 2 SMA (n = 10), with a mean age at diagnosis of cryptorchidism of 6.4 ± 4.4 (range 1.2–14.3) years [26]. All patients with Type 3 SMA (n = 7) had descended testes. In a study of patients with Type 1 SMA, Bach et al. reported bilateral cryptorchidism in 52% (13 of 25) of male patients whose testes were examined, and unilateral cryptorchidism was observed in an additional two male patients [27]. The prevalence of cryptorchidism in males with SMA in observational studies is significantly higher than the incidence (2–4%) reported in the general population for full-term males [28].
Testicular descent during embryonic development requires intra-abdominal muscles to provide adequate pressure to facilitate the migration of the testes from the abdomen into the scrotum [28, 29]. It is suspected that weakness in the intra-abdominal muscles could inhibit or prevent the testes from properly descending, thus leading to cryptorchidism. As muscle weakness is more profound in severe forms of SMA, the risk of cryptorchidism is significantly higher in males with early-onset SMA.
Effects of Cryptorchidism on Fertility
Properly descended testes maintained in a cooler environment within the scrotum (~ 4 °C below body temperature) are necessary for optimal spermatogenesis [28, 29]. Elevated testes temperatures are associated with male infertility and impaired semen quality [28, 29]. In patients with cryptorchidism, the undescended testes remain near or at body temperature. If the testes do not descend by 6 months of age, then surgical intervention to bring the testis into the scrotum is ideally recommended within 12–18 months [30]. Without surgical correction, children with bilateral cryptorchidism will become permanently sterile. Therefore, in children with uncorrected bilateral cryptorchidism who have become sterile, the impact of any SMA DMT on male fertility may not be relevant. Cryptorchidism is also associated with a significantly increased risk (3.7–7.5 times higher) for testicular cancer [31], and surgical placement of the undescended testis in the scrotum is often performed to preserve testosterone production as well as providing access for the ongoing physical examinations required for long-term cancer screening. As men with SMA are expected to live longer with DMTs, it is imperative that urological surveillance and monitoring of undescended male gonads for testis cancer with advanced imaging be undertaken.
Summary of SMA and Male Fertility
These studies collectively demonstrate the critical nature of SMN protein in the male reproductive system. In animal studies, low levels of SMN protein in the testis showed a detrimental effect on the development of male organs, a reduction in fertility, and diminution of the spermatogonia germ-cell population [13,14,15, 21].
In a study of healthcare usage, significantly higher rates of infertility and testicular hypofunction were noted in men with adult-onset SMA where symptoms are usually milder compared with infantile-onset SMA. Furthermore, in a small cohort of seven adult men with SMA who attempted sperm cryopreservation prior to initiating risdiplam therapy, 43% had no sperm in the ejaculate. In more severe forms of SMA, there is also a markedly increased prevalence of cryptorchidism. Collectively, these findings demonstrate the potential for a direct and negative impact of SMA on the male reproductive system, and men with SMA are at a higher risk for reduced fertility.
SMN2 Splicing Modifiers and Male Fertility
Risdiplam and Male Fertility
Prior to the development of DMTs, treatment of SMA was focused primarily on the management of disease symptoms driven by the loss of motor neurons and improvements in standard of care [32]. Risdiplam is one of three DMTs available for SMA and is approved for the treatment of pediatric and adult patients with SMA [5, 33]. Risdiplam is an oral pre-mRNA splicing modifier that promotes the inclusion of exon 7 in SMN2 mRNA to produce stable SMN protein [34, 35]. As a small molecule, risdiplam was specifically designed to distribute evenly in the body, including the central nervous system. This leads to increased levels of functional SMN protein in tissues throughout the body [36], i.e., in the central nervous system and elsewhere, including the testes. Another small molecule with a similar mechanism of action, known as RG7800, was evaluated in patients with SMA when risdiplam was still in preclinical development [37]. In trials of healthy adults and patients with SMA, RG7800 increased SMN protein levels; however, studies in patients were put on hold because of safety findings in animal toxicology studies [38]. Due to improvements in drug metabolism (suitable half-life and wide tissue distribution), improved in vitro potency on SMN2 splicing, and a favorable preclinical safety profile, risdiplam was selected for subsequent clinical development [35, 39]. Risdiplam shows high selectivity to two binding sites in exon 7 of SMN2 pre-mRNA, namely the exonic splicing enhancer 2 and a 5’ splice site [39, 40]. The combination of binding to exonic splicing enhancer 2 and the 5’ splice site provides risdiplam high selectivity to SMN2 pre-mRNA [40].
During the drug development process, non-clinical toxicologic findings were reported in male germ cells of animals exposed to risdiplam and RG7800. An analysis of risdiplam, RG7800, and related SMN2 gene splicing compounds identified splicing events in genes other than SMN2 [35] as animals do not have the SMN2 gene. In a few genes, similar exon inclusion events were seen in the mRNA transcripts as with SMN2 in human cells at drug concentrations relevant for the male germ-cell target organ and other tissues with toxicologically relevant findings in animal studies. Although it remains difficult to attribute such tissue-specific effects to any single off target, it was plausible to focus on Forkhead Box M1 (FOXM1) and MAP kinase-activating death domain protein (MADD) among the affected genes, as the toxicologically relevant features were seen exclusively in proliferating and/or self-renewing tissues [35, 39]. Of the affected genes and their known exon inclusion variants, only FOXM1 and MADD are associated with regulation of the cell cycle and apoptosis [35, 39]. FOXM1 can be considered of particular relevance to male fertility due to its expression pattern in male reproductive tissues and across a specific stage of spermatogenesis [41, 42].
