Abstract
Introduction
A higher levodopa dose is a risk factor for motor complications in Parkinson’s disease (PD). Istradefylline (IST) is used as adjunctive treatment to levodopa in PD patients with off episodes, but its impact on levodopa dose titration remains unclear. The objective of this study was to investigate the effect of IST on levodopa dose escalation in PD patients with wearing-off.
Methods
This was a multicenter, open-label, randomized, parallel-group controlled study (ISTRA ADJUST PD) in which PD patients experiencing wearing-off (n = 114) who were receiving levodopa 300–400 mg/day were randomized to receive IST or no IST (control). Levodopa dose was escalated according to clinical severity. The primary endpoint was cumulative additional levodopa dose, and secondary endpoints were changes in symptom rating scales, motor activity determined by a wearable device, and safety outcomes.
Results
The cumulative additional levodopa dose throughout 37 weeks and dose increase over 36 weeks were significantly lower in the IST group than in the control group (both p < 0.0001). The Movement Disorder Society Unified Parkinson’s Disease Rating Scale Part I and device-evaluated motor activities improved significantly from baseline to 36 weeks in the IST group only (all p < 0.05). Other secondary endpoints were comparable between the groups. Adverse drug reactions (ADRs) occurred in 28.8% and 13.2% of patients in the IST and control groups, respectively, with no serious ADRs in either group.
Conclusion
IST treatment reduced levodopa dose escalation in PD patients, resulting in less cumulative levodopa use. Adjunctive IST may improve motor function more objectively than increased levodopa dose in patients with PD.
Trial Registration
Japan Registry of Clinical Trials: jRCTs031180248.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Levodopa is the mainstay of Parkinson’s disease (PD) treatment; however, high doses are a risk factor for motor complications. |
Istradefylline (IST) is an A2A receptor antagonist used as adjunctive treatment to levodopa in PD patients with off episodes; however, its impact on the increase in levodopa dosing is unclear. |
In this study, we hypothesized that IST would modulate increases in the levodopa dose and result in less levodopa use overall. |
What was learned from this study? |
IST therapy had a significant effect on modulating levodopa dose increases, with lower overall levodopa use compared with no IST therapy. |
IST therapy significantly improved device-evaluated motor activities to 36 weeks; no effect was seen in the group without IST therapy. |
IST therapy adjunctive to levodopa may provide greater benefit compared with increased levodopa doses alone. |
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder associated with motor dysfunction, including akinesia, resting tremor, and rigidity, as a result of dopaminergic neuronal degeneration [1]. Levodopa is the mainstay treatment for motor dysfunction in PD, by supplementing decreased dopamine levels. However, levodopa treatment is usually associated with motor fluctuations, which present a problem for PD patients [2]. Furthermore, the medium spiny neurons in the striatum connect not only with dopaminergic neurons but also with cortical glutamatergic and cholinergic interneurons. Non-dopaminergic modulation is therefore also a useful therapeutic approach for patients with PD. In this context, several non-dopaminergic drugs, including anti-cholinergic drugs, amantadine [3], zonisamide [4], and istradefylline (IST) [5], have been developed for the treatment of PD.
The adenosine A2A receptor predominantly localizes to the striatum and notably modulates the indirect pathway, which is important for the control of voluntary movement [6]. Patients with PD have hyperactivation of this indirect pathway, leading to reduced voluntary movements and bradykinesia [7]. The adenosine A2A receptor antagonist IST can improve parkinsonism by normalizing basal ganglia function [8], and is currently indicated as adjunctive treatment to levodopa/decarboxylase inhibitors in patients with PD with wearing-off/off episodes [5].
Previous nonclinical and clinical results also indicated that adjunctive IST may have long-lasting anti-Parkinsonian effects without the need for increased levodopa doses [9,10,11]. However, the ability of IST to prevent the elevation of levodopa dose in the treatment of patients with PD experiencing wearing-off remains unclear. To address this question, we conducted a multicenter, open-label, randomized, parallel-group controlled study (ISTRA ADJUST PD) [12]. The objective of this trial was to investigate the effect of adjunctive IST on the cumulative dose of medications containing levodopa in PD patients experiencing wearing-off. Additionally, we employed a triaxial accelerometry monitoring system to reduce the risk of bias, especially performance bias, in the open-label assessment of the effectiveness of IST in relation to the daily movements of patients. The efficacy and safety of IST were also evaluated.
Methods
Details of the methods can be found in the previously published study protocol [12].
Study Design
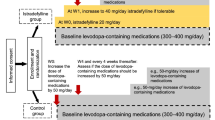
A 37-week, multicenter, open-label, randomized, parallel-group controlled study was conducted as the ISTRA ADJUST PD study from February 2019 to May 2022 at 20 sites in Japan (with the registration period starting from May 2019 lasting until November 2020). Most observations were carried out at 4- or 12-week intervals throughout the study period.
Standard Protocol Approvals, Registrations, and Patient Consent
We carried out the present study in compliance with the Japan Clinical Trials Act and all related national and international guidelines for human trials, and with the Declaration of Helsinki. The Juntendo University Certified Review Board (J18-006) reviewed and approved the study protocol (Version 6.0, 1 November 2021) and all other study documentation (approval number CRB3180012). This trial was registered with the Japan Registry of Clinical Trials (jRCTs031180248).
