Abstract
Introduction
The current therapeutic landscape of Alzheimer's disease (AD) is evolving rapidly. Our treatment options include new anti-amyloid-β protein disease-modifying therapies (DMTs) that decrease cognitive decline in patients with early AD (prodromal and mild AD dementia). Despite these advances, we have limited information on how neurologists would apply the results of recent DMT trials to make treatment decisions. Our goal is to identify factors associated with the use of new AD DMTs among neurologists applying concepts from behavioral economics.
Methods
This non-interventional, cross-sectional, web-based study will assess 400 neurologists with expertise in AD from across Spain. Participants will start by completing demographic information, practice settings, and a behavioral battery to address their tolerance to uncertainty and risk preferences. Participants will then be presented with 10 simulated case scenarios or vignettes of common encounters in patients with early AD to evaluate treatment initiation with anti-amyloid-β DMTs (e.g., aducanumab, lecanemab, etc.). The primary outcomes will be therapeutic inertia and suboptimal decisions. Discrete choice experiments will be used to determine the weight of factors influencing treatment choices.
Results
The results of this study will provide new insights into a better understanding of the most relevant factors associated with therapeutic decisions on the use of DMTs, assessing how neurologists handle uncertainty when making treatment choices, and identifying the prevalence of therapeutic inertia in the management of early AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recent approval of agents targeting amyloid-β protein are changing the current treatment landscape and management of early-stage Alzheimer's disease (AD). |
There is a need to know how neurologists make treatment decisions in a context of uncertainties related to new agents' clinical benefit and safety problems. |
This study will provide evidence for understanding the current gaps in pharmacological treatment decision-making in patients with early AD applying principles of behavioral economics. |
Introduction
Alzheimer's disease (AD) is the most common type of primary degenerative dementia, with a negative impact on patients, their families, and society from the earliest stages [1, 2]. The global number of people with AD across the spectrum (pre-clinical, prodromal, and AD dementia) is estimated at 416 million (22% of all people over 50), and this prevalence is expected to more than double every 5 years [3, 4].
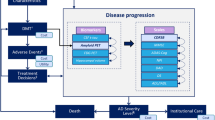
The current diagnostic and therapeutic landscape of AD is evolving rapidly. The access to early specific diagnosis through core biomarkers in cerebrospinal fluid (CSF) and positron emission tomography has been an important advance in improving the right of patients to plan their lives and start pharmacological and non-pharmacological therapies earlier [5, 6]. However, there are other aspects to consider, such as the psychological problems associated with receiving the diagnosis, predicting the risk of dementia, misconception, or stigmatization [7, 8]. However, the clinical development of disease-modifying treatments (DMTs) has been more complicated [9,10,11]. Aducanumab was the first monoclonal antibody-targeting amyloid-β protein in early AD patients, and was approved by the FDA in 2021 [12]. This approval was controversial and was followed by a restriction of the drug's coverage by the Centers for Medicare and Medicaid Services in the US and the denial of marketing authorization in Europe, due to the contradictory results of pivotal studies (ENGAGE and EMERGE), and to the lack of a clear relationship between the anti-amyloid-β protein effect and clinical benefit [10]. Recently, a second anti-amyloid-β protein agent (lecanemab) has also been approved in the US for prodromal and mild AD [13]. Patients randomized to aducanumab and lecanemab showed a significant 22% and 27%, respectively, slowed cognitive and functional decline compared to placebo [14, 15]. Despite recent advances, only a small proportion of patients are being diagnosed using AD biomarkers and treated according to the best clinical practice recommendations [6, 16, 17]. Even traditional recommendations, such as regular physical exercise and cognitive training and offering cholinesterase inhibitors, are little used in clinical practice in early AD patients [16]. Some potential explanations include the challenge faced by practitioners, regulatory agencies, and decision-makers to determine whether significant findings from trials are clinically meaningful [9,10,11, 18]. Different publications have attempted to address some of the objections by defining cut-off points for measurement instruments used in clinical trials associated with significant benefit to patients and caregivers [19,20,21]. Concerns have also been raised about the safety profile of these new treatments, in view of cases of amyloid-related imaging abnormalities (ARIA), and how they should be monitored and managed symptomatically in routine clinical practice [10, 22]. There is still a need to contextualize multidimensional assessments of clinical outcomes of new anti-amyloid-β DMTs with the perspective of patients and their caregivers, especially in the long term [10, 11]. This includes the management of different manifestations of the disease during its course (e.g., behavioral, psychiatric, cognitive) among other gaps (Fig. 1) [1].
