Abstract
Introduction
Recent studies have suggested a potential association between methotrexate use and an increased risk of dementia. However, the causal relationship between methotrexate and dementia remains unclear. This study aims to investigate the potential causal effect of methotrexate use on the risk of dementia using a two-sample Mendelian randomization (TSMR) approach.
Methods
We conducted a TSMR study using summary statistics from genome-wide association studies (GWAS) of methotrexate use and dementia. We obtained genetic instruments for methotrexate use from a large-scale GWAS meta-analysis and genetic instruments for dementia from a separate GWAS meta-analysis. We performed several statistical analyses, including inverse-variance weighted (IVW), weighted median (WM1), weighted mode (WM2), and MR-Egger regression methods, to estimate the causal effect of methotrexate on dementia risk.
Results
Our TSMR analysis showed a significant positive association between genetic predisposition to methotrexate use and dementia risk. The IVW method estimated a causal odds ratio (OR) of 0.476 [95% confidence interval (CI) 0.362–0.626] per unit increase in the log odds ratio of methotrexate use. WM1, WM2, and MR-Egger methods provided consistent results.
Conclusion
The findings of this mendelian randomization (MR) study suggest a potential causal effect of methotrexate use on the risk of dementia. However, further research is needed to validate these findings and explore the underlying mechanisms. Since methotrexate is widely prescribed for various autoimmune diseases, a better understanding of its potential impact on dementia risk is crucial for optimizing treatment strategies and addressing potential adverse effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A Mendelian randomized study was used to investigate the existence of a causal relationship between methotrexate use and dementia risk. |
The results of the study suggest that there is a positive causal relationship between methotrexate and the risk of dementia. |
The use of methotrexate has a protective effect on the risk of dementia. |
Methotrexate may reduce the risk of dementia. |
Introduction
Methotrexate, the most common disease-modifying antirheumatic drug, is the cornerstone of therapy for many autoimmune diseases and is commonly used in the treatment of rheumatoid arthritis (RA), osteoarthritis, and psoriasis [1,2,3]. Several studies have shown that methotrexate inhibits the dividing and proliferative activity of lymphocytes and reduces systemic inflammation and tissue damage [2, 4, 5]. Meanwhile, in a systematic evaluation and meta-analysis by Micha et al. [6], methotrexate treatment of patients with RA was found to be effective in reducing the risk of cardiovascular events by 21%. A study by Zhu et al. [7] reported that conjugates of methotrexate may have a positive effect on the treatment of prostate cancer. Even in a study by Zhou et al. [8] it was claimed that methotrexate acts as a hydrophobic drug that inhibits drug resistance in cancer cells and helps in cancer diagnosis and treatment. The wide use of methotrexate stems from its good therapeutic effect on multiple organs, systems, and diseases; methotrexate can effectively inhibit the development of the disease and improve the prognosis of patients. Therefore, it is one of the most commonly used drugs in clinical practice.
However, the use of methotrexate also carries the potential for adverse effects on a variety of organs and systems, with hematologic and gastrointestinal adverse effects being the most common [1, 3]. Its common side effects also include elevated liver enzymes, decreased blood counts, opportunistic infections, dermatologic manifestations, and memory impairment [9, 10]. Zorlu et al. and Lim et al. estimated that the prevalence of hematologic toxicity in methotrexate-treated patients with RA was 3% [3, 11]. A case report and review of the literature suggests that methotrexate use also has an increased risk of pericarditis in patients with rheumatic or other diseases [9]. A study by Yamamoto et al. [12] concluded that methotrexate, one of the commonly used chemotherapeutic agents, may induce gastrointestinal mucositis. Methotrexate use therefore has pros and cons. Attaching great importance to the potential adverse effects of methotrexate and using the drug rationally is an effective way to reduce and eliminate the occurrence of its side effects.