All patients provided written informed consent before enrollment. We anonymized all patient data so that no personal information was associated with the wearable devices.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
The study protocol has been published previously [12], and the statistical analysis plan file has been uploaded as a supplemental file (Data S1).
Inclusion and Exclusion Criteria
Inclusion Criteria
Eligible patients met the following criteria: receiving levodopa-containing medications ≥ three times daily (total daily dose of levodopa, 300–400 mg); experiencing wearing-off; age, 30–84 years; PD diagnosis in accord with the International Parkinson and Movement Disorder Society criteria; stage ≤ 3 modified Hoehn & Yahr scale (mH&Y) (on); and provision of written informed consent. Patients concomitantly receiving anti-PD drugs other than levodopa (e.g., dopamine agonists, catechol-O-methyltransferase inhibitors, and monoamine oxidase type B inhibitors) were also included in this study [12].
Exclusion Criteria
Patients were excluded if they met any of the following criteria: prior treatment with IST; taking any study drug ≤ 4 months prior to enrollment; having dementia or a Mini-Mental State Examination (MMSE) Japanese ver. score ≤ 23; previous neurosurgery for PD such as stereotactic surgery, deep brain stimulation, gamma knife; ongoing or prospective treatment with levodopa/carbidopa hydrate enteral suspension; initiation of PD treatment or any changes in regimen ≤ 4 weeks prior to enrollment; taking strong CYP3A4 inhibitors such as itraconazole and clarithromycin ≤ 14 days prior to enrollment; moderate or severe hepatic disorder; lactation or pregnancy; and the investigator’s discretion for ineligibility.
Randomization and Masking
Patients were randomized in a 1:1 ratio to the IST group (20 mg/day, escalating to 40 mg/day) or control group (without IST treatment). Randomization was performed centrally using a minimization method by computer allocation, with stratification according to age (< 60 or ≥ 60 years), presence or absence of dyskinesia, and levodopa equivalent dose (< 400 or ≥ 400 mg/day). Participants and study investigators were not blinded to treatment allocation.
Procedures
Patients in the IST group received orally administered IST (20 mg tablet, once daily) starting at week 0. The IST daily dose was increased to 40 mg at week 1 if the patient tolerated the treatment well and sustained motor symptoms. If treatment with 40 mg IST was not tolerated, dose reduction was allowed. The dosage of levodopa-containing drugs was increased by 50 mg/day at week 0 in patients in the control group. The attending physician then assessed whether the dose needed to be increased by 50 mg/day every 4 weeks, based on the following criteria: an increase of 50 mg/day was indicated if the clinical global impression of severity (CGI-S) scale was ≥ 4 on the observation day. If an intolerable adverse drug reaction (ADR) occurred because of the dose increase, the dose of medication containing levodopa was reduced at the discretion of the physician. The dose or dosing regimens of other adjunctive anti-PD drugs were not changed ≤ 4 weeks prior to enrollment and throughout the 37-week treatment period. The dose of a specific drug was reduced in the event of intolerable ADRs causally related to that drug.
Outcomes
The primary endpoint was the cumulative additional dose of medications containing levodopa throughout the treatment period of 37 weeks as the area under the curve (AUC) in patients in the IST group compared with that in the control group.
Secondary efficacy endpoints were comparisons of additional levodopa doses on each observation day in weeks 4–36 in the IST and control groups; the number of days until the first dose increase after week 4; CGI-S score; patient global impression of severity (PGI-S) score; mH&Y staging scale (on/off) score; Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [13] Part I score (non-motor experiences of daily living), Part II score (motor experiences of daily living), Part III score (motor examination), and Part IV score (motor complications); and Parkinson’s Disease Questionnaire-39 (PDQ-39).
During the study, patients wore a wristband-type triaxial accelerometry system (UW-301BT, Hitachi Systems, Ltd., Tokyo, Japan) on their non-dominant wrist for 7 days every 12 weeks. Data on movement frequency and intensity, and gait (step count, pace, and laterality) were obtained from the wearable device. Correlations between CGI-S and other outcomes such as MDS-UPDRS Parts I, II, III, and IV, PDQ-39, PGI-S, mH&Y, and device data were evaluated to confirm whether decisions on the dose escalation differed based on CGI-S and other outcomes.
Secondary safety endpoints were adverse events and ADRs classified based on System Organ Class and Preferred Terms defined in the Medical Dictionary for Regulatory Activities (Japanese edition, version 24.1). Correlations between the motor information obtained using the wearable device and the MDS-UPDRS Part II or III were evaluated as exploratory endpoints.