Suboptimal management is a common phenomenon in medicine, defined as lack of treatment initiation or escalation when recommended according to the best practice guidelines [23]. The consequences of suboptimal management and treatment inertia among patients with early AD may lead to poorer patient outcomes, greater disability, and diminished quality of life [4, 24, 25]. Despite the evolving management scenario of early AD, there is limited information regarding neurologists’ treatment choices under uncertainty [11, 26]. Given physicians’ limited education in risk management and formal training in decision-making, the aim of this study will be evaluate the most critical factors influencing pharmacological treatment decisions and those leading to practice gaps in the management of early AD among neurologists.
Methods
Design and Participants
We have designed a non-interventional, cross-sectional, web-based pilot study in collaboration with the Spanish Society of Neurology (SEN). The selection criteria include: (1) neurologists (with or without specialization in cognitive disorders/dementia) and (2) active practice either in an academic or non-academic setting. Our protocol follows previous work completed in other therapeutic areas [27,28,29]. Participants will be recruited by receiving an e-mail invitation by SEN from June 1 to November 30, 2023. The Qualtrics web-platform will be used for the survey. Information on security and data protection can be found at www.qualtrics.com.
Objectives
The primary objective is to evaluate factors associated with treatment choices in the pharmacological management of patients with early AD (prodromal and mild AD dementia) by practicing neurologists from across Spain. Specifically, we are interested in determining the most common factors associated with suboptimal therapeutic decisions in early AD-simulated case scenarios or vignettes (e.g., not receiving adequate treatment as per the results of clinical trials or when warranted by best practice guidelines) [10, 15, 17, 30]. Secondary objectives include participants’ perspectives regarding the role of biomarkers in the diagnosis and treatment of early AD [27, 31, 32].
Outcome Measures and Definitions
The main outcome of interest is therapeutic inertia (TI), traditionally defined as the failure of the healthcare professional to initiate or intensify treatment despite a clear indication based on available evidence-based guidelines [33]. In this study, a TI score representing the number of simulated case scenarios or vignettes where treatment initiation is warranted over the ten simulated case scenarios will be used [27,28,29]. These scenarios were created by neurologists with expertise in the field of dementia and senior methodologists from our research team (Jorge Maurino, Elena Garcia-Arcelay, Gustavo Saposnik, David A. Perez-Martinez, Emilio Franco-Macias, Gonzalo Sanchez-Benavidez, and Ricardo F. Allegri) based on the inclusion and exclusion criteria, efficacy, and safety of anti-amyloid-β DMTs clinical trials and on the current recommendations from the US neurologists (Supplementary Material) [15, 17, 30]. This score ranges from 0 to 10, where higher values represent a higher degree of TI. Participants with a TI score ≥ 1 (e.g., therapeutic inertia in at least one case scenario) will be considered to calculate the presence of therapeutic inertia.
Study Flow
The study design consists of three parts (Fig. 2), as follows:
Part 1: Neurologists will complete demographics, practice setting information, and a behavioral battery regarding their tolerance to uncertainty. Tolerance to uncertainty will be assessed using the standardized physician’s reaction to an uncertainty test [34]. Participants will rate their level of agreement with each question from 0 (strongly disagree) to 5 (strongly agree), and a total score will be calculated [34, 35]. Higher values indicate lower tolerance to uncertainty. Ambiguity aversion is defined as dislike for events with unknown probability over events with known probability. Participants will be asked to choose between a visual option represented by bars with a known 50/50 probability of winning €400 (blue bar) or €0 (red bar) and an option with unknown probability of the same outcomes in one of the following degrees of uncertainty representing 10%, 30%, 50%, 70%, and 90% of the winning probability (illustrated by a gray area covering in the bar) (Supplementary Material) [31, 36]. The degree of ambiguity aversion is defined as the proportion of times participants chose the 50/50 option over the ambiguous option combining all five uncertainty options. Details of the battery can be found elsewhere [29, 32].