Effects of SMN2 Splicing Modifiers on Secondary Splice Targets
The FOXM1 gene produces several splice variants, which are present in animals and humans (see Table 1). These include a transcriptionally inactive FOXM1a variant with exon A2 and transcriptionally active variants FOXM1b and FOXM1c, which lack exon A2 [43,44,45]. FOXM1 is a transcription factor that regulates genes that control G1/S (interphase of the cell cycle). Thus, the FOXM1b/c isoforms promote the cell cycle, whereas the FOXM1a variant results in a stop of the cell cycle. Depending on the stage of the cell cycle, interference of FOXM1 or these splice variants can impact mitosis and meiosis. Accordingly, changes in FOXM1 splice variants are expected to interfere with spermatogenesis. An additional splice target, MADD, is likely responsible for the induction of apoptosis observed as cytoplasmic vacuoles because of increased expression of a pro-apoptotic splice variant known as IG20 [35, 46].
Small-molecule SMN2 pre-mRNA splicing modifiers can interact with the FOXM1 mRNA transcript by upregulating the FOXM1a variant via exon inclusion with concomitant downregulation of the FOXM1b/c variants. In vitro experiments of SMN2 splicing modifiers using mouse cell lines and human cells derived from patients with SMA showed evidence of an increased frequency of micronucleated cells and an increase in apoptosis (manifested as cells with large cytoplasmic vacuoles potentially due to incomplete apoptotic processes), which likely indicated changes in the expression of FOXM1 and MADD splice variants [35]. Experiments using cells derived from patients with SMA demonstrated splicing impacts in the mRNA transcripts of secondary splice targets in human genes after treatment with SMN2 splicing modifiers [35].
In non-clinical in vivo experiments of rats and mice with both RG7800 and risdiplam, similar concomitant up- and downregulation of different FOXM1 splice variants were observed which caused mitotic arrest and appeared as micronucleated cells [35]. In monkey studies with RG7800, FOXM1b/c was downregulated in a single monkey at the highest dose; however, no effects were observed at lower doses [47]. In another study, FOXM1b/c was downregulated in five of seven monkeys after treatment with RG7800, and FOXM1a was upregulated in the remaining two monkeys [47]. These changes in the expression of splice variants of this specific cell cycle gene likely resulted in damage to sperm-producing tissues of rats and monkeys in non-clinical experiments with RG7800 and risdiplam [47], with stage-specific and reversible effects as outlined below.
Effects of SMN2 Splicing Modifiers on Spermatogenesis
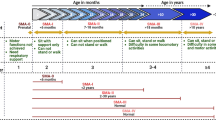
SMN2 splicing modifiers can impact spermatogenesis, which is the process where new sperm are produced in the seminiferous tubules of the testes [48, 49]. Spermatogenesis can be divided into three stages: the mitotic stage, where spermatogonia (2n) undergo mitosis to maintain the stem cell pool and differentiate into primary spermatocytes; the meiotic stage (which begins at puberty), where primary spermatocytes (2n) divide into secondary spermatocytes (n); and spermiogenesis, where mature sperm are produced [48]. SMN1 is critical for the preservation of spermatogonia and paramount for prepubertal and postpubertal germ cell survival. In contrast, FOXM1 is highly expressed in spermatocytes during meiosis 1, a process which only occurs postpuberty, where primary spermatocytes differentiate into secondary spermatocytes (Fig. 1). Interactions of SMN2 splicing modifiers with FOXM1 during meiosis 1 can interrupt the development of mature sperm and thus negatively impact male fertility [50].
Proposed mechanism of off-target effects of SMN2 splicing modifiers on spermatogenesis. Spermatogenesis takes place over three stages: the mitotic stage, where spermatogonia (2n) undergo mitosis to maintain the stem cell pool and differentiate into primary spermatocytes; the meiotic stage (which beings during puberty), where primary spermatocytes (2n) divide into secondary spermatocytes (n); and spermiogenesis, where mature sperm are produced. SMN protein is highly expressed in spermatogonia and is essential for normal spermatogenesis. The impact of SMN2 pre-mRNA splicing modifiers is proposed to impact spermatocytes during meiosis 1 as a result of splicing changes in FOXM1. FOXM1 Forkhead Box M1, SMN survival of motor neuron
Evidence of Stage-specific Degeneration During Spermatogenesis
A staging study of monkeys exposed to RG7800 was conducted to determine whether treatment affected specific cells and/or specific stages of spermatogenesis [35]. Microscopic analysis of testicular tissue samples showed evidence of stage-specific degeneration in the seminiferous tubules and the presence of micronucleated cells and cytoplasmic vacuoles but mature sperm cells were still observed (Fig. 2) [47]. The presence of these micronucleated cells (and vacuoles) were similar to those described in in vitro experiments reported by Ratni et al. [35]. Testicular degeneration was observed to be specific to germ-cell maturation, and the arrest occurred during stages of spermatogenesis after meiosis 1 (postpuberty), with no impact on spermatogonia observed [35, 47]. More specifically, germ-cell degeneration was observed almost exclusively in spermatocytes during meiosis 1, a stage when FOXM1 is highly expressed, and in tissue where alternative splicing of FOXM1 was observed [47].