Statistical Analysis
To the best of our knowledge, no previous studies have investigated the effect of IST treatment on the flexibility of the doses of medications containing levodopa. In the present study, we therefore utilized real-world data from the medical claims database (Medical Data Vision Co., Ltd., Tokyo, Japan) to estimate the minimum between-group difference by simulating cumulative levodopa doses in PD patients (non-IST treatment) using Mann–Whitney U tests with 50 patients in each group, ensuring a power of 80% at the two-sided significance level of 5%. Under these conditions, a between-group difference of approximately 21.3% over 9 months (270 days) was estimated as the AUC for cumulative additional levodopa doses between groups (data on file; Kyowa Kirin Co., Ltd., Tokyo, Japan). It was assumed that IST might reduce the levodopa dose escalation of medications containing levodopa by at least 20% on the basis of the mean additional levodopa dose over 9 months (approximately 265 mg) in a previous study [14], and recommendation from the Expert Medical Advisory board for this study based on the clinical experiences in Japan. Based on the above, we set the sample size at 111 patients, assuming a 10% dropout rate and to ensure that 50 patients were evaluable in each group in the efficacy analyses.
The efficacy and safety analysis sets included all patients except those who withdrew consent before the start of the observation period (week 0), were withdrawn by the study investigator, or those in the IST group who did not initiate IST administration.
The Mann–Whitney U test was used for between-group comparisons for the primary and secondary endpoints. For the primary endpoint, we calculated the cumulative additional dose (AUC throughout the 37-week treatment period) as the total dose (daily dose × number of days) added to the dose of medications containing levodopa (300–400 mg/day) at randomization. Wilcoxon’s signed-rank test was used to evaluate changes from baseline. The log-rank test and Cox proportional hazards model were used to compare the number of days from the start of the observation (week 0) to the time of levodopa dose increase between the groups, and time-to-event curves were prepared using the Kaplan–Meier method. The correlation of the secondary endpoint scores was examined using Spearman’s correlation coefficient.
A two-sided 5% significance level was set for between-group comparisons, and two-sided 95% confidence intervals were calculated. We made no imputation for missing data. A detailed statistical analysis plan was prepared before the database was finalized and locked. In this study, SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses.
Results
Patients
A total of 115 patients were enrolled between May 2019 and November 2020 (Fig. 1). One patient withdrew from the trial before the baseline assessment, and 114 patients were thus randomized (participation rate 99%) to the IST or control group (57 patients per group). Five patients were excluded from the IST group because they did not start IST treatment, withdrew consent, or were withdrawn by the study investigator, and four patients were excluded from the control group because they did not meet the inclusion criteria or met the exclusion criteria, withdrawal of consent, or were withdrawn by the study investigator. Therefore, 52 and 53 patients in the IST group and control group, respectively, were included in the efficacy and safety analyses. During the study period, three patients in the IST group failed to increase the dosage of IST to 40 mg, four patients in the IST group dropped out of the study because of withdrawal of consent, adverse events, or withdrawal by the study investigator, and five patients in the control group dropped out because of withdrawal of consent. Forty-eight patients in each group thus completed the 37-week trial. Overall, there were no significant differences in baseline demographic and clinical characteristics between the IST group and control group (Table 1).
Outcomes
Primary Outcome
The mean (± standard deviation [SD]) cumulative additional levodopa dose as the AUC for 37 weeks was lower in the IST group (3229.8 ± 5692.7 mg) compared with that in the control group (15,056.6 ± 5187.1 mg) (p < 0.0001).
Secondary Outcomes
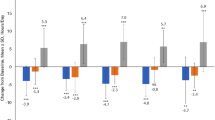
The levodopa dose increase was significantly reduced over 36 weeks in the IST group compared with the control group (p < 0.0001). The mean (± SD) add-on levodopa dose was significantly lower in the IST group (25.0 ± 40.2 mg/day) than in the control group (73.6 ± 43.9 mg/day) at week 36 (p < 0.0001) (Fig. 2).
Additional levodopa doses in the IST and control groups. Data shown as mean ± SD. *p < 0.0001, Mann–Whitney U test (vs. control). Add-on dose at week 36 (mean ± SD): IST group = 25.0 ± 40.2 mg/day; control group = 73.6 ± 43.9 mg/day (p < 0.0001, Mann–Whitney U test). IST istradefylline, SD standard deviation
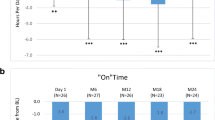
The percentage of patients who added levodopa after 4 weeks tended to be lower in the IST group than in the control group, but there was no significant difference between the two groups (Fig. 3). The levodopa dose was increased in 19 patients in the IST group (36.5%) and 25 patients (47.2%) in the control group at week 36. The mean (± SD) number of days until the first levodopa dose increase after week 4 tended to be longer in the IST group (202.1 ± 87.6) than in the control group (191.8 ± 88.8).
Number of days until first levodopa dose increase after week 4. Vertical axis indicates percentage of patients who first increased the levodopa dose after week 4. Data shown as mean ± SD. Number of days until first levodopa dose increase: IST group = 202.1 ± 87.6 days; control group = 191.8 ± 88.8 days (p = 0.2912, log-rank test; p = 0.2946, Cox proportional hazards model). IST istradefylline, SD standard deviation
The CGI-S score, as an indicator of levodopa dose increase, decreased at all time points after week 4 (vs. week 0) in both the IST and control groups (p < 0.05), with no significant difference between the groups (Table S1). There were no significant differences in the scores for other secondary outcomes between the two groups during the study.