Part 2: Participants will be exposed to 10 simulated case scenarios or vignettes assessing therapeutic decisions in the management of early AD (US National Institute on Aging and Alzheimer’s Association criteria) [5]. Specifically, we created six simulated case scenarios of patients with different metrics in their cognitive assessments, two longitudinal case scenarios of patients with a follow-up of 6–20 months sequentially, adding medical information to determine the step by which participants would initiate an anti-amyloid-β DMT, and two control cases (e.g., a patient with advanced AD and another with cognitive and extrapyramidal symptoms and negative AD CSF biomarkers) where treatment with an anti-amyloid-β agent should not be initiated.
Part 3: Discrete choice experiments (DCEs) to determine the weight of different factors and attributes that influence therapeutic choices as carried out previously by our team [32, 36]. A DCE is a standard technique in economic research to estimate the factors affecting neurologists’ decisions when considering treatment choice for early AD [37]. DCE analysis is an accurate and insightful approach that relies on data from an experiment in which respondents choose between pairs of options. The model identifies patterns across the choice sets. Relevant variables to be included in the analysis were selected based on data from the literature and anti-amyloid-β DMTs clinical trials, including patients’ demographics, time since diagnosis, disease progression (fast vs. slow), Functional Assessment Questionnaire score, AD core biomarker determination, brain imaging, living and functional status, presence of neuropsychiatric symptoms (e.g., irritability, apathy, depressive symptoms), route of treatment administration, the total treatments risk of ARIA, and the number of ARIA lesions to make a therapeutic decision (Table 1, Panel A) [11, 22, 38,39,40]. Participants will be exposed to pairs of patient profiles to choose the use of one of the new recently approved anti-amyloid-β DMTs. They would be able to choose between a patient profile 1 or 2, or neither. The matrix of the DCE (13 attributes or factors, 2 to 4 levels per attribute, and 12 tasks) and an example choice task are described in Table 1, Panel B.
There are no standards for the determination of the minimum sample size in DCE [37]. Based on the number of attributes, levels, and possible combinations, we would require 200 participants. We are increasing our sample size to 400 neurologists to account for pre-specified subgroup analysis (general neurologists vs. cognitive disorders/dementia specialists) and the presence of early psychiatric symptoms, among others [41]. To the best of our knowledge, there are scant published studies using DCE in neurologists (or other physicians) making therapeutic decisions in AD [42]. A few studies were limited to patients instead of healthcare providers [43, 44].
Statistical Considerations
Descriptive statistics will be used to report frequency distributions of qualitative variables, measures of central tendency, and dispersion of quantitative variables using non-parametric tests, and 95% confidence intervals. Factors associated with TI will be determined using linear regression analysis with backward selection. DCE uses a multinomial logit model to estimate the “utility” of a given option. The model relies on data from an experiment in which respondents choose between pairs of options. Each option is a hypothetical combination of patient attributes chosen by an experimental design procedure. The model identifies patterns across the choice sets. A disaggregate DCE estimates a separate model for each respondent, calculating utilities at the individual level. An experimental design of patient scenarios will be generated using the SAS PROC FACTEX and PROC OPTEX procedures. Prespecified group comparisons will include (general neurologist vs. behavioral specialist), sex (male/female neurologist), presence of neuropsychiatric symptoms, living and functional status, and ARIA.
All tests will be 2-tailed, and p values < 0.05 will be considered significant. Unavailable data will be described as missing, without any imputation/allocation. Statistical analysis will be performed using Stata Statistical Software 17.0 (StataCorp, College Station, TX, USA) and considering a significant level of 0.05.
Ethical Considerations
This study will be conducted according to the Guidelines for Good Pharmacoepidemiologic Practice published by the International Society of Pharmacoepidemiology, the ethical principles laid down in the World Medical Association Declaration of Helsinki of 1964 and its later amendments, and applicable national regulations. The study will be submitted to the ethics committee of Hospital Clínico San Carlos (Madrid, Spain), and all participants will provide an electronic informed consent before the collection of any study data.
Discussion
Despite the ongoing advances in the management of patients with early AD, there is a dearth of information on how neurologists weight patients’ attributes when making diagnostic and therapeutic choices [11]. This non-interventional study will contribute to better understanding of the therapeutic decision-making process of neurologists who provide care for early AD patients in Spain. The uniqueness of this study is the application of a behavioral paradigm approach to examine the association between the participants’ tolerance to uncertainty and pharmacological treatment choices. Furthermore, our study will provide evidence on the weight of demographic, clinical, behavioral, biomarker, social, and brain-imaging factors associated with such decisions. Finally, we will be able to quantify therapeutic inertia and suboptimal decisions for new anti-amyloid-β DMTs among practicing neurologists.
