Abstract
Introduction
Tonic motor activation (TOMAC) therapy is a novel non-pharmacologic treatment approach for patients suffering from medication-refractory restless legs syndrome (RLS). The objective of this study was to explore the potential cost-effectiveness of TOMAC in the US healthcare system.
Methods
A decision-analytic Markov model was constructed to project strategy-specific treatment costs and benefits over 3 years and lifetime. Cohort characteristics (mean age 57.4 years, 39.8% male) and treatment effects were derived from the sham-controlled RESTFUL study. Study-observed International RLS Study Group (IRLS) scores were used to estimate changes in healthcare resource utilization and quality of life based on mapping algorithms informed by published data. The incremental cost-effectiveness ratio (ICER) was evaluated against established willingness-to-pay thresholds of $50,000/$150,000 per QALY to determine cost-effectiveness. Extensive scenario analyses were performed, including longer-term extension study data.
Results
TOMAC and sham reduced IRLS scores from baseline 25.3 to 18.10 and 21.60, respectively, at 4 weeks (treatment effect – 3.4 vs. sham), with an increase in utility from 0.80 to 0.84 (0.75–0.84 vs. baseline). Over 3 years and lifetime, the TOMAC vs. sham effect size corresponded to an added 0.10 and 0.49 QALYs (2.36 vs. 2.26; 12.59 vs. 12.10) at incremental costs of $8061 and $36,373 ($36,707 vs. $28,646; $224,040 vs.$187,667), resulting in ICER estimates of $83,822 and $73,600, respectively. Compared to baseline, TOMAC resulted in ICER estimates of $29,569 and $23,690 over 3 years and lifetime, respectively. TOMAC remained cost-effective or dominant across all scenarios, with ICERs ranging from $10,530–$83,822 and − $8061 to $29,569 vs. sham and baseline, respectively. Larger TOMAC effect sizes, achieved per extension study data, further increased cost-effectiveness.
Conclusion
Based on this exploratory analysis of published trial data, TOMAC therapy appears to offer meaningful improvements in patient health-related quality at net costs that render it a cost-effective intervention. Further analyses are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The current treatment regimen for RLS patients relies primarily upon pharmacologic interventions; however, standard treatment does not always result in meaningful symptom relief nor is it an option for medication-refractory RLS patients |
TOMAC therapy, most recently studied in the RESTFUL trial, provides a novel non-invasive neurostimulation treatment for RLS now cleared by the United States Food and Drug Administration (FDA) |
The potential cost-effectiveness of TOMAC therapy has not previously been studied |
What was learned from the study? |
In the current exploratory cost-effectiveness evaluation, TOMAC therapy was found to improve patient outcome at incremental cost, which renders it a cost-effective treatment strategy for medication-refractory RLS patients, providing good value to healthcare payers |
Future studies will benefit from further detailed data collection about resource utilization and costs incurred by RLS patients at different symptom severity |
Introduction
Restless legs syndrome (RLS) is characterized by uncontrollable movement urges of the lower limb, primarily during nocturnal hours [1,2,3]. As a result, patients with this chronic neurologic disorder experience frequent sleep disruptions and reduced quality of life and incur a heightened risk for the development of other chronic conditions and comorbidities like diabetes or cardiovascular disease [3]. RLS is more common among women, but individuals diagnosed with the condition range in age from childhood to the elderly.
The current treatment regimen for clinical RLS consists of pharmacologic interventions, primarily with the prescription of dopamine agonists or alpha-2-delta ligands, while alternate treatment modalities consist of lifestyle interventions, most commonly through the encouragement of good sleep habits or iron supplementation [4, 5]. However, pharmacologic interventions often do not provide long-term benefits; dopamine agonists frequently lead to augmentation—paradoxical worsening of RLS severity [2]—and alpha-2-ligands and opiates have side effects that limit tolerability. Patients with medication-refractory RLS are considered to be those who experience the aforementioned effects, with one or more RLS pharmacologic treatment options deemed to be intolerable or non-efficacious [6]. As a result, medication-refractory RLS patients have limited available therapeutic alternatives. Approximately 2–3% of the adult US population suffers from moderate-severe RLS, and > 50% of patients develop resistance to medication [2,3,4,5, 7]. As a result, medication-refractory RLS represents a substantial unmet need.