MDS-UPDRS Part I only improved in the IST group, whereas MDS-UPDRS Parts III and IV improved in both groups from baseline (week 0) to week 36 (all p < 0.05) (Table 2). MDS-UPDRS Part II, mH&Y (on/off), PDQ-39, and PGI-S showed no significant improvement at week 36 compared with those at baseline in either group (Table 2, Table S1). However, temporary but significant improvements were observed for mH&Y (off), PDQ-39, or PGI-S in the IST group and MDS-UPDRS Part II in the control group (p < 0.05, Table 2 and Table S1).
Motor activity indicators, including motion frequency and intensity measured using the triaxial accelerometry system, improved significantly only in the IST group at week 36 compared with that at baseline (all p < 0.05, Table 3). Furthermore, light exercise intensity (≥ 1.5, < 3 metabolic equivalents) time increased significantly, whereas low exercise intensity (< 1.5 metabolic equivalents) time decreased significantly (both p < 0.05, Table 3). CGI-S, as an indicator of levodopa dose increase, correlated with PGI-S and MDS-UPDRS Part III (Table S2). Most of the motor activity parameters from wearable devices were correlated with MDS-UPDRS Part III and moderately correlated with MDS-UPDRS Part II (Table S3). However, the number of steps per day, and duration of moderate or more-intense exercise (≥ 3 metabolic equivalents) were not correlated with MDS-UPDRS Part II (Table S3).
ADRs were observed in 15 patients (28.8%) and seven patients (13.2%) in the IST and control groups, respectively (Table 4), and one patient discontinued the study in the IST group because of dyskinesia. The most common ADRs were dyskinesia, somnolence, and nausea (5.8% each) in the IST group, and nausea (3.8%) in the control group (Table 4). There were no serious ADRs in either group. Overall, there was no clear difference between the groups in the type of ADRs.
Classification of Evidence
This study provides level II evidence that IST treatment is effective in the adjustment of levodopa dose escalation with less cumulative levodopa use in PD patients with wearing-off.
Discussion
This study demonstrated a significant reduction in the cumulative additional levodopa dose throughout the 37-week treatment period in patients with PD receiving IST. There was no notable difference in the backgrounds of participants treated with and without IST, indicating appropriate randomization. MDS-UPDRS Part I and device-evaluated motor activities were significantly improved from baseline to 36 weeks only in the IST group. We also confirmed that CGI-S correlated with PGI-S and MDS-UPDRS Part III in both groups, suggesting that it was appropriate to judge increased levodopa dose based on CGI-S. Although IST suppressed levodopa dose escalation in PD patients, ADRs were more common in the IST group than in the control group. However, there were no serious ADRs in either group and no difference in dropout rates between the groups, indicating that IST was well tolerated for the treatment of PD.
The current study showed that the cumulative additional levodopa dose was significantly reduced by IST throughout the 37-week treatment period. The increase in daily levodopa dose from baseline to 36 weeks was 25.0 ± 40.2 mg in the IST group and 73.6 ± 43.9 mg in the control group. Given that the first levodopa administration at week 0 was additional 50 mg in the control group, IST may have a similar impact on preventing escalation of levodopa dose as an additional 50 mg/day of levodopa. There was no significant difference between the two groups in the number of days until the first levodopa dose increase after week 4. However, fewer patients in the IST group had a first levodopa dose increase before week 36 (IST: 36.5% vs. control: 47.2%) and the largest difference was found at week 28 (IST: 25.3% vs. control: 38.2%). The observation periods in most previous studies were approximately 12 weeks [8], and the long-term efficacy of IST for parkinsonism was thus unclear. In contrast, the present results indicated that IST may not only be useful for treating parkinsonism, but may also reduce the need for increased levodopa doses for long periods. Watts et al. investigated whether the addition of ropinirole prolonged-release delayed the onset of levodopa-induced dyskinesia, compared with levodopa administration alone [14]. Their study design was similar to the current study, but the additional levodopa dose until 6–9 months was 245 mg, which was approximately three times that in the present study. This suggests that the parkinsonism experienced by the participants in our study might have been too mild to require an increased dose of levodopa. Further studies are thus needed to confirm whether IST can prevent increases in levodopa dosage in patients with advanced PD.
Several clinical endpoints were similarly improved in both groups compared with those at baseline, and no endpoints deteriorated. Investigator objective scores, including MDS-UPDRS Part III and CGI-S, both decreased significantly during the trial period in both groups. Notably, the MDS-UPDRS Part III fell by > 3.25 in the IST group, which was considered a clinically meaningful improvement [15]. Furthermore, the mH&Y (off) was significantly improved in the IST group at weeks 12 and 24.