Dementia is a group of disorders with behavioral and psychological symptoms of dementia (BPSD) or neuropsychiatric symptoms (NPS) as the core symptoms [13]. Its main clinical manifestations are apathy, depression, anxiety or hallucinations, delusions, sleep disorders, and cognitive impairment [14]. The increasing severity of dementia has led to a corresponding increase in the cost of care, which is estimated at $81.8 billion per year globally [14,15,16]. Therefore, actively searching for potential risk factors for dementia is important for preventing the onset of dementia and improving the prognosis, care, and quality of life of patients. Existing studies have shown that there are multiple potential risk factors for dementia. Among them, Alzheimer’s disease is the leading cause of dementia in the elderly and remains incurable [17]. Cerebrovascular diseases, including chronic cerebral hypoperfusion (CCH), are considered to be high risk factors for cognitive decline in vascular dementia (VaD) [18]. Meanwhile, underlying diseases such as hypertension and diabetes mellitus have been repeatedly mentioned as risk factors for dementia in the studies by Zülke et al. [19, 20]. Even chronic digestive diseases such as inflammatory bowel disease (IBD) and Crohn’s disease (CD) have emerged as new risk factors for dementia [21]. Moreover, the development of dementia has been associated with individual psychological and social factors, alcohol consumption, gender, sleep quality, and care [15, 22,23,24,25].
There are many opinions on the pros and cons of methotrexate use. However, its effect on dementia is currently unclear. Therefore, this study investigated whether there is a causal relationship between methotrexate and dementia and whether methotrexate plays a protective or risk-increasing role in dementia, using a Mendelian randomization (MR) study method.
MR studies are instrumental variable methods that use genetic data to determine causal relationships between relevant exposures and various endpoints [26]. This approach addresses the major limitations of confounding and reverse causality that exist in traditional observational studies and randomized controlled trials [27]. At the same time, under the premise of satisfying the necessary assumptions, MR can provide strong evidence for our study of each causal relationship [27].
Methods
Codes for datasets related to methotrexate and dementia were obtained from the genome-wide association studies (GWAS) website (https://gwas.mrcieu.ac.uk/), and the corresponding datasets were obtained from the Medical Research Council Integrated Epidemiology Unit (MRC-IEU) 2018. Single nucleotide polymorphisms (SNPs) associated with methotrexate were identified and screened for valid instrumental variables. Genome-wide association data associated with dementia were subsequently obtained. After excluding confounders, MR analysis and sensitivity analysis were performed. The entire flowchart of the study is shown in Fig. 1. Our studies are accompanied by authoritative open databases (https://gwas.mrcieu.ac.uk/datasets/ukb-b-12209 and https://gwas.mrcieu.ac.uk/datasets/ukb-b-14699), so there was no need to obtain ethics committee approval.
Instrumental Variables Associated with Methotrexate
Methotrexate-associated dataset codes (ID: ukb-b-12209) were obtained and analyzed as exposure factors through the GWAS website. The methotrexate-associated phenotype includes methotrexate treatment and medication. The dataset contained data on a total of 462,933 individuals (2454 cases and 460,479 controls; European ancestry; male and female). Methotrexate-associated SNPs that met the screening criteria were selected [P < 5 × 10−8; linkage disequilibrium (LD): r2 < 0.001, window size = 10 mB] [28, 29]. PhenoScanner (http://www.phenoscanner.medschl.cam.ac.uk/phenoscanner) was utilized to exclude confounders. Eventually, after palindromic sequences, SNPs with moderate allele frequencies, and SNPs with potential pleiotropy were removed, five SNPs were selected as instrumental variables for methotrexate, namely rs112923147, rs1230666, rs2894316, rs6605556, and rs9276609 (Table 1).
GWAS Datasets Related to Dementia
Dementia-related dataset codes (ID: ukb-b-14699) were also obtained from the GWAS website and used as outcome variables. Dementia phenotypes included Alzheimer’s disease and dementia. The dataset contained data on a total of 423,738 individuals (36,548 cases and 387,190 controls; European ancestry; male and female). The outcome variables were finally analyzed in combination with the acquired exposure factors (methotrexate-associated SNPs).