Histology of testicular tissue in a non-clinical monkey study. a A schematic of the process of spermatogenesis within the seminiferous tubules. Image adapted from Allais-Bonnet A and Pailhoux E (2014) Role of the prion protein family in the gonads. Front. Cell Dev. Biol. 2:56. b Testis sample from a control animal; note a layer of spermatocytes (arrows). c Testis tissue from a cynomolgus monkey dosed at 6 mg/kg/day of RG7800. The seminiferous tubules depict an insult to the specific spermatocyte layer; arrows denote germ cells with abnormal morphology and vacuoles at a germinal epithelial location compatible with spermatocytes. Note that mature sperm can be observed in the center of the tubule in both images. Histology images reproduced from Reproductive Toxicology, Vol 118, Mueller L. et al. Reproductive findings in male animals exposed to selective survival of motor neuron 2 (SMN2) gene splicing-modifying agents. Copyright 2023, with permission from Elsevier. FOXM1 Forkhead Box M1
Evidence of Reversibility of SMN2 Splicing Modifiers
The impact of RG7800 and risdiplam on male reproductive tissues was further investigated in non-clinical studies in which animals were exposed to the drug and then assessed after a drug-free recovery period. In non-clinical studies of rats, after a 28- to 56-day recovery period, full reversibility of germ-cell degeneration was observed in half of the rats assessed following exposure to either risdiplam or RG7800 [47]. Consistent with the reversibility of germ-cell degeneration, male rats exposed to risdiplam for 13 weeks and allowed to recover for 8 weeks were able to breed successfully and did not show impaired fertility when paired with non-treated females [47]. Monkeys dosed with RG7800 and then allowed to recover for at least 55 days did not show any impaired testicular findings and did not demonstrate alternative splicing of FOXM1 and MADD [47]. No testicular degeneration was observed in a longer-term study where monkeys were exposed with RG7800 for 39 weeks and allowed to recover for 22 weeks [47]. No testicular findings were reported in any studies involving immature prepubertal monkeys [47].
The period of recovery following drug withdrawal occurs over a short timescale relative to the length of the spermatogenic cycle (56 days in rats [51]; 42 days in monkeys [52]). This contrasts with the long-lasting or at times irreversible effects on fertility observed following exposure to cytotoxic agents used in chemotherapy or radiotherapy [53]. The short recovery period observed with SMN2 splicing modifiers is consistent with the proposed stage-specific mechanism of action, which implies minimal or no damage to the primary germ-cell population of spermatogonia. Damage induced by cytotoxic agents, such as chemotherapy or radiotherapy, usually damages all stages of spermatogenesis including spermatogonia and thus results in long-term or permanent damage that can reduce sperm counts, often to azoospermic levels [54]. The time course of damage from cytotoxic agents depends on the cell types impacted, with the spermatogonia stem cells being most sensitive to damage and their loss resulting in the most severe and long-lasting damage [54]. It is important to note that damage to spermatogonia was not evident in any of the non-clinical studies of SMN2 splicing modifiers reported by Mueller et al., including studies in prepubertal monkeys [47].
Summary of Findings from Non-clinical Animal Studies
These findings provide evidence that the effects of oral SMN2 pre-mRNA splicing modifiers were reversible in non-clinical animal experiments. As SMN2 is absent in animals, the effects are attributed to off-target splicing events. The stage-specific nature of the damage to spermatocytes in meiosis 1, with no evidence of damage to spermatogonia, suggests the drug exposure did not impact the spermatogonia stem cell line. Following cessation of drug exposure, the impact on FOXM1 splicing is removed, and spermatogonial division and sperm maturation appear to resume unimpaired.
Assessment and Preservation of Male Fertility
Treatment and Technological Advancements
With the availability of multiple therapies for SMA, many patients are now living longer and have or will consider family-building opportunities. As the effects of SMA disease progression or SMN2 splicing modifiers are not fully elucidated, it has been recommended for male patients to consider options to screen and preserve fertility. It is also important to highlight that infertility in the general population has been estimated as high as 17.5% worldwide [20]. This section provides an overview of several advancements, recommendations, and options to assess and optimize fertility, particularly in male patients who may be on treatment or have experienced infertility.
Infertility is defined by the failure to achieve pregnancy following a 12-month period of regular unprotected sexual intercourse [55], which can be caused by a variety of factors in either the male or female reproductive system. The American Urological Association/American Society for Reproductive Medicine recommends evaluating both partners concurrently per their guidelines for infertility [56]. For males, the American Urological Association/American Society for Reproductive Medicine evaluation incorporates both a male reproductive history and a semen analysis that measures key parameters including semen volume and sperm concentration, motility, and morphology. This initial assessment should guide the physician on determining baseline male fertility status, consider additional testing if needed, and provide the most appropriate and targeted therapy.