Previous studies showed improvements in MDS-UPDRS Part II following treatment with 40 mg IST, but most only revealed improved on-phase motor function and off-time reduction [16, 17]. IST is a non-dopaminergic drug and may thus have potential effectiveness against levodopa-resistant symptoms. The present study also evaluated patient-reported outcomes, which reflect patient quality of life (QoL) [18, 19]. The PGI-S improved significantly at weeks 4, 16, 20, and 24, and the MDS-UPDRS Part I improved at weeks 12 and 36 in the IST group. Previous studies showed that IST improved non-motor symptoms such as daytime sleepiness, apathy, and fatigue, which are difficult to treat with levodopa [20,21,22]. These effects may contribute to the improvement of MDS-UPDRS Part I. Although MDS-UPDRS Part II, which includes QoL-related items [23], was ameliorated in the control group at weeks 12 and 24, PDQ-39 decreased in the IST group at week 12. Overall, patients in the IST group reported more improved patient-reported outcome items than those in the control group, suggesting that the administration of IST may provide better QoL in patients with PD.
This study was designed as an open-label trial and as such was subject to critical performance bias. We therefore analyzed data from a triaxial accelerometry monitoring system, which did not include evaluator subjectivity, to overcome this issue and confirmed a correlation between CGI-S and accelerometry parameters, such as frequency of motion while awake and intensity of daily exercise. These parameters improved significantly from baseline to week 36 in the IST group and were correlated with MDS-UPDRS Part III. IST may thus have a greater effect on motor performance than levodopa administration alone for 36 weeks. Furthermore, the improvement in motor activity parameters from wearable devices may reflect this amelioration in off-time. Walk pitch and walk balance decreased in the IST group; however, these parameters were not correlated with CGI-S or MDS-UPDRS Part III, suggesting that deterioration of these parameters might have minimal influence on the patient’s activities of daily life. A previous single-arm, open-label, prospective, multicenter study revealed that the administration of IST ameliorated the gait-related total scores of MDS-UPDRS Part III from baseline, with significant improvements in gait, gait freezing, and postural stability [24]. In this context, the decrease in walk pitch may reflect the improvement of small steps. IST might be effective on gait disturbance, which is sometimes difficult to treat with levodopa.
This study had several limitations. First, this was an open-label study with performance bias, and the increase in levodopa dose may have been affected by the participants’ judgment. If a patient disliked the idea of increasing their drug treatment, the administration of levodopa may have been suppressed. In the present study, there was no improvement in accelerometry parameters in the control group. This might be associated with a hesitation to increase the levodopa dose. However, the participation by many centers reduces investigator bias. Second, the diagnosis of PD was based on clinical features rather than pathology, and some patients with atypical parkinsonism might thus have been included. However, there was no notable difference in the backgrounds of the two groups, suggesting that this did not influence the analysis. Third, we did not validate the association between the accelerometry parameters and motor symptoms in PD patients. However, the accelerometry parameters were correlated with CGI-S and MDS-UPDRS Part III scores, indicating that the system may reflect motor performance in patients with PD. Fourth, we used CGI as a criterion for levodopa dose escalation based on methods such as those described by Watts et al. [14], who determined the optimal dose while observing the patient's condition. Finally, we set the research period as 37 weeks because this period was long enough for levodopa dose escalation to occur based on a report by Watts et al. [14]. Furthermore, levodopa dose escalation without changing other drugs over a long period could lead to problems, such as the onset of levodopa-induced ADRs. However, an observation period of 37 weeks might not provide adequate time to identify alterations in the necessary levodopa dose [12]. Therefore, long-term studies will be needed in the future. Despite these limitations, we believe that the current results show that IST administration can reduce levodopa dose escalation in patients with PD.
Conclusion
Treatment with IST effectively reduced levodopa dose escalation in patients with PD, resulting in less cumulative levodopa use during the study period. Data from a wearable accelerometry device suggested that IST resulted in greater objective improvements in motor function than increased levodopa dose. This study clarified the effectiveness of adjunctive IST compared with increased doses of levodopa in PD patients with wearing-off.
Data Availability
Taku Hatano takes responsibility for the integrity of the data and the accuracy of the data analysis. The datasets generated during and/or analyzed during the current study are not publicly available because permission for their secondary use, including data sharing, has not been obtained from the participants in this study.
References
Cilia R, Akpalu A, Sarfo FS, et al. The modern pre-levodopa era of Parkinson’s disease: insights into motor complications from sub-Saharan Africa. Brain. 2014;137:2731–42. https://doi.org/10.1093/brain/awu195.
Athulya RT, Jayakrishnan S, Iype T, Rajan R, Alapatt PJ. Predictors of levo-dopa induced dyskinesias in Parkinson’s disease. Ann Indian Acad Neurol. 2020;23:44–7. https://doi.org/10.4103/aian.AIAN_460_18.
Rascol O, Fabbri M, Poewe W. Amantadine in the treatment of Parkinson’s disease and other movement disorders. Lancet Neurol. 2021;20:1048–56. https://doi.org/10.1016/S1474-4422(21)00249-0.
Tsuboi Y, Nakamura M, Maruyama H, Matsumoto Y. Zonisamide improves wearing off in Parkinson’s disease without exacerbating dyskinesia: post hoc analysis of phase 2 and phase 3 clinical trials. J Neurol Sci. 2021;430: 120026. https://doi.org/10.1016/j.jns.2021.120026.
Ijzerman AP, Jacobson KA, Müller CE, Cronstein BN, Cunha RA. International union of basic and clinical pharmacology. CXII: adenosine receptors: a further update. Pharmacol Rev. 2022;74:340–72. https://doi.org/10.1124/pharmrev.121.000445.