Previous studies have used similar approaches to evaluate how physicians make therapeutic decisions for different medical conditions (multiple sclerosis, spinal muscular atrophy, breast cancer, spondylolisthesis, use of antibiotics, life support) [27, 29, 32, 45,46,47], but limited evidence is available for AD [26, 42]. A recent study evaluated the main factors associated with the use of anti-amyloid-β therapy versus standard of care among AD caregivers (n = 117), neurologists (n = 90), and payers (n = 90) from the US [38]. The authors categorized relevant factors into four domains: need for an intervention (disease severity, unmet needs), intervention outcomes (safety and efficacy of DMTs), type of benefit (public health or patient), and economic impact (direct and indirect costs). Of the neurologists, 40–45% practiced in academic institutions and had over 20 years of experience, and 60% treated over 75 AD patients in the last year. Interestingly, neurologists and payers placed the highest value on the efficacy of anti-amyloid therapy in clinical outcomes, whereas caregivers prioritized the need for a therapeutic intervention based on disease severity and unmet needs. The economic impact of DMTs did not make the top three factors associated with the decision to use them.
We expect our study will provide more detailed information by identifying the weight of specific patient-level factors directly influencing neurologists’ therapeutic choices. More specifically, we would expect that disease severity, degree of disease progression, and ARIA will be the top factors associated with the decision to prescribe a new anti-amyloid-β DMT.
We acknowledge some study limitations. First, our sample size may be relatively small when compared to the total population of practicing neurologists. However, we would expect a homogeneous distribution from across Spain given the support provided by the SEN. Second, our study will not cover the whole spectrum of patients with AD, as the clinical criteria for the use of anti-amyloid-β agents (as per clinical trials and labels) only include patients with early AD with AD core biomarkers [5, 6, 10, 11]. Finally, we cannot rule out the possibility of residual confounders in models derived from the regression analysis. Despite the aforementioned limitations, our study should provide new insights into factors influencing neurologists’ therapeutic decisions using new agents for patients with early AD. In addition, we will be able to provide estimates on the prevalence of suboptimal decisions and therapeutic inertia, and to identify existing gaps. The application of concepts from behavioral economics will facilitate the identification of unconscious biases and determine how participants handle uncertainty. Our results will allow the development of educational interventions and health policy strategies aimed at improving neurologists’ treatment choices and the well-being and outcomes of patients with early AD and their families.
In conclusion, our study may provide new information regarding treatment decisions by neurologists in the management of early AD with new anti-amyloid-β DMTs. Moreover, we will be able to identify the top factors associated with treatment choices and how participants’ tolerance to uncertainly may affect suboptimal decisions or therapeutic inertia. Finally, our results may also contribute to the growing evidence on the relevance of value-based shared decision-making for the current management of patients with AD at earlier stages.
References
Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397:1577–90.
Villarejo-Galende A, Garcia-Arcelay E, Pinol-Ripoll G, et al. Quality of life and the experience of living with early-stage alzheimer’s disease. J Alzheimers Dis. 2022;90:719–26.
Gustavsson A, Norton N, Fast T, et al. Global estimates on the number of persons across the Alzheimer's disease continuum. Alzheimers Dement. 2022 https://doi.org/10.1002/alz.12694
Cummings J, Aisen PS, DuBois B, et al. Drug development in alzheimer’s disease: The path to 2025. Alzheimers Res Ther. 2016;8:39.
Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62.
Dubois B, Villain N, Frisoni GB, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet Neurol. 2021;20:484–96.
van der Schaar J, Visser LNC, Bouwman FH, et al. Considerations regarding a diagnosis of Alzheimer’s disease before dementia: a systematic review. Alzheimers Res Ther. 2022;14:31.
Largent EA, Grill J, O'Brien K, Wolk D, Harkins K, Karlawish J. Testing for Alzheimer disease biomarkers and disclosing results across the disease continuum. Neurology. 2023 https://doi.org/10.1212/WNL.0000000000206891. Epub ahead of print.
Messner DA, Rabins P, Downing AC, et al. Designing trials of disease modifying agents for early and preclinical Alzheimer’s disease intervention: What evidence is meaningful to patients, providers, and payers? J Prev Alzheimers Dis. 2019;6:20–6.