Tonic motor activation (TOMAC) therapy, a novel form of noninvasive peripheral nerve stimulation, presents a promising treatment alternative for medication-refractory RLS patients [2, 6, 8]. The TOMAC therapy device (Noctrix NTX100 system, Noctrix Health Inc., Pleasanton, CA, USA), which was recently studied in the sham-controlled RESTFUL study (NCT04874155), consists of two wearable stimulation units, which are worn bilaterally around the lower leg [2, 6]. The high-frequency noninvasive peroneal nerve stimulation reduces RLS symptoms by evoking tonic increases in tibialis anterior muscle tone [6, 9]. By comparison, TOMAC causes neuromuscular behavior similar to that achieved through voluntary leg movements [6]. Treatment is self-administered and involves 30 min of stimulation administered by the patient when RLS symptoms are present [2, 6].
Over the last decade, the significant health-related quality of life and economic burden of RLS have received increasing attention, with several studies conducted in the US and European healthcare systems consistently documenting the substantial challenge associated with RLS treatment [10,11,12,13]. Novel solutions such as TOMAC may help to meaningfully address this clinical and economic burden. The objective of the current analysis was to provide an early directional assessment of the potential cost-effectiveness of TOMAC therapy, building on and complementing the recent clinical evidence collected in the sham-controlled clinical study that formed the basis for market authorization by the US Food and Drug Administration (FDA).
Methods
Overview
A decision-analytic Markov model was developed from the perspective of the US healthcare system to project therapy-specific cost and quality-of-life outcomes over 36 months and lifetime for both TOMAC and status quo. Cohort characteristics were based on the RESTFUL study. The relationships between RLS severity—as measured through International RLS Study Group (IRLS) scores—and health-related quality of life and resource utilization were encoded based on prior study data. These functional relationships were subsequently used to calculate therapy-induced gains in quality-adjusted life years (QALYs) and reductions in healthcare utilization commensurate with the IRLS effect size observed in the RESTFUL study. Costs were derived from a systematic search of published literature, current fee schedules, and the anticipated reimbursement of TOMAC therapy. Cost-effectiveness was evaluated at 36 months (the anticipated lifetime of the stimulation device before a new device needs to be acquired/reimbursed) and over the patients’ lifetime, with extensive scenario analyses conducted.
Model Framework
The decision-analytic model incorporated a cycle length of 3 months. Modeled health states were ‘on treatment,’ ‘off treatment’ (applying to TOMAC only, where a patient would discontinue therapy), and death, where quality of life and costs incurred in the ‘on treatment’ and ‘off treatment’ states were further defined by IRLS level. Cohort survival was assumed to not differ between the treatment strategies and was informed by gender-specific general population lifetables adjusted by an RLS-associated mortality hazard ratio. The primary outcome was the incremental cost-effectiveness ratio (ICER), in US dollars per QALY gained, which was evaluated against the established cost-effectiveness thresholds of $50,000 per QALY gained (cost-effective, high value) and $150,000 per QALY gained (cost-effective, of value). All costs and effects were discounted at 3% p.a.
Model Inputs
Detailed model inputs are shown in Table 1.