Men with male factor infertility now have many options for assessing and managing their fertility [56]. Historically, a standard semen analysis would require the patient to attend a fertility clinic and produce an ejaculate onsite. For many men this activity was psychologically challenging and created a barrier and delay for a proper fertility evaluation [57]. This coupled with financial constraints and lack of adequate access to fertility care can make navigating infertility challenging for couples. Male infertility has traditionally been overlooked and often stigmatized and may be a barrier to individuals accessing available resources [58, 59]. In addition, people with disabilities may experience greater difficulties in accessing reproductive services, and specifically for men with SMA upper limb weakness may limit their ability to produce an ejaculate.
Fortunately, recent advances in fertility telehealth, home male fertility testing, and sperm cryopreservation techniques have emerged, and their utilization and acceptance have dramatically accelerated. It should be noted that there are wide differences in the availability of and access to services both within and between countries. These advances have included convenient technologies to test semen parameters at home which can provide a preliminary general assessment of sperm concentration as well as newer technologies that provide a real-time video and determine motile sperm concentration [60]. Home-collected samples can also be maintained using optimized containers and buffered with media which then enables them to be shipped to a centralized andrology laboratory for a complete semen analysis and possible cryopreservation [61]. These diagnostic advances have been complemented by the broad acceptance and proven benefit of fertility telehealth services. These developments are uniquely applicable to the specific population of men with SMA where mobility and access to fertility care are relevant and emerging issues.
For male patients who find it difficult to produce a semen sample, particularly among men who are experiencing physical weakness, techniques such as vibratory stimulation can be utilized [62]. For patients who are unable to ejaculate or upon semen analysis have no viable or detectable sperm in their semen, numerous surgical sperm retrieval techniques can be used to extract mature sperm which can then be utilized in conjunction with in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) [63].
When appropriate, sperm cryopreservation is considered the gold standard for male fertility preservation [64], and options for semen samples to be collected at home and then transported to centralized facilities are available. Cryopreservation of multiple vials as well as a post-thaw test of the sample is recommended at the time of initial cryopreservation to establish confidence that the sperm will survive future cryo-thaw cycles. Testing for communicable diseases is performed at the time of cryopreservation to enable the sperm to be utilized in the future with assisted reproductive technologies (ARTs). A discussion or meeting between the patient and a fertility specialist is recommended to discuss fertility preservation options and implications as well as review the current quality and viability of the cryopreserved semen samples.
ARTs are the mainstay and have been successfully utilized to treat male factor infertility. These include intrauterine inseminations and IVF with ICSI, which can be used to markedly improve the chances of successful conception. ICSI has become the gold standard for treating male factor infertility during in vitro fertilization as it offers the profound benefit of requiring only a single viable sperm cell to be directly injected under a microscope and using advanced reproductive technologies into every retrieved oocyte [65].
Depending on the resources available to patients and healthcare professionals, in this population of men with SMA, a pre-pregnancy genetic carrier screening panel for both partners and genetic counseling has been recommended for couples considering pregnancy [66]. If both partners have mutations in SMN1 and IVF is utilized, the embryos created can subsequently undergo preimplantation genetic testing for monogenic/single gene defects (PGT-M).Embryos that are homozygous or compound heterozygous for SMN1 mutations and at risk of developing SMA can be screened out [67]. PGT-M for single gene disorders is limited by the incredibly small amount of DNA obtained in an embryo biopsy where the usual techniques for analysis of the SMA gene are not possible. As a result, PGT-M for SMA requires the creation of a custom genetic test using linkage analysis prior to beginning IVF treatment. To establish informative linkage markers, DNA from both the sperm and oocyte contributors and their biological parents (or offspring, if any) is typically required to facilitate test creation. It is now also more common for couples to consider pre-emptively undergoing IVF/ICSI, PGT testing, and embryo-cryopreservation to optimize current and future family-building opportunities [68, 69].
Discussion
There are robust animal data highlighting the importance of SMN protein for normal spermatogenesis, male reproductive organ development, and male fertility [13,14,15, 21]. Evidence from animal models of SMA demonstrates abnormalities in male reproduction which affect both sperm development and fertility [15]. There is limited information on how SMA may impact the male reproductive system in humans. Higher rates of male infertility have been reported among healthcare claims from patients with SMA [23], and higher prevalence of azoospermia has also been observed among men with SMA [24]. Markedly higher rates of cryptorchidism have been observed in males with Type 1 and Type 2 SMA, and, when bilateral and not surgically corrected, results in future sterility [26].
Effects of SMN2 splicing modifiers on male sperm cell production were identified in non-clinical toxicologic studies in animals [35, 47]. Impacts on spermatogenesis isolated to postpubertal sperm maturation differentiation are proposed to be the result of off-target effects from oral SMN2 splicing modifiers primarily via a cell cycle controlling gene, FOXM1, during spermatogenesis. This insult appears to be cell stage specific to the postpubertal maturation event of meiosis 1 and does not appear to impair the spermatogonia germ-cell line [47]. Consistent with the proposed mechanism of action, these effects were observed to be reversible following cessation of exposure to SMN2 splicing modifiers. Furthermore, in non-clinical experiments, exposure to SMN2 splicing modifiers did not result in a complete arrest of sperm production. Further research is needed to explore if there is a benefit from restoration of and/or increasing the levels of SMN protein in testicular tissues by SMN2 splicing modifiers that can reach the male testis.