Villain N, Planche V, Levy R. High-clearance anti-amyloid immunotherapies in Alzheimer's disease. Part 1: Meta-analysis and review of efficacy and safety data, and medico-economical aspects. Rev Neurol (Paris). 2022;178:1011–30.
Assunção SS, Sperling RA, Ritchie C, Kerwin DR, Aisen PS, Lansdall C, et al. Meaningful benefits: a framework to assess disease-modifying therapies in preclinical and early Alzheimer’s disease. Alzheimers Res Ther. 2022;14:54.
US Food and Drug Administration 2021. https://www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-new-treatment-alzheimers-disease
US Food and Drug Administration 2023. https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment
Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9:197–210.
van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in Early Alzheimer’s disease. N Engl J Med. 2023;388:9–21.
Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:126–35.
Cummings J, Rabinovici GD, Atri A, et al. Aducanumab: appropriate use recommendations update. J Prev Alzheimers Dis. 2022;9:221–30.
European Medicines Agency 2021. https://www.ema.europa.eu/en/documents/medicine-qa/questions-answers-refusal-marketing-authorisation-aduhelm-aducanumab_en.pdf
Andrews JS, Desai U, Kirson NY, Zichlin ML, Ball DE, Matthews BR. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer’s disease clinical trials. Alzheimers Dement (N Y). 2019;5:354–63.
Borland E, Edgar C, Stomrud E, Cullen N, Hansson O, Palmqvist S. Clinically relevant changes for cognitive outcomes in preclinical and prodromal cognitive stages: implications for clinical Alzheimer trials. Neurology. 2022;99:e1142–53.
Cohen S, Cummings J, Knox S, Potashman M, Harrison J. Clinical trial endpoints and their clinical meaningfulness in early stages of Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9:507–22.
Filippi M, Cecchetti G, Spinelli EG, Vezzulli P, Falini A, Agosta F. Amyloid-related imaging abnormalities and β-amyloid-targeting antibodies: a systematic review. JAMA Neurol. 2022;79:291–304.
Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138.
Mauricio R, Benn C, Davis J, et al. Tackling gaps in developing life-changing treatments for dementia. Alzheimers Dement (N Y). 2019;5:241–53.
Galvin JE, Aisen P, Langbaum JB, et al. Early stages of Alzheimer’s disease: evolving the care team for optimal patient management. Front Neurol. 2021;11: 592302.
Saposnik G, Ismail Z, Rivard AM, et al. Decision making under uncertainty in the diagnosis and management of Alzheimer’s Disease in primary care: A study protocol applying concepts from neuroeconomics. Front Med (Lausanne). 2022;9: 997277.
Saposnik G, Sempere AP, Prefasi D, et al. Decision-making in Multiple Sclerosis: The role of aversion to ambiguity for therapeutic inertia among neurologists (DIScUTIR MS). Front Neurol. 2017;8:65.
Sposato LA, Stirling D, Saposnik G. Therapeutic decisions in atrial fibrillation for stroke prevention: The role of aversion to ambiguity and physicians’ risk preferences. J Stroke Cerebrovasc Dis. 2018;27:2088–95.
Saposnik G, Camacho A, Díaz-Abós P, et al. Therapeutic decision-making under uncertainty in the management of spinal muscular atrophy: results from DECISIONS-SMA study. Neurol Ther. 2022;11:1209–19.
Villain N, Planche V, Levy R. High-clearance anti-amyloid immunotherapies in Alzheimer's disease. Part 2: putative scenarios and timeline in case of approval, recommendations for use, implementation, and ethical considerations in France. Rev Neurol (Paris). 2022;178:999–1010.
Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182:E73–7.
Saposnik G, Andhavarapu S, Fernández Ó, et al. Factors associated with treatment escalation among MS specialists and general neurologists: Results from an International cojoint study. Mult Scler Relat Disord. 2022;58: 103404.
Lavoie KL, Rash JA, Campbell TS. Changing provider behavior in the context of chronic disease management: focus on clinical inertia. Annu Rev Pharmacol Toxicol. 2017;57:263–83.
Politi MC, Légaré F. Physicians’ reactions to uncertainty in the context of shared decision making. Patient Educ Couns. 2010;80:155–7.
Cunningham BA, Bonham VL, Sellers SL, et al. Physicians’ anxiety due to uncertainty and use of race in medical decision making. Med Care. 2014;52:728–33.