Jenner P, Mori A, Kanda T. Can adenosine A2A receptor antagonists be used to treat cognitive impairment, depression or excessive sleepiness in Parkinson’s disease? Parkinsonism Relat Disord. 2020;80(Suppl 1):S28–36. https://doi.org/10.1016/j.parkreldis.2020.09.022.
Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27:27–42. https://doi.org/10.1111/ene.14108.
Jenner P, Mori A, Hauser R, Morelli M, Fredholm BB, Chen JF. Adenosine, adenosine A2A antagonists, and Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:406–13. https://doi.org/10.1016/j.parkreldis.2008.12.006.
Bara-Jimenez W, Sherzai A, Dimitrova T, et al. Adenosine A(2A) receptor antagonist treatment of Parkinson’s disease. Neurology. 2003;61:293–6. https://doi.org/10.1212/01.wnl.0000073136.00548.d4.
Uchida S, Tashiro T, Kawai-Uchida M, Mori A, Jenner P, Kanda T. The adenosine A2A-receptor antagonist istradefylline enhances the motor response of L-DOPA without worsening dyskinesia in MPTP-treated common marmosets. J Pharmacol Sci. 2014;124:480–5. https://doi.org/10.1254/jphs.13250fp.
Yabe I, Kitagawa M, Takahashi I, Matsushima M, Sasaki H. The efficacy of istradefylline for treating mild wearing-off in Parkinson disease. Clin Neuropharmacol. 2017;40:261–3. https://doi.org/10.1097/WNF.0000000000000249.
Hatano T, Kano O, Sengoku R, et al. Evaluating the impact of adjunctive istradefylline on the cumulative dose of levodopa-containing medications in Parkinson’s disease: study protocol for the ISTRA ADJUST PD randomized, controlled study. BMC Neurol. 2022;22:71. https://doi.org/10.1186/s12883-022-02600-w.
Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–70. https://doi.org/10.1002/mds.22340.
Watts RL, Lyons KE, Pahwa R, et al. Onset of dyskinesia with adjunct ropinirole prolonged-release or additional levodopa in early Parkinson’s disease. Mov Disord. 2010;25:858–66. https://doi.org/10.1002/mds.22890.
Horváth K, Aschermann Z, Ács P, et al. Minimal clinically important difference on the motor examination part of MDS-UPDRS. Parkinsonism Relat Disord. 2015;21:1421–6. https://doi.org/10.1016/j.parkreldis.2015.10.006.
Li ZJ, Wu Q, Yi CJ. Clinical efficacy of istradefylline versus rTMS on Parkinson’s disease in a randomized clinical trial. Curr Med Res Opin. 2015;31:2055–8. https://doi.org/10.1185/03007995.2015.1086994.
Tao Y, Liang G. Efficacy of adenosine A2A receptor antagonist istradefylline as augmentation for Parkinson’s disease: a meta-analysis of randomized controlled trials. Cell Biochem Biophys. 2015;71:57–62. https://doi.org/10.1007/s12013-014-0162-7.
Schwarzschild MA, Ascherio A, Casaceli C, et al. Effect of urate-elevating inosine on early Parkinson disease progression: the SURE-PD3 randomized clinical trial. JAMA. 2021;326:926–39. https://doi.org/10.1001/jama.2021.10207.
Gray R, Patel S, Ives N, et al. Long-term effectiveness of adjuvant treatment with catechol-o-methyltransferase or monoamine oxidase B inhibitors compared with dopamine agonists among patients with Parkinson disease uncontrolled by levodopa therapy: the PD MED randomized clinical trial. JAMA Neurol. 2022;79:131–40. https://doi.org/10.1001/jamaneurol.2021.4736.
Nagayama H, Kano O, Murakami H, et al. Effect of istradefylline on mood disorders in Parkinson’s disease. J Neurol Sci. 2019;396:78–83. https://doi.org/10.1016/j.jns.2018.11.005.
Suzuki K, Miyamoto M, Miyamoto T, et al. Istradefylline improves daytime sleepiness in patients with Parkinson’s disease: an open-label, 3-month study. J Neurol Sci. 2017;380:230–3. https://doi.org/10.1016/j.jns.2017.07.045.
Abe K, Fujita M, Yoshikawa H. Effectiveness of istradefylline for fatigue and quality of life in Parkinson’s disease patients’ and of their caregivers’. Adv Parkinson’s Dis. 2016;5:24–8. https://doi.org/10.4236/apd.2016.52004.
Tu XJ, Hwang WJ, Ma HI, Chang LH, Hsu SP. Determinants of generic and specific health-related quality of life in patients with Parkinson’s disease. PLoS ONE. 2017;12: e0178896. https://doi.org/10.1371/journal.pone.0178896.
Iijima M, Orimo S, Terashi H, et al. Efficacy of istradefylline for gait disorders with freezing of gait in Parkinson’s disease: a single-arm, open-label, prospective, multicenter study. Expert Opin Pharmacother. 2019;20:1405–11. https://doi.org/10.1080/14656566.2019.1614167.