Clinical Data
The cohort and base case effectiveness assumption was based on the published findings from the RESTFUL study (NCT04874155) [6]. This multicenter, randomized, double-blind, sham-controlled trial enrolled patients with primary moderate to severe RLS [International RLS Study Group Rating Scale (IRLS) total score ≥ 15], symptoms occurring at least 2 nights per week, and with the frequency of symptoms occurring most in the lower legs and near bedtime [2, 6]. The RESTFUL trial further specified patients with medication-refractory RLS as an inclusion criteria [6]. Trial participants were required to maintain a stable dose of RLS medication during the study and for 30 days prior to study entry [2, 6]. During the first phase in RESTFUL, N = 133 study participants were randomly assigned 1:1 to TOMAC (n = 68) or sham control (n = 65) [6]. After 4 weeks of treatment, all participants remaining in the study (n = 128) were then assigned to active TOMAC treatment for an additional 4 weeks [6]. The primary safety and efficacy endpoints included the documentation of adverse events and the Clinical Global Impressions-Improvement (CGI-I) responder rate at 4 weeks [6]. The change in total IRLS score, which is most relevant for the current health economic study because of its previously reported implication for patient quality of life and resource utilization, was collected as a prespecified key secondary endpoint. Additional data from the recently published extension study reporting on TOMAC treatment through 24 weeks informed additional scenario calculations, as further described below [14].
Cost and Resource Utilization
Cost and resource utilization for RLS patient was sourced from published survey data [15]. The functional relationship was explored between data corresponding to the rate of ER visits, hospitalizations, and healthcare provider visits by IRLS score, which then allowed for the derivation of resource utilization specific to the trial-observed IRLS score at 4 weeks for both the TOMAC and status quo cohorts. Published literature and current fee schedules informed the health event costs relied upon in the model [15]. However, as unit costs in the published study appeared to be high—possibly as a result of using commercial payment amounts—the model base case conservatively assumes only 50% of the published ER costs and 40% of hospitalization and alternate provider visit costs. This adjustment in the base case was informed by an analysis of the cost to Medicare for an inpatient treatment episode with primary diagnosis of RLS and by the current Medicare payment amount for a Level 5 neurology office visit and resulted in more conservative cost estimates throughout. Monthly medication costs were estimated using utilization data from trial-collected medication logs and published RLS treatment regimens. These costs are accounted for in the model, but given the costs are applied equally to both TOMAC and the comparator, there is no direct impact on the ICER reported under the current or any alternate cost assumption. Further information on the methods utilized to estimate resource utilization and alternate costs, as well as the derivation of monthly medication costs, can be found in the Supplemental Materials, S.3, S.5, and S.4, respectively.
Survival and Quality-of-Life Assumptions
Gender- and age-specific mortality rates from US lifetable data informed the long-term cohort survival projected in the model. Mortality rates observed were further adjusted to reflect the RLS population, with the application of a literature-informed hazard ratio of 1.52 [16]. Both the TOMAC and status-quo cohorts were assumed to incur the same elevated risk of mortality.
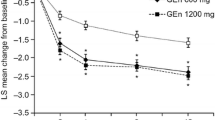
The utility estimates relied upon in the model were informed by EQ-5D scores specific to RLS severity, as denoted by the IRLS scale. Two relevant published sources—specific to German and UK RLS patient samples—were identified through the systematic search conducted [17, 18]. A regression analysis was conducted separately for each dataset to derive a predictive function that allowed for the estimation of EQ-5D scores for a specific IRLS score. Figure 1a illustrates the trial-observed IRLS effect sizes informing the model, while the relationship between EQ-5D and IRLS scores is shown in Fig. 1b. The health-related quality of life data from Happe et al. were utilized to inform the utility values relied upon in the model base case, given the cohort was most comparable to the RESTFUL cohort, though the alternate utilities from Lees et al. were explored in sensitivity analyses. Additional background information on the systematic search and underlying methods utilized in the derivation of EQ-5D utilities are detailed in the Supplemental Materials, S.1 and S.2, respectively.