Mendelian Randomization Analysis
In this study, we used four MR analysis methods to elucidate the existence of a causal relationship between methotrexate and dementia, including inverse variable weighting (IVW), MR-Egger regression, weighted median (WM1), and weighted mode (WM2) estimation. Our analytic approach was consistent with the three basic assumptions of MR analysis: (I) genetic variation is strongly associated with methotrexate; (II) genetic variation is independent of other confounders; and (III) genetic variation is associated with outcome variables only through methotrexate [30]. Of these, IVW was our main reference method for causal estimation. Three other MR analysis methods and sensitivity analyses (leave-one-out analysis and MR-PRESSO test) complemented IVW to ensure the robustness of causal effect estimates. Heterogeneity analysis was performed via Cochran’s Q statistic.
Statistical Analysis
All data analyses were performed in R software (version 4.3.1) using the “Two SampleMR”, “plyr”, “MRInstruments”, and “MR-PRESSO” packages. Two-tailed p values less than 0.05 were considered statistically significant.
Results
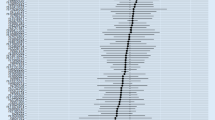
As shown in Table 2, IVW analysis showed a causal odds ratio (OR) of 0.476 [95% confidence interval (CI) 0.362–0.626] for each unit increase in the log odds of methotrexate use, suggesting that there is a positive causal association between the genetic variant of methotrexate and the risk of dementia, and that methotrexate is protective against dementia risk. WM1 (OR 0.457, 95% CI 0.336–0.623), WM2 (OR 0.443, 95% CI 0.317–0.620), and MR-Egger (OR 0.389, 95% CI 0.234–0.645) methods yielded consistent results. The p values of all the aforementioned estimation methods were less than 0.05. Heterogeneity analysis (Q = 1.489, P = 0.829) indicated that there was little heterogeneity between the genes of the two samples (Fig. 2). A forest plot of the effect values and 95% CIs estimated by MR analysis is illustrated in Fig. 3. MR-Egger intercept analysis (Q = 0.616, P = 0.893) did not indicate genetic pleiotropy. Sensitivity analyses showed robustness of causal effect estimates (Fig. 4).
Discussion
We obtained codes for datasets related to methotrexate and dementia from the genome-wide association studies (GWAS) website and used these codes to screen for valid instrumental variables and obtain genome-wide association data related to dementia. After confounders were excluded, Mendelian randomization (MR) analysis was performed. The results of our MR analyses indicated a positive causal association between methotrexate and dementia risk, and that methotrexate may be associated with a reduced risk of dementia.
In a cohort study by Judge et al. [31], a total of 3876 patients with rheumatoid arthritis (RA) receiving classical disease-modifying antirheumatic drugs (cDMARD) were observed, of whom 2355 received methotrexate. The effect was strongest in methotrexate users [hazard ratio 0.52; 95% CI 0.34–0.82] [31]. Methotrexate had a protective effect against dementia in patients with RA [31]. This coincides with our findings.
However, in a case–control study, Chou et al. concluded that the use of disease-modifying antirheumatic drugs (DMARDs), including methotrexate, was associated with a higher risk of dementia in patients with RA after studying 957 patients with RA and dementia, as well as 957 patients with RA but not dementia (95% CI 1.33–2.00) [32].
A randomized controlled trial by Zhao et al. [33] demonstrated greater efficacy, safety, and cost-effectiveness in reducing disease recurrence in combination with other conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) compared to methotrexate alone. Another of their studies reached the same conclusion [34]. In addition, in a study by Zhao et al. [35], dissolving microneedle patch (DMNP) with transdermal administration of methotrexate was found to be safe, convenient, and more efficient than conventional administration in the treatment of RA. A study by Yan et al. [36] concluded that methotrexate, as an anticancer drug, could be more effective in inhibiting tumor growth by applying modified functional layered double hydroxide (LDH) nanomaterials to deliver methotrexate. In a pilot study by Yang et al. [37], they described intrathecal methotrexate as a potentially effective adjunctive therapy for the treatment of patients with severe anti-N-methyl-d-aspartate receptor (anti-NMDAR) encephalitis.