Saposnik G, Andhavarapu S, Fernández Ó, et al. Effect of desire for pregnancy on decisions to escalate treatment in multiple sclerosis care: Differences between MS specialists and non-MS specialists. Mult Scler Relat Disord. 2022;57: 103389.
de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21:145–72.
Cohen CI, Reisberg B, Yaffee R. Global cognitive trajectory patterns in Alzheimer's disease. Int Psychogeriatr. 2022 Epub ahead of print.
Adkins-Jackson PB, Belsky DW. Alzheimer’s disease risk biomarkers: progress and challenges. Lancet Healthy Longev. 2022;3:e575–6.
Roberto N, Portella MJ, Marquié M, et al. Neuropsychiatric profiles and conversion to dementia in mild cognitive impairment, a latent class analysis. Sci Rep. 2021;11:6448.
Orme BK. Getting started with conjoint analysis: Strategies for product design and pricing research. Research Publishers, LLC; 1st edition, 2005.
Dranitsaris G, Zhang Q, Quill A, et al. Treatment preference for Alzheimer’s disease: A multicriteria decision analysis with caregivers, neurologists, and payors. Neurol Ther. 2023;12:211–27.
Mühlbacher A, Johnson FR, Yang JC, Happich M, Belger M. Do you want to hear the bad news? the value of diagnostic tests for Alzheimer’s disease. Value Health. 2016;19:66–74.
Johnson FR, DiSantostefano RL, Yang JC, Reed SD, Streffer J, Levitan B. Something is better than nothing: the value of active intervention in stated preferences for treatments to delay onset of Alzheimer’s disease symptoms. Value Health. 2019;22:1063–9.
Drewniak D, Brandi G, Buehler PK, et al. Key factors in decision making for ECLS: a binational factorial survey. Med Decis Making. 2022;42:313–25.
Sydenham RV, Jarbøl DE, Hansen MP, Justesen US, Watson V, Pedersen LB. Prescribing antibiotics: Factors driving decision-making in general practice. A discrete choice experiment. Soc Sci Med. 2022;305:115033.
Schneider N, Fisher C, Glennie A, et al. Lumbar degenerative spondylolisthesis: factors associated with the decision to fuse. Spine J. 2021;21:821–8.
Acknowledgements
The authors would like to thank the Spanish Society of Neurology whose support and collaboration are making this study possible.
Funding
This study is funded by the Medical Department of Roche Farma Spain. The sponsor also funded the journal's Rapid Service Fee. The funding source had no role in the design, analysis and interpretation of the data, review or approval of the manuscript, and decision to submit for publication.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors made a significant contribution to the work reported, whether in study conception, design, execution, analysis and interpretation, or in all these areas; all took part in drafting, revising or critically reviewing the article; all gave final approval of the version to be published; all have agreed on the journal to which the article has been submitted; and all agree to be accountable for all aspects of the work.
Disclosures
Gustavo Saposnik is being supported by the Heart and Stroke Foundation of Canada Scientist Award following a peer-reviewed competition and reported receiving unrestricted grants and personal fees from Hoffman La Roche (Canada) and Roche Farma (Spain). Elena Garcia-Arcelay and Jorge Maurino are employees of Roche Farma Spain. Catalina Bensi and Sebastian Carmelingo are employees of Roche Farma Argentina. Gonzalo Sanchez-Benavidez, Emilio Franco-Macias, Ricardo F. Allegri, and David A. Perez Martinez declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Compliance with Ethics Guidelines
This study will be conducted according to the Guidelines for Good Pharmacoepidemiologic Practice published by the International Society of Pharmacoepidemiology, the ethical principles laid down in the World Medical Association Declaration of Helsinki of 1964 and its later amendments, and applicable national regulations. The study will be submitted to the ethics committee of Hospital Clínico San Carlos (Madrid, Spain), and all participants will provide an informed consent before collecting any study data.
Data Availability
Data sharing is not applicable to this article as no datasets have yet been generated or analyzed.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Saposnik, G., Sánchez-Benavidez, G., García-Arcelay, E. et al. Design of a Non-Interventional Study to Assess Neurologists’ Perspectives and Pharmacological Treatment Decisions in Early Alzheimer's Disease. Neurol Ther 12, 995–1006 (2023). https://doi.org/10.1007/s40120-023-00466-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-023-00466-9