Implications for Human Patients
In the absence of any clinical data from human patients, the effects of SMN2 splicing modifiers on fertility in male patients are not fully understood. Due to the conserved nature of these secondary splice targets between species, the testicular impacts and the reversible nature observed from the animal studies would be expected to be translatable to humans [47]. It is important to note these effects of risdiplam are relevant to post-pubescent males with SMA, as the observed impairments in spermatogenesis are specific to postpubertal meiosis 1. Although these data suggest normal fertility function might be restored within 4 months (encompassing the length of the human sperm cycle, drug transit time, and six half-lives of the drug), the US Prescribing Information and European Summary of Product Characteristics contain recommendations that male patients with SMA who desire to father a child may consider sperm preservation before commencing risdiplam treatment [5, 33]. Physicians should be aware of these recommendations and refer to the following section for a discussion of procedures that can assess or preserve sperm in patients with SMA. Although the full impact of SMN2 splicing modifiers on the male reproductive system in humans is unknown, at the time of this writing the authors have been made aware of three reports of men with SMA who have conceived while treated with risdiplam.
Conclusions
Current understanding of how SMN protein impacts the male reproductive system in individuals with SMA is limited by the lack of research into this aspect of the disease; however, there are robust animal data demonstrating the importance of SMN protein in spermatogenesis and testicular function. Evidence on the impact of SMA on male fertility in humans primarily comes from observational reports that did not seek to identify the underlying mechanistic reasons. Yet, some additional credible mechanistic/experimental evidence with human male germ cells in vitro is available to attribute a significant role of SMN protein in human spermatogenesis.
Male patients of reproductive age should be counseled about the potential effects of treatment and may consider sperm preservation prior to starting treatment or after a sufficient treatment-free period of 4 months [33]. With increased access to male fertility testing via telehealth and home-collection capabilities, there are more options for men with SMA to explore their fertility status. Significant advances in assisted reproductive technologies such as IVF, ICSI, and PGT-M will enable many men with SMA and male factor infertility to achieve their hope of parenthood.
Further research is needed to better understand the effects of SMA on the human male reproductive system and male fertility and the potential impact resulting from available DMTs.
References
Mercuri E, Pera MC, Scoto M, Finkel R, Muntoni F. Spinal muscular atrophy—insights and challenges in the treatment era. Nat Rev Neurol. 2020;16(12):706–15.
Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–65.
Monani UR, Lorson CL, Parsons DW, Prior TW, Androphy EJ, Burghes AH, McPherson JD. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum Mol Genet. 1999;8(7):1177–83.
Burnett BG, Munoz E, Tandon A, Kwon DY, Sumner CJ, Fischbeck KH. Regulation of SMN protein stability. Mol Cell Biol. 2009;29(5):1107–15.
Genentech, EVRYSDI® (risdiplam) US prescribing information, 2020. https://www.gene.com/download/pdf/evrysdi_prescribing.pdf. Accessed Mar 2024.
Biogen, SPINRAZA (nusinersen) US prescribing information, 2020. https://www.spinraza.com/PI. Accessed Mar 2024.
AveXis Inc., ZOLGENSMA® (onasemnogene abeparvovec-xioi) US prescribing information, 2019. https://www.fda.gov/media/126109/download. Accessed Mar 2024.
Coovert DD, Le TT, McAndrew PE, Strasswimmer J, Crawford TO, Mendell JR, Coulson SE, Androphy EJ, Prior TW, Burghes AH. The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet. 1997;6(8):1205–14.
Otsuki N, Arakawa R, Kaneko K, Aoki R, Arakawa M, Saito K. A new biomarker candidate for spinal muscular atrophy: Identification of a peripheral blood cell population capable of monitoring the level of survival motor neuron protein. PLoS One. 2018;13(8): e0201764.
Hamilton G, Gillingwater TH. Spinal muscular atrophy: going beyond the motor neuron. Trends Mol Med. 2013;19(1):40–50.
Nash LA, Burns JK, Chardon JW, Kothary R, Parks RJ. Spinal muscular atrophy: more than a disease of motor neurons? Curr Mol Med. 2016;16(9):779–92.
Singh NN, Hoffman S, Reddi PP, Singh RN. Spinal muscular atrophy: broad disease spectrum and sex-specific phenotypes. Biochim Biophys Acta Mol Basis Dis. 2021;1867(4): 166063.
Chang WF, Xu J, Chang CC, Yang SH, Li HY, Hsieh-Li HM, Tsai MH, Wu SC, Cheng WT, Liu JL, Sung LY. SMN is required for the maintenance of embryonic stem cells and neuronal differentiation in mice. Brain Struct Funct. 2015;220(3):1539–53.