Change in International RLS Study Group Rating Scale (IRLS) score at 4 weeks from the RESTFUL Study (a) and EQ-5D utility estimates calculated from mean IRLS scores in RESTFUL Study and literature-derived relationship between IRLS and EQ-5D (b). The boxplot shows TOMAC in orange and sham in blue, denoting the median and first and third quartile values, and whisker length is the lesser of 1.5*interquartile range or outermost data point
TOMAC Costs and Adherence Assumptions
TOMAC device cost, based on manufacturer-provided information, was assumed to be $7500. The device cost was assumed to be incurred in equal amounts over the first 13 months of use, in line with the anticipated durable medical equipment (DME) payment schedule. The stimulation device has a lifetime of 36 months. Hence, costs for a new device were accounted for every 3 years for those patients remaining on therapy. The cost of consumables was assumed to be $75 per month.
Subsequent device and consumable costs beyond the index billing cycle were assumed to be incurred only for patients on active therapy use, which was defined as a minimum of one 30-min treatment session per month [6]. The model conservatively assumed 80% of the cohort remained active users, based on evidence from the RESTFUL extension study, which demonstrated that 91% of extension trial participants continued to utilize the device over 6+ months of follow-up [14]. Patients who discontinued treatment were assumed to retain some limited quality of life and resource utilization benefit on grounds that trial evidence suggests that patients continued to retain partial symptom improvement for at least 24 weeks after cessation of device usage [14]. The base case assumed 50% of quality of life and resource utilization benefits were maintained, while scenarios explored a range of different assumptions, including no maintained benefit.
Base Case
The analysis base case relied on a − 3.4 change in IRLS score vs. sham (corresponding change from baseline of − 7.2 for TOMAC vs. − 3.8 for sham at 4 weeks, which was assumed to be maintained in the long-term). ICERs were calculated for a 36-month horizon (one device lifecycle) and for the patient’s lifetime. In light of limited data to date, the base case conservatively assumed no reduction in medication utilization for TOMAC users.
Scenario Analysis
Comprehensive one- and multi-way sensitivity analyses were conducted, exploring the impact of varied model inputs for both the IRLS effect size observed between TOMAC vs. baseline and vs. sham. The one-way sensitivity analyses explored included varied assumptions surrounding the device and health events costs as well as the utilities. In addition, the 36-month and lifetime resulting ICERs from a multi-way sensitivity analysis, varying both events costs and utilities, are presented in a heat map, encoded corresponding to the cost-effectiveness favorability of the resulting ICER. The incremental percent difference between the base case and literature-sourced values informed the range of inputs explored. Additionally, further scenarios were calculated using IRLS reductions reported in the recent extension study, which reported on IRLS reductions through 24 weeks of TOMAC treatment [14]. The range of inputs explored in all sensitivity analyses is presented in Table 1.
Ethics Compliance
The underlying RESTFUL clinical study from which data for this analysis were obtained was conducted in accordance with the International Conference on Harmonization guidelines on good clinical practice and the Declaration of Helsinki and was designed with input from the US Food and Drug Administration (FDA). A blinded independent medical monitor was responsible for adjudicating adverse events (AEs). The study protocol and informed consent were approved by a central institutional review board (Advarra, Columbia, MD, USA). All participants provided informed consent. The trial was preregistered (ClinicalTrials.gov number, NCT04874155) on May 5, 2021.
Results
Base Case
Compared to sham control, TOMAC added 0.10 QALYs over 3 years and 0.49 QALYs over lifetime, at concurrent incremental cost of $8061 and $36,373, respectively. The resulting ICER at 36 months and over lifetime was $83,822 and $73,600, respectively. See Table 2.
Scenario Analysis
Key results from the one-way deterministic sensitivity and scenario analyses are shown in Fig. 2a–c. As shown in Fig. 2a, utilities from the alternative IRLS-EQ-5D function based on the study by Lees et al. led to more pronounced QALY gains over time, as did the exploratory effect size comparison vs. baseline (as opposed to sham). Figure 2b shows corresponding incremental costs over time. The stepwise function reflects the TOMAC device costs incurred for device replacement every 3 years. As is visible from the chart, incremental costs decrease over time. The decreased cost difference observed when modeling the larger IRLS effect size (TOMAC vs. baseline) can be attributed to larger reductions in healthcare resource utilization. Figure 2 plots the resulting ICERs over time. Notably, TOMAC was found cost-effective or dominant (associated with lower total cost than the comparator, at concurrent outcome improvement) across all tested scenarios. See Supplementary Materials Figures S.6.2.a–c for further detailed results of the full range of scenario analyses conducted.