Acknowledgements
The authors thank the patients who participated in the clinical trial, and all study investigators, study coordinators, and other personnel for their contributions to the study.
Medical Writing, Editorial, and Other Assistance
Editorial assistance in the preparation of this article was provided by Kazuyoshi Masuda, PhD, of ASCA Corporation (www.asca-co.com) funded by Kyowa Kirin Co., Ltd.
Funding
Sponsorship for this study was funded by Kyowa Kirin Co., Ltd., Tokyo, Japan. The sponsor was not involved in the study processes, including data management, monitoring/audits, statistical analysis, and interpretation of results, except for providing the wearable device. The Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Conceptualization: Taku Hatano, Osamu Kano, Renpei Sengoku, Hiroshi Nagayama; Organization: Taku Hatano; Execution: Taku Hatano, Renpei Sengoku, Hiroshi Nagayama, Naotake Yanagisawa, Asako Yoritaka, Keisuke Suzuki, Noriko Nishikawa, Yohei Mukai, Kyoichi Nomura, Norihito Yoshida, Morinobu Seki, Miho Kawabe Matsukawa, Hiroo Terashi, Katsuo Kimura, Jun Tashiro, Shigeki Hirano, Hidetomo Murakami, Hideto Joki, Tsuyoshi Uchiyama, Hideki Shimura, Kotaro Ogaki, Jiro Fukae, Yoshio Tsuboi, Kazushi Takahashi, Toshimasa Yamamoto, Kenichi Kaida, Ryoko Ihara, Kazutomi Kanemaru, and Osamu Kano; Statistical Analysis: Naotake Yanagisawa; Manuscript Writing – First Draft: Taku Hatano; Manuscript Review and Critique: Taku Hatano, Renpei Sengoku, Hiroshi Nagayama, Naotake Yanagisawa, Asako Yoritaka, Keisuke Suzuki, Noriko Nishikawa, Yohei Mukai, Kyoichi Nomura, Norihito Yoshida, Morinobu Seki, Miho Kawabe Matsukawa, Hiroo Terashi, Katsuo Kimura, Jun Tashiro, Shigeki Hirano, Hidetomo Murakami, Hideto Joki, Tsuyoshi Uchiyama, Hideki Shimura, Kotaro Ogaki, Jiro Fukae, Yoshio Tsuboi, Kazushi Takahashi, Toshimasa Yamamoto, Kenichi Kaida, Ryoko Ihara, Kazutomi Kanemaru, and Osamu Kano; Approval of the Final Version of the Manuscript: Taku Hatano, Renpei Sengoku, Hiroshi Nagayama, Naotake Yanagisawa, Asako Yoritaka, Keisuke Suzuki, Noriko Nishikawa, Yohei Mukai, Kyoichi Nomura, Norihito Yoshida, Morinobu Seki, Miho Kawabe Matsukawa, Hiroo Terashi, Katsuo Kimura, Jun Tashiro, Shigeki Hirano, Hidetomo Murakami, Hideto Joki, Tsuyoshi Uchiyama, Hideki Shimura, Kotaro Ogaki, Jiro Fukae, Yoshio Tsuboi, Kazushi Takahashi, Toshimasa Yamamoto, Kenichi Kaida, Ryoko Ihara, Kazutomi Kanemaru, and Osamu Kano.
Corresponding author
Ethics declarations
Conflict of Interest
Taku Hatano reports receiving grants from the Setsuro Fujii Memorial, the Osaka Foundation for Promotion of Fundamental Medical Research, JSPS KAKENHI (under grant number 21K07424), Japan Agency for Medical Research and Development (grant number 20dm0107156, 21wm0425015, and 21dk0207055); speaker’s honoraria from Sumitomo Pharma Co., Ltd., Takeda Pharmaceutical Co. Ltd., Novartis Pharma K.K., Sanofi K.K., Eisai Co. Ltd. and Otsuka Pharmaceutical Co., Ltd. during the conduct of the study. Taku Hatano also reports receiving grants and speaker’s honoraria from Kyowa Kirin Co., Ltd. during the conduct of the study. Renpei Sengoku reports no relevant disclosures. Renpei Sengoku, Hiroshi Nagayama, Naotake Yanagisawa, Keisuke Suzuki, and Hiroo Terashi report grants and consultation fees from Kyowa Kirin Co., Ltd. during the conduct of the study. Asako Yoritaka received speaker’s honoraria from Kyowa Kirin Co., Ltd. during the conduct of the study. Noriko Nishikawa, Kyoichi Nomura, Norihito Yoshida, Morinobu Seki, Miho Kawabe Matsukawa, Shigeki Hirano, Hidetomo Murakami, Hideto Joki, Tsuyoshi Uchiyama, Kotaro Ogaki, Jiro Fukae, Kazushi Takahashi, and Toshimasa Yamamoto report grants from Kyowa Kirin Co., Ltd. during the conduct of the study. Hiroshi Nagayama reports no relevant disclosures. Naotake Yanagisawa reports no relevant disclosures. Asako Yoritaka received lecture fees from Sumitomo Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eisai Co., Ltd. and Kyowa Kirin Co., Ltd. Keisuke Suzuki reports receiving speaker honoraria from Kyowa Kirin Co. Ltd, Otsuka Pharmaceutical, Co. Ltd, Sumitomo Pharma Co. Ltd, Takeda Pharmaceuticals Co. Ltd, Eisai Co. Ltd, and Novartis Pharma K.K. Noriko Nishikawa reports no relevant disclosures. Yohei Mukai reports no relevant disclosures. Yohei Mukai reports receiving honoraria fee for lectures from Kyowa Kirin Co., Ltd. during the conduct of the study. Kyoichi Nomura reports no relevant disclosures. Norihito Yoshida reports no relevant disclosures. Morinobu Seki received a Takeda