Chang WF, Xu J, Lin TY, Hsu J, Hsieh-Li HM, Hwu YM, Liu JL, Lu CH, Sung LY. Survival motor neuron protein participates in mouse germ cell development and spermatogonium maintenance. Int J Mol Sci. 2020;21(3):794.
Ottesen EW, Howell MD, Singh NN, Seo J, Whitley EM, Singh RN. Severe impairment of male reproductive organ development in a low SMN expressing mouse model of spinal muscular atrophy. Sci Rep. 2016;6:20193.
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419.
Karlsson M, Zhang C, Méar L, Zhong W, Digre A, Katona B, Sjöstedt E, Butler L, Odeberg J, Dusart P, Edfors F, Oksvold P, von Feilitzen K, Zwahlen M, Arif M, Altay O, Li X, Ozcan M, Mardinoglu A, Fagerberg L, Mulder J, Luo Y, Ponten F, Uhlén M, Lindskog C. A single-cell type transcriptomics map of human tissues. Sci Adv. 2021;7(31): eabh2169.
Abati E, Corti S. Pregnancy outcomes in women with spinal muscular atrophy: a review. J Neurol Sci. 2018;388:50–60.
Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, Arafa M, Panner Selvam MK, Shah R. Male infertility. Lancet. 2021;397(10271):319–33.
Cox CM, Thoma ME, Tchangalova N, Mburu G, Bornstein MJ, Johnson CL, Kiarie J. Infertility prevalence and the methods of estimation from 1990 to 2021: a systematic review and meta-analysis. Hum Reprod Open. 2022;2022(4): hoac051.
Chang WF, Peng M, Hsu J, Xu J, Cho HC, Hsieh-Li HM, Liu JL, Lu CH, Sung LY. Effects of survival motor neuron protein on germ cell development in mouse and human. Int J Mol Sci. 2021;22(2):661.
Chen YC, Chang JG, Jong YJ, Liu TY, Yuo CY. High expression level of Tra2-beta1 is responsible for increased SMN2 exon 7 inclusion in the testis of SMA mice. PLoS One. 2015;10(3): e0120721.
Lipnick SL, Agniel DM, Aggarwal R, Makhortova NR, Finlayson SG, Brocato A, Palmer N, Darras BT, Kohane I, Rubin LL. Systemic nature of spinal muscular atrophy revealed by studying insurance claims. PLoS One. 2019;14(3): e0213680.
Ribault S, Le Goff L, Rippert P, De Montferrand C, Morard M, Barriere A, Bernard E, Pegat A, Vuillerot C. Implementation of SMN restoring therapies in symptomatic patients with spinal muscular atrophy: a description of new phenotypes in children and adults, 7th European Congress of NeuroRehabilitation, Lyon, France, 2023.
Cocuzza M, Alvarenga C, Pagani R. The epidemiology and etiology of azoospermia. Clinics (Sao Paulo). 2013;68(Suppl 1):15–26.
Brener A, Lebenthal Y, Shtamler A, Levy S, Stein R, Fattal-Valevski A, Sagi L. The endocrine manifestations of spinal muscular atrophy, a real-life observational study. Neuromuscul Disord. 2020;30(4):270–6.
Bach JR. Medical considerations of long-term survival of Werdnig-Hoffmann disease. Am J Phys Med Rehabil/Assoc Acad Physiatrists. 2007;86(5):349–55.
Ferguson L, Agoulnik AI. Testicular cancer and cryptorchidism. Front Endocrinol. 2013;4:32.
Rodprasert W, Virtanen HE, Makela JA, Toppari J. Hypogonadism and cryptorchidism. Front Endocrinol. 2019;10:906.
Kolon TF, Herndon CD, Baker LA, Baskin LS, Baxter CG, Cheng EY, Diaz M, Lee PA, Seashore CJ, Tasian GE, Barthold JS, A. American Urological. Evaluation and treatment of cryptorchidism: AUA guideline. J Urol. 2014;192(2):337–45.
Thorup J, McLachlan R, Cortes D, Nation TR, Balic A, Southwell BR, Hutson JM. What is new in cryptorchidism and hypospadias—a critical review on the testicular dysgenesis hypothesis. J Pediatr Surg. 2010;45(10):2074–86.
Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, Mazzone ES, Vitale M, Snyder B, Quijano-Roy S, Bertini E, Davis RH, Meyer OH, Simonds AK, Schroth MK, Graham RJ, Kirschner J, Iannaccone ST, Crawford TO, Woods S, Qian Y, Sejersen T, S.M.A.C. Group. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103–15.
Roche, EVRYSDI® (risdiplam) Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/evrysdi-epar-product-information_en.pdf. Accessed Mar 2024.
Naryshkin NA, Weetall M, Dakka A, Narasimhan J, Zhao X, Feng Z, Ling KK, Karp GM, Qi H, Woll MG, Chen G, Zhang N, Gabbeta V, Vazirani P, Bhattacharyya A, Furia B, Risher N, Sheedy J, Kong R, Ma J, Turpoff A, Lee CS, Zhang X, Moon YC, Trifillis P, Welch EM, Colacino JM, Babiak J, Almstead NG, Peltz SW, Eng LA, Chen KS, Mull JL, Lynes MS, Rubin LL, Fontoura P, Santarelli L, Haehnke D, McCarthy KD, Schmucki R, Ebeling M, Sivaramakrishnan M, Ko CP, Paushkin SV, Ratni H, Gerlach I, Ghosh A, Metzger F, Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science. 2014;345(6197):688–93.