Incremental quality-adjusted life year (QALY) gains (a), costs (b), and resulting incremental cost-effectiveness ratios (ICERs) (c) for TOMAC therapy in the base case and scenario analyses. Blue denotes scenarios with base case costs and Happe utilities; fuchsia details average utilities (a), average costs (b), and base costs with Lees utilities (c); teal represents base costs and Lees utilities (a), Full Durgin/Literature costs (b), and Full Durgin/Literature costs and Happe utilities (c); purple illustrates Full Durgin/Lit costs and Lees utilities (c). Scenarios comparing TOMAC vs. Sham are denoted by solid lines, while dashed lines represent comparisons to baseline. In panel c, the horizontal lines represent ranging WTP thresholds including dominant (black), highly cost-effective (light green), and maroon (cost-effective)
Multi-way sensitivity analyses explored the impact of varied health event costs and utilities over 3 years and the lifetime horizon. The resulting ICERs are presented as ‘heat maps’ in Figs. 3a–f. All alternative scenarios explored resulted in lower and therefore more cost-effective ICERs than the base case. Results ranged from a cost-effective finding for the base case at a 3-year ICER of around $84,000 per QALY—substantially below the $150,000 per QALY willingness-to-pay threshold—and dominance for TOMAC (lower cost at concurrent QALY gain) when the literature costs from Durgin et al. were considered in conjunction with the Lees et al. EQ-5D utility values. Using the 24-week effectiveness data from the extension study (– 5.9 treatment effect, TOMAC vs. control) further increased cost-effectiveness findings throughout, based on the higher effect sizes observed during longer follow-up [14]. See Supplementary Materials S.6 for additional figures detailing the change in QALYs and costs corresponding to key scenarios included in the heat maps.
Scenario analyses: Incremental cost-effectiveness ratios (ICERs) for TOMAC vs. sham over 3 years (a) and lifetime (b) and for TOMAC vs. baseline over 3 years (c) and lifetime (d) for different cost and utility assumptions. Panels (e) and (f) show the potential cost-effectiveness based on 24-week data from the extension study. The color gradient displayed corresponds to the favorability of the resulting ICER, with yellow indicating moderate cost-effectiveness and dark green being highly cost-effective
Results from a threshold analysis suggest TOMAC, under the base case assumptions, would remain cost-effective up to a device cost of $14,363. Similarly, TOMAC remained cost-effective at an IRLS reduction of 1.84 between TOMAC and sham.
Discussion
This exploratory study assessed the potential cost-effectiveness of TOMAC therapy for the treatment of refractory RLS. TOMAC was found to provide good value for money, meaningfully improving patient outcomes at an overall net cost difference that renders TOMAC a cost-effective intervention, compared to sham and baseline, and both in the shorter-term and—if therapy is maintained—even more so over the lifetime horizon. Findings of the comprehensive additional scenarios suggest cost-effectiveness findings might be even more favorable than reported for the base case, which was based on a set of conservative assumptions on costs, utility, and treatment effect.
As demonstrated in the randomized, double-blind, sham-controlled RESTFUL study, TOMAC stimulation therapy provides a safe, effective, and novel noninvasive peripheral nerve stimulation that provides a valuable treatment alternative for medication-refractory RLS patients. The therapy has recently received FDA authorization and, as such, additional real-world experience and data will become available in the future that will help to further corroborate the therapy’s clinical value proposition. Additionally, the publication and conduct of a 24-week extension study provided further insight into the therapy’s long-term safety and effectiveness [14]. In addition, scenario analyses explored relying upon these data further confirm TOMAC to be a cost-effective intervention.