Japan Medical Affairs Funded Research Grant 2018, and grants from Kanae Foundation for the Promotion of Medical Science, outside the submitted work. Miho Kawabe Matsukawa reports no relevant disclosures. Hiroo Terashi reports no relevant disclosures. Katsuo Kimura reports personal fees from Medtronic Japan Co., Ltd., Boston Scientific Corporation, Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., AbbVie GK, Eisai Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Sumitomo Pharma Co., Ltd., and grants from Novartis AG, outside the submitted work. Katsuo Kimura reports grants and lecture fees from Kyowa Kirin Co., Ltd. during the conduct of the study. Jun Tashiro reports grants and non-financial support from Kyowa Kirin Co., Ltd. during the conduct of the study. Hideki Shimura reports grants and lecture fees from Kyowa Kirin Co., Ltd. during the conduct of the study. Yoshio Tsuboi, Kenichi Kaida, Ryoko Ihara, Kazutomi Kanemaru, and Osamu Kano report no relevant disclosures during the conduct of the study. Jun Tashiro reports grants and non-financial support from CSL Behring K.K. and personal fees and non-financial support from Kyowa Kirin Co., Ltd., Takeda Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., FP Pharmaceutical Corporation, Ono Pharmaceutical Co., Ltd., Eisai Co., Ltd., and AbbVie GK outside the submitted work. Shigeki Hirano reports grants from Eli Lilly Japan K.K., and grants and lecture fees from Nihon Medi-Physics Co., Ltd. outside the submitted work. Hidetomo Murakami reports no relevant disclosures. Hideto Joki reports no relevant disclosures. Tsuyoshi Uchiyama reports no relevant disclosures. Hideki Shimura reports no relevant disclosures. Kotaro Ogaki reports receiving grants from JSPS KAKENHI (under grant number 19K17047), speakers honoraria from Sumitomo Pharma Co., Ltd, Takeda Pharmaceutical Co. Ltd., Kyowa Kirin Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., FP Pharmaceutical Co. Ltd., Mochida Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd, and Eli Lilly Japan K.K. Jiro Fukae reports no relevant disclosures. Yoshio Tsuboi received personal fees from Eisai Co., Ltd., Takeda Pharmaceutical Co., Ltd., Novartis Pharma K.K., Sumitomo Pharma Co., Ltd., AbbVie GK, and Otsuka Pharmaceutical Co., Ltd., and Kyowa Kirin Co., Ltd., outside the submitted work; and were supported by a grant from Nipro Corporation. Kazushi Takahashi reports no relevant disclosures. Toshimasa Yamamoto reports no relevant disclosures. Kenichi Kaida reports no relevant disclosures. Ryoko Ihara reports no relevant disclosures. Kazutomi Kanemaru reports no relevant disclosures. Osamu Kano reports honoraria from AbbVie GK, Alexion Pharmaceuticals, Inc., Biogen Japan Ltd., Biogen Inc., Chugai Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Eisai Co., Ltd, Kyowa Kirin Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and scholarship grants from Eisai Co., Ltd., and Otsuka Pharmaceutical Co., Ltd., and writing fees from Mitsubishi Tanabe Pharma Co., outside the submitted work, and grants for commissioned work from Alexion Pharmaceuticals, Inc., and Mitsubishi Tanabe Pharma Co.
Ethical Approval
We carried out the present study in compliance with the Japan Clinical Trials Act and all related national and international guidelines for human trials, and with the Declaration of Helsinki. The Juntendo University Certified Review Board (J18-006) reviewed and approved the study protocol (Version 6.0, 1 November 2021) and all other study documentation (approval number CRB3180012). This trial was registered with the Japan Registry of Clinical Trials (jRCTs031180248). All patients provided written informed consent before enrollment. We anonymized all patient data so that no personal information was associated with the wearable devices. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Additional information
Prior Presentation: Results reported here were presented as posters at the International Congress of Parkinson’s Disease and Movement Disorders (September 15–18, 2022, Madrid, Spain) and the American Academy of Neurology Annual Meeting (April 22–27, 2023, Boston, MA).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hatano, T., Sengoku, R., Nagayama, H. et al. Impact of Istradefylline on Levodopa Dose Escalation in Parkinson’s Disease: ISTRA ADJUST PD Study, a Multicenter, Open-Label, Randomized, Parallel-Group Controlled Study. Neurol Ther 13, 323–338 (2024). https://doi.org/10.1007/s40120-023-00574-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00574-6