The use of different doses of methotrexate can also produce completely different clinical manifestations in humans. High doses of methotrexate induce neurological, hematologic, gastrointestinal, and other systemic toxicities [38]. Whereas, low doses of methotrexate induce liver diseases such as hepatic fibrosis, gastrointestinal reactions, and skin diseases [9]. In conclusion, as far as the effectiveness and safety of methotrexate use are concerned, the dosage of the drug, the route of action, and the therapeutic regimen are important influences that cannot be ignored. These factors may influence the pathogenesis of dementia by affecting the cardiovascular system, the digestive system, and many other systems and have led to differences in the results of many related studies.
As far as cancerous diseases are concerned, Zabłocka et al. [39] stated that Alzheimer’s disease, which is the main cause of dementia, is able to reduce many types of cancer by interfering with vascular function, insulin and glucose metabolism, pathways such as the Wnt signaling pathway, and inflammatory processes. However, our findings suggest that methotrexate, which is one of the recognized anticancer drugs, is able to reduce the risk of dementia, and the study by Zabłocka et al. seems to be contrary to our results. Therefore, the possible interplay between mechanisms relating to methotrexate, dementia, and cancer need to be further explored in this context.
Therefore, on the basis of many existing studies, we hypothesize that the effect of methotrexate on the course of dementia is also closely related to the dosage of methotrexate, the route of action, and the treatment regimen. The rational use of methotrexate has some clinical significance in the prevention of dementia. In connection with the results of this study, the correct use of methotrexate may fulfill its protective potential against the risk of dementia. However, further exploration of the potential mechanisms of action between methotrexate and dementia is needed.
Of course, our study has some shortcomings. The genetic data only came from the corresponding dataset from the GWAS website, meaning our sample may have some deficiencies. In addition, the instrumental variable screening process made it difficult for us to ensure a strong correlation (F < 10) between instrumental variables and exposure factors. However, our study used genome-wide association data to visually and scientifically explore the existence of a causal relationship between methotrexate and dementia through MR analysis methods, providing strong direct evidence of the association between the two. Methotrexate is widely used to treat a variety of autoimmune diseases, so a better understanding of its potential impact on dementia risk is critical for optimizing treatment strategies and addressing potential adverse effects.
Conclusions
This two-sample Mendelian randomization (TSMR) study provides evidence supporting a potential causal association between methotrexate use and a reduced risk of dementia. Future studies should focus on larger sample sizes and further exploration of the biological pathways involved to corroborate these findings and improve patient care.
Data Availability
The data supporting the findings of this study are available within the article and/or its supplementary material.
References
Zhu Z, Yu Q, Leng X, et al. Can low-dose methotrexate reduce effusion-synovitis and symptoms in patients with mid- to late-stage knee osteoarthritis? Study protocol for a randomised, double-blind, and placebo-controlled trial. Trials. 2020;21:795.
Zimmerman MC, Clemens DL, Duryee MJ. Direct antioxidant properties of methotrexate: inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588–93.
Zorlu M, Kiskac M, Karatoprak C, et al. Pott’s disease and hypercalcemia in a patient with rheumatoid arthritis receiving methotrexate monotherapy. Indian J Pharmacol. 2013;45:631–3.
Brown PM, Pratt AG, Isaacs JD. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol. 2016;12:731–42.
Zhu H, Li R, Da Z, et al. Remission assessment of rheumatoid arthritis in daily practice in China: a cross-sectional observational study. Clin Rheumatol. 2018;37:597–605.
Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362–70.
Zhu S, Wang Q, Jiang J, Luo Y, Sun Z. A conjugate of methotrexate and an analog of luteinizing hormone releasing hormone shows increased efficacy against prostate cancer. Sci Rep. 2016;6:33894.
Zhou M, Zhang X, Yang Y, et al. Carrier-free functionalized multidrug nanorods for synergistic cancer therapy. Biomaterials. 2013;34:8960–7.