The objective of the current study was to conduct an exploratory rather than a definitive cost-effectiveness analysis. As such, it provides an early perspective about the main drivers of TOMAC’s expected health-economic value and early orientation about the potential cost-effectiveness. These insights—particularly the relevance of quality of life improvement with achieved symptom relief and resulting reductions in resource utilization—will be useful to inform future more definitive analyses that should be conducted as more experience is gained with the therapy and further study data become available.
The analysis is subject to several limitations. First, the assumed effectiveness was based on the 4-week results of the RESTFUL study, the protocol-defined time frame for the primary analysis of the therapy’s safety and effectiveness. Projecting this short-term effect over lifetime is subject to significant uncertainty. However, follow-up data from the RESTFUL study beyond 4 weeks demonstrate an increase in treatment effect rather than decrease over time [14]. Furthermore, prior cost-effectiveness analyses of neurostimulation treatments and therapies, such as continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea, do rely on comparable short-term data to project long-term effects. The current study, however, explored the potential effect of reduced and increased effectiveness in a threshold analysis and presented a scenario based on 24-week outcomes reported in the recent extension study. Second, as in any patient-used therapy and in neurostimulation treatments, issues of therapy adherence and potential non-response to treatment affect long-term patient outcomes and costs and need to be properly reflected. The current study assumed 80% therapy compliance in the long-term, which seems well supported by data available to date. In this context, it is important to keep in mind that patients treated with TOMAC are medication-refractory RLS patients who have exhausted other treatment options and who have a substantial maintained disease burden. It can reasonably be expected that these patients will continue to use the therapy if it provides sufficient symptom improvement. The assumption that the overall cohort effect will only gradually be affected by 20% of the cohort discontinuing treatment (implemented in the analysis by assuming patients off therapy retain 50% of the prior modeled benefit) seems reasonable for this very reason—those discontinuing therapy are more likely than not to be non-responders, and their results already affected the therapy effectiveness of the full cohort. Nevertheless, alternative assumptions of zero and full benefit maintained in those discontinuing therapy were explored and did not materially change the results. Third, quality of life and cost data were derived based on previously published studies and survey data. While these were large sample studies in reasonably comparable RLS cohorts, this approach introduces uncertainty. However, this was addressed by exploring different sources for the IRLS-to-EQ-5D mapping and choosing the more conservative source for the base case. Future, more definitive TOMAC cost-effectiveness studies will benefit from study-collected EQ-5D quality of life data. Further, the analysis relied on data from Durgin et al. to establish a relationship between IRLS severity and utilization of provider visits, ED treatments, and inpatient admissions, but a conscious choice was made to down-adjust the unit cost data per visit, ED treatment, and hospitalization. This deviation from previously reported cost was grounded in the authors’ assessment that Medicare-incurred costs might be lower, as supported by a review of the current fee schedule and recent Medicare cost data. The use of these lower cost assumptions in the base case can be regarded as conservative, as using the data as previously reported in the Durgin study leads to much more sizable savings with TOMAC therapy and, in consequence, to substantially more favorable cost-effectiveness findings that even included TOMAC dominance. Furthermore, in the absence of an established DME reimbursement for TOMAC, the analysis assumed a manufacturer-provided cost estimate of $7500 plus monthly incurred cost of $75 per device. While it is likely that reimbursement will be provided in this magnitude, some uncertainty remains. This was addressed by conducting additional threshold and scenario analyses. Future studies will benefit from TOMAC trial-collected information about resource utilization and costs in both arms of the study. Additionally, detailed data about the contemporary long-term RLS-specific health care utilization and costs, possibly analyzed and provided from current ongoing RLS registries in the US, could further benefit TOMAC and—more broadly—any future cost-effectiveness evaluation of RLS therapies. Finally, RLS-specific healthcare costs are primarily driven by prescription and outpatient treatment costs, which would suggest that the model assumption of no change in prescription utilization with TOMAC therapy may be conservative [10]. For example, recent data suggest a potential reduction in opioid utilization for patients on TOMAC treatment [19]. Broadly, a reduced need for pharmacologic treatments might also lower the risk of augmentation or side effects for patients receiving standard treatments, which could lead to additional benefits not accounted for in the current analysis [1, 20,21,22]. Further evidence is warranted to model any potential reduction with necessary certainty.
