Abstract
Introduction
Preclinical studies have indicated insulin-like growth factor 1 (IGF1) as a novel therapeutic target in the treatment of migraines. We aimed to investigate the causal effect of circulating IGF1 levels on migraine risk using the two-sample Mendelian randomization method.
Methods
A total of 431 independent variants from 363,228 unrelated individuals in the UK Biobank were used as genetic instruments for circulating IGF1 levels. Summary-level data for migraines were obtained from two independent studies with 10,536 and 28,852 migraine cases, respectively.
Results
Mendelian randomization using inverse-variance weighting showed that increased IGF1 levels were significantly associated with decreased risk of migraines in both outcome datasets (odds ratio 0.905, 95% confidence interval 0.842–0.972, p = 0.006; odds ratio 0.929, 95% confidence interval 0.882–0.979, p = 0.006). Although some other robust Mendelian randomization methods did not demonstrate a significant association, no unbalanced horizontal pleiotropy was found by Mendelian randomization–Egger regression (p