Guevara NA, Hernandez TE, Rosado F, Gupta S. Pericarditis induced by methotrexate: a case report and literature review. Cureus. 2023;15:e40257.
KharfanDabaja MA, Morgensztern D, Markoe AM, Bartlett-Pandite L. Radiation recall dermatitis induced by methotrexate in a patient with Hodgkin’s disease. Am J Clin Oncol. 2000;23:531–3.
Lim AY, Gaffney K, Scott DG. Methotrexate-induced pancytopenia: serious and under-reported? Our experience of 25 cases in 5 years. Rheumatology (Oxford). 2005;44:1051–5.
Yamamoto A, Itoh T, Nasu R, Kajiwara E, Nishida R. Sodium alginate inhibits methotrexate-induced gastrointestinal mucositis in rats. Biol Pharm Bull. 2013;36:1528–34.
Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264–71.
Magierski R, Sobow T, Schwertner E, Religa D. Pharmacotherapy of behavioral and psychological symptoms of dementia: state of the art and future progress. Front Pharmacol. 2020;11:1168.
Mellinger TJ, Forester BP, Vogeli C, et al. Impact of dementia care training on nurse care managers’ interactions with family caregivers. BMC Geriatr. 2023;23:16.
Rattinger GB, Schwartz S, Mullins CD, et al. Dementia severity and the longitudinal costs of informal care in the Cache County population. Alzheimers Dement. 2015;11:946–54.
Zulfiqar S, Garg P, Nieweg K. Contribution of astrocytes to metabolic dysfunction in the Alzheimer’s disease brain. Biol Chem. 2019;400:1113–27.
Zheng Y, Zhang J, Zhao Y, et al. Curcumin protects against cognitive impairments in a rat model of chronic cerebral hypoperfusion combined with diabetes mellitus by suppressing neuroinflammation, apoptosis, and pyroptosis. Int Immunopharmacol. 2021;93:107422.
Zülke A, Luppa M, Köhler S, Riedel-Heller SG. What does the population know about risk and protective factors for dementia? An international review of the current state of knowledge in various countries. Nervenarzt. 2023;94:384–91.
Zülke AE, Luppa M, van Boxtel M, et al. Older adults’ awareness of modifiable risk and protective factors for dementia and interest in eHealth interventions for brain health: a comparison between the Netherlands and Germany. BMC Public Health. 2023;23:2321.
Zuin M, De Giorgio R, Capatti E, Boschetti E, Zuliani G. Inflammatory bowel disease as a new risk factor for dementia. Aging Clin Exp Res. 2022;34:1725–8.
Clare L, Nelis SM, Martyr A, et al. The influence of psychological, social and contextual factors on the expression and measurement of awareness in early-stage dementia: testing a biopsychosocial model. Int J Geriatr Psychiatry. 2012;27:167–77.
Fauria K, Minguillon C, Knezevic I, et al. Exploring cognitive and biological correlates of sleep quality and their potential links with Alzheimer’s disease (ALFASleep project): protocol for an observational study. BMJ Open. 2022;12:e067159.
Kilian C, Klinger S, Rehm J, Manthey J. Alcohol use, dementia risk, and sex: a systematic review and assessment of alcohol-attributable dementia cases in Europe. BMC Geriatr. 2023;23:246.
Klingenberg M, Stark D, Eitel F, Budding C, Habes M, Ritter K. Higher performance for women than men in MRI-based Alzheimer’s disease detection. Alzheimers Res Ther. 2023;15:84.
Mukamal KJ, Stampfer MJ, Rimm EB. Genetic instrumental variable analysis: time to call mendelian randomization what it is. The example of alcohol and cardiovascular disease. Eur J Epidemiol. 2020;35:93–7.
Katikireddi SV, Green MJ, Taylor AE, Davey Smith G, Munafò MR. Assessing causal relationships using genetic proxies for exposures: an introduction to Mendelian randomization. Addiction. 2018;113:764–74.