Conclusion
Based on available trial data, this exploratory health-economic analysis found TOMAC therapy to offer meaningful improvements in patient health-related quality at net costs that render it a cost-effective intervention across a broad range of therapy effectiveness, costs, and quality of life assumptions. Further analyses are warranted as more data become available.
Data Availability
Supporting data can be found in supplementary materials, with more information available from the corresponding author on reasonable request.
References
Buchfuhrer MJ. Strategies for the treatment of restless legs syndrome. Neurotherapeutics. 2012;9(4):776–90.
Buchfuhrer MJ, Baker FC, Singh H, Kolotovska V, Adlou B, Anand H, et al. Noninvasive neuromodulation reduces symptoms of restless legs syndrome. J Clin Sleep Med. 2021;17(8):1685–94.
Becker PM, Novak M. Diagnosis, comorbidities, and management of restless legs syndrome. Curr Med Res Opin. 2014;30(8):1441–60.
Guo S, Huang J, Jiang H, Han C, Li J, Xu X, et al. Restless legs syndrome: from pathophysiology to clinical diagnosis and management. Front Aging Neurosci. 2017;9:171.
Silber MH, Buchfuhrer MJ, Earley CJ, Koo BB, Manconi M, Winkelman JW, et al., editors. The management of restless legs syndrome: an updated algorithm. In: Mayo Clinic proceedings. 2021; Elsevier.
Bogan RK, Roy A, Kram J, Ojile J, Rosenberg R, Hudson JD, et al. Efficacy and safety of tonic motor activation (TOMAC) for medication-refractory restless legs syndrome: a randomized clinical trial. Sleep. 2023. https://doi.org/10.1093/sleep/zsad190.
Trotti LM, Goldstein CA, Harrod CG, Koo BB, Sharon D, Zak R, et al. Quality measures for the care of adult patients with restless legs syndrome. J Clin Sleep Med. 2015;11(3):293–310.
Roy A, Ojile J, Kram J, Rosenberg R, Olin J, Hudson JD, et al. 0713 Long-term response to tonic motor activation (TOMAC) therapy for refractory restless legs syndrome. Sleep. 2023;46(Supplement_1):A313.
Charlesworth JD, Adlou B, Singh H, Buchfuhrer MJ. Bilateral high-frequency noninvasive peroneal nerve stimulation evokes tonic leg muscle activation for sleep-compatible reduction of restless legs syndrome symptoms. J Clin Sleep Med. 2023;19:1199–209.
Allen RP, Bharmal M, Calloway M. Prevalence and disease burden of primary restless legs syndrome: results of a general population survey in the United States. Mov Disord. 2011;26(1):114–20.
Trenkwalder C, Tinelli M, Sakkas G, Dauvilliers Y, Ferri R, Rijsman R, et al. Socioeconomic impact of restless legs syndrome and inadequate restless legs syndrome management across European settings. Eur J Neurol. 2021;28(2):691–706.
Reinhold T, Müller-Riemenschneider F, Willich SN, Brüggenjürgen B. Economic and human costs of restless legs syndrome. Pharmacoeconomics. 2009;27:267–79.
Reese JP, Stiasny-Kolster K, Oertel WH, Dodel RC. Health-related quality of life and economic burden in patients with restless legs syndrome. Expert Rev Pharmacoecon Outcomes Res. 2007;7(5):503–21.
Roy A, Ojile J, Kram J, Olin J, Rosenberg R, Hudson JD, et al. Long-term efficacy and safety of tonic motor activation (TOMAC) for treatment of medication-refractory restless legs syndrome: a 24-week open-label extension study. Sleep. 2023. https://doi.org/10.1093/sleep/zsad188.