Ratni H, Ebeling M, Baird J, Bendels S, Bylund J, Chen KS, Denk N, Feng Z, Green L, Guerard M, Jablonski P, Jacobsen B, Khwaja O, Kletzl H, Ko CP, Kustermann S, Marquet A, Metzger F, Mueller B, Naryshkin NA, Paushkin SV, Pinard E, Poirier A, Reutlinger M, Weetall M, Zeller A, Zhao X, Mueller L. Discovery of risdiplam, a selective survival of motor neuron-2 (SMN2) gene splicing modifier for the treatment of spinal muscular atrophy (SMA). J Med Chem. 2018;61(15):6501–17.
Poirier A, Weetall M, Heinig K, Bucheli F, Schoenlein K, Alsenz J, Bassett S, Ullah M, Senn C, Ratni H, Naryshkin N, Paushkin S, Mueller L. Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol Res Perspect. 2018;6: e00447.
ClinicalTrials.gov, NCT02240355: a study of RO6885247 in adult and pediatric patients with spinal muscular atrophy (MOONFISH), 2016. https://clinicaltrials.gov/ct2/show/NCT02240355. Accessed Mar 2024.
Kletzl H, Marquet A, Günther A, Tang W, Heuberger J, Groeneveld G, Birkhoff W, Mercuri E, Lochmüller H, Wood C, Fischer D, Gerlach I, Heinig K, T B, Dziadek S, Kinch R, Czech C, Khwaja O. The oral splicing modifier RG7800 increases full length survival of motor neuron 2 mRNA and survival of motor neuron protein: results from trials in healthy adults and patients with spinal muscular atrophy. Neuromuscul Disord. 2019;29:21–9.
Sivaramakrishnan M, McCarthy KD, Campagne S, Huber S, Meier S, Augustin A, Heckel T, Meistermann H, Hug MN, Birrer P, Moursy A, Khawaja S, Schmucki R, Berntenis N, Giroud N, Golling S, Tzouros M, Banfai B, Duran-Pacheco G, Lamerz J, Hsiu Liu Y, Luebbers T, Ratni H, Ebeling M, Clery A, Paushkin S, Krainer AR, Allain FH, Metzger F. Binding to SMN2 pre-mRNA-protein complex elicits specificity for small molecule splicing modifiers. Nat Commun. 2017;8(1):1476.
Ratni H, Scalco RS, Stephan AH. Risdiplam, the first approved small molecule splicing modifier drug as a blueprint for future transformative medicines. ACS Med Chem Lett. 2021;12(6):874–7.
Ye H, Kelly TF, Samadani U, Lim L, Rubio S, Overdier DG, Roebuck KA, Costa RH. Hepatocyte nuclear factor 3/fork head homolog 11 is expressed in proliferating epithelial and mesenchymal cells of embryonic and adult tissues. Mol Cell Biol. 1997;17(3):1626–41.
O’Donnell L, Pratis K, Wagenfeld A, Gottwald U, Muller J, Leder G, McLachlan RI, Stanton PG. Transcriptional profiling of the hormone-responsive stages of spermatogenesis reveals cell-, stage-, and hormone-specific events. Endocrinology. 2009;150(11):5074–84.
Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007;388(12):1257–74.
Wierstra I. The transcription factor FOXM1 (Forkhead box M1): proliferation-specific expression, transcription factor function, target genes, mouse models, and normal biological roles. Adv Cancer Res. 2013;118:97–398.
Katzenellenbogen BS, Guillen VS, Katzenellenbogen JA. Targeting the oncogenic transcription factor FOXM1 to improve outcomes in all subtypes of breast cancer. Breast Cancer Res. 2023;25(1):76.
Al-Zoubi AM, Efimova EV, Kaithamana S, Martinez O, El-Idrissi Mel A, Dogan RE, Prabhakar BS. Contrasting effects of IG20 and its splice isoforms, MADD and DENN-SV, on tumor necrosis factor alpha-induced apoptosis and activation of caspase-8 and -3. J Biol Chem. 2001;276(50):47202–11.
Mueller L, Barrow P, Jacobsen B, Ebeling M, Weinbauer G. Reproductive findings in male animals exposed to selective survival of motor neuron-2 (SMN2) gene splicing modifying agents. Reprod Toxicol. 2023;118: 108360.
de Kretser DM, Loveland KL, Meinhardt A, Simorangkir D, Wreford N. Spermatogenesis. Hum Reprod. 1998;13(Suppl 1):1–8.
Linn E, Ghanem L, Bhakta H, Greer C, Avella M. Genes regulating spermatogenesis and sperm function associated with rare disorders. Front Cell Dev Biol. 2021;9: 634536.
Martin-du Pan RC, Campana A. Physiopathology of spermatogenic arrest. Fertil Steril. 1993;60(6):937–46.