Weng LC, Roetker NS, Lutsey PL, et al. Evaluation of the relationship between plasma lipids and abdominal aortic aneurysm: a Mendelian randomization study. PLoS One. 2018;13:e0195719.
Zhu J, Niu Z, Alfredsson L, Klareskog L, Padyukov L, Jiang X. Age at menarche, age at natural menopause, and risk of rheumatoid arthritis—a Mendelian randomization study. Arthritis Res Ther. 2021;23:108.
Zheng J, Baird D, Borges MC, et al. Recent developments in mendelian randomization studies. Curr Epidemiol Rep. 2017;4:330–45.
Judge A, Garriga C, Arden NK, et al. Protective effect of antirheumatic drugs on dementia in rheumatoid arthritis patients. Alzheimers Dement (N Y). 2017;3:612–21.
Chou MH, Wang JY, Lin CL, Chung WS. DMARD use is associated with a higher risk of dementia in patients with rheumatoid arthritis: a propensity score-matched case-control study. Toxicol Appl Pharmacol. 2017;334:217–22.
Zhao J, Zhou W, Wu Y, Yan X, Yang L, Zhang Z. Efficacy, safety, and cost-effectiveness of triple therapy in preventing relapse in rheumatoid arthritis: a randomized controlled trial (ESCoRT study). Chin Med J (Engl). 2022;135:2200–9.
Zhao J, Zhou W, Wu Y, et al. The efficacy, safety and cost-effectiveness of hydroxychloroquine, sulfasalazine, methotrexate triple therapy in preventing relapse among patients with rheumatoid arthritis achieving clinical remission or low disease activity: the study protocol of a randomized controlled clinical Trial (ESCoRT study). BMC Med Inform Decis Mak. 2021;21:83.
Zhao W, Zheng L, Yang J, Ma Z, Tao X, Wang Q. Dissolving microneedle patch-assisted transdermal delivery of methotrexate improve the therapeutic efficacy of rheumatoid arthritis. Drug Deliv. 2023;30:121–32.
Yan L, Zhou M, Zhang X, et al. A novel type of aqueous dispersible ultrathin-layered double hydroxide nanosheets for in vivo bioimaging and drug delivery. ACS Appl Mater Interfaces. 2017;9:34185–93.
Yang XZ, Zhu HD, Ren HT, et al. Utility and safety of intrathecal methotrexate treatment in severe anti-N-methyl-d-aspartate receptor encephalitis: a pilot study. Chin Med J (Engl). 2018;131:156–60.
Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–35.
Zabłocka A, Kazana W, Sochocka M, et al. Inverse correlation between Alzheimer’s disease and cancer: short overview. Mol Neurobiol. 2021;58:6335–49.
Acknowledgements
We would like to acknowledge the researchers who conducted the original GWAS studies and made their summary statistics publicly available.
Medical Writing and Editorial Assistance
The authors did not use any medical writing or editorial assistance for this article.
Funding
This study was supported in part by: Natural Science Foundation of Department of Education of Guangdong Province [Grant Number 2022KTSCX030].
Author information
Authors and Affiliations
Contributions
Xiao-Na Ma and Wei Feng: Data collection, analysis, and visualization; Xiao-Na Ma, Wei Feng, and Shu-Lin Chen: The first draft writing; Xiao-Qin Zhong: Software and references; Chang-Song Lin: Financial access and resources; Qiang Xu: Study design, management, acquisition of funds and resources, and review and editing of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest in the research process.
Ethical Approval
Our data were sourced from public databases (https://gwas.mrcieu.ac.uk/datasets/ukb-b-12209 and https://gwas.mrcieu.ac.uk/datasets/ukb-b-14699) and therefore do not require approval from the ethics committee.
Additional information
Xiao-Na Ma, Wei Feng and Shu-Lin Chen are Co-first author.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ma, XN., Feng, W., Chen, SL. et al. Methotrexate and the Risk of Dementia: A Two-Sample Mendelian Randomization Study. Neurol Ther 13, 715–725 (2024). https://doi.org/10.1007/s40120-024-00609-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-024-00609-6