Durgin T, Witt EA, Fishman J. The humanistic and economic burden of restless legs syndrome. PLoS ONE. 2015;10(10): e0140632.
Cubo E, Gallego-Nieto C, Elizari-Roncal M, Barroso-Pérez T, Collazo C, Calvo S, et al. Is restless legs syndrome associated with an increased risk of mortality? A meta-analysis of cohort studies. Tremor Other Hyperkinet Mov. 2019. https://doi.org/10.5334/tohm.50.
Happe S, Reese JP, Stiasny-Kolster K, Peglau I, Mayer G, Klotsche J, et al. Assessing health-related quality of life in patients with restless legs syndrome. Sleep Med. 2009;10(3):295–305.
Lees M, Roberts G, Tabberer M, DasGupta R, Finnern H, Group RHES. Cost-effectiveness of licensed treatment options for restless legs syndrome in the UK and Sweden. Curr Med Res Opin. 2008;24(10):2919–30.
Buchfuhrer M, Rodriguez S, Charlesworth J. Enabling opioid dose reduction for refractory restless legs syndrome through adjunctive tonic motor activation (TOMAC) therapy (P13–11.002). AAN Enterprises; 2023.
Mackie S, Winkelman JW. Long-term treatment of restless legs syndrome (RLS): an approach to management of worsening symptoms, loss of efficacy, and augmentation. CNS Drugs. 2015;29:351–7.
Manconi M, Garcia-Borreguero D, Schormair B, Videnovic A, Berger K, Ferri R, et al. Restless legs syndrome. Nat Rev Dis Primers. 2021;7(1):80.
Drogan D, Schüssel K, Berger K, Trenkwalder C, et al. l-Dopa-Pharmakotherapie bei der Behandlung des Restless Leg Syndroms. In: Günster C, et al., editors. Versorgungs-Report Leitlinien: Evidenz für die Praxis. Berlin: MWV Medizinisch Wissenschaftliche Verlagsgesellschaft; 2023.
US Department of Veterans Affairs. Office of Procurement, Acquisition and Logistics (OPAL) Pharmaceutical Prices 2023. https://www.va.gov/opal/nac/fss/pharmPrices.asp.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103.
International Restless Legs Syndrome Study Group. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–32.
Medical Writing and Editorial Assistance
The authors thank Drs. Jessica Preciado and Jonathan Charlesworth (Noctrix Health, Inc.) for their review of the final manuscript and supporting materials.
Funding
This analysis was supported by funding from Noctrix Health, Inc., which also paid the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Jan B. Pietzsch conceived of the study. Anne M. Ryschon, Khoa N. Cao, and Jan B. Pietzsch developed the analysis model and performed data analyses. All authors contributed to the drafting and review of the manuscript, and all authors read and approved of the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Wing Tech Inc. (Anne M. Ryschon, Khoa N. Cao, Jan B. Pietzsch) provided health-economic consulting services to Noctrix, Inc. AR is an investigator in the underlying RESTFUL study.
Ethical Approval
The underlying RESTFUL clinical study from which data for this analysis were obtained was conducted in accordance with the International Conference on Harmonization guidelines on good clinical practice and the Declaration of Helsinki. The study protocol and informed consent were approved by a central institutional review board (Advarra, Columbia, MD, USA). All participants provided informed consent.
Additional information
Prior Presentation: This manuscript is based on work that, in part, has previously been presented at the SLEEP 2023 annual meeting (June 5, 2023, Indianapolis, IN).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ryschon, A.M., Cao, K.N., Roy, A. et al. Cost-Effectiveness of Tonic Motor Activation (TOMAC) Therapy for Patients with Restless Legs Syndrome: An Exploratory Analysis. Neurol Ther 12, 2133–2146 (2023). https://doi.org/10.1007/s40120-023-00551-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00551-z