Picut CA, Remick AK, de Rijk EP, Simons ML, Stump DG, Parker GA. Postnatal development of the testis in the rat: morphologic study and correlation of morphology to neuroendocrine parameters. Toxicol Pathol. 2015;43(3):326–42.
Dreef HC, Van Esch E, De Rijk EP. Spermatogenesis in the cynomolgus monkey (Macaca fascicularis): a practical guide for routine morphological staging. Toxicol Pathol. 2007;35(3):395–404.
Delessard M, Saulnier J, Rives A, Dumont L, Rondanino C, Rives N. Exposure to chemotherapy during childhood or adulthood and consequences on spermatogenesis and male fertility. Int J Mol Sci. 2020;21(4):1454.
Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril. 2013;100(5):1180–6.
Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406.
Schlegel PN, Sigman M, Collura B, De Jonge CJ, Eisenberg ML, Lamb DJ, Mulhall JP, Niederberger C, Sandlow JI, Sokol RZ, Spandorfer SD, Tanrikut C, Treadwell JR, Oristaglio JT, Zini A. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part II. Fertil Steril. 2021;115(1):62–9.
Gonzalez D, Narasimman M, Best JC, Ory J, Ramasamy R. Clinical update on home testing for male fertility. World J Mens Health. 2021;39(4):615–25.
Petok WD. Infertility counseling (or the lack thereof) of the forgotten male partner. Fertil Steril. 2015;104(2):260–6.
Hammarberg K, Collins V, Holden C, Young K, McLachlan R. Men’s knowledge, attitudes and behaviours relating to fertility. Hum Reprod Update. 2017;23(4):458–80.
Kobori Y. Home testing for male factor infertility: a review of current options. Fertil Steril. 2019;111(5):864–70.
Agarwal A, Sharma R, Gupta S, Sharma R. NextGen home sperm banking kit: outcomes of offsite vs onsite collection-preliminary findings. Urology. 2015;85(6):1339–45.
Ibrahim E, Brackett NL, Lynne CM. Penile vibratory stimulation for semen retrieval in men with spinal cord injury: patient perspectives. Res Reports Urol. 2022;14:149–57.
Dabaja AA, Schlegel PN. Microdissection testicular sperm extraction: an update. Asian J Androl. 2013;15(1):35–9.
Grin L, Girsh E, Harlev A. Male fertility preservation-methods, indications and challenges. Andrologia. 2021;53(2): e13635.
Haddad M, Stewart J, Xie P, Cheung S, Trout A, Keating D, Parrella A, Lawrence S, Rosenwaks Z, Palermo GD. Thoughts on the popularity of ICSI. J Assist Reprod Genet. 2021;38(1):101–23.
Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129(3):e41–55.
Khorshid A, Boyd ALH, Behr B, Zhao Q, Alvero R, Bavan B. Cost-effectiveness of IVF with PGT-M/A to prevent transmission of spinal muscular atrophy in offspring of carrier couples. J Assist Reprod Genet. 2023;40(4):793–801.
Christianson MS, Stern JE, Sun F, Zhang H, Styer AK, Vitek W, Polotsky AJ. Embryo cryopreservation and utilization in the United States from 2004–2013. F&S reports. 2020;1(2):71–7.
Bortoletto P. Impending ice age of assisted reproductive technology. F&S Reports. 2020;1(2):60.
Medical Writing and Editorial Assistance
Medical writing support was provided by Jack Curran, PhD, of Nucleus Global, an Inizio Company, and was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland, in accordance with Good Publication Practice (GPP) 2022 guidelines (http://www.ismpp.org/gpp-2022).
Funding
The Rapid Service Fee was sponsored by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Author information
Authors and Affiliations
Contributions
Natan Bar-Chama, Bakri Elsheikh, Channa Hewamadduma, Carol Jean Guittari, Ksenija Gorni, and Lutz Mueller were involved in the concept for the article, participated in manuscript development and writing, and approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of Interests
Natan Bar-Chama is the recipient of an Investigator-Initiated Study with Genentech, Inc. and F. Hoffmann-La Roche Ltd and has served on a Medical Advisory Board for WINFertility. Bakri Elsheikh received research funding from Alexion Pharmaceuticals, Avidity, Biogen, Genentech, Inc., NMD Pharma, and Pharnext; and served as a consultant for Argenx, Biogen, and Genentech, Inc. Channa Hewamadduma has received speaker and advisory honoraria from Biogen and Roche. His clinical studies are supported by the NIHR BRC Neuroscience centre grant. He conducts patient-reported outcome measures-based natural history studies in SMA via Adult SMA REACH UK funded via Biogen and Roche. Carol Jean Guittari is an employee of Genentech, Inc. and a shareholder of F Hoffmann-La Roche Ltd. Ksenija Gorni and Lutz Mueller are employees and shareholders of F. Hoffmann-La Roche Ltd.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bar-Chama, N., Elsheikh, B., Hewamadduma, C. et al. Male Reproduction in Spinal Muscular Atrophy (SMA) and the Potential Impact of Oral Survival of Motor Neuron 2 (SMN2) Pre-mRNA Splicing Modifiers. Neurol Ther (2024). https://doi.org/10.1007/s40120-024-00626-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40120-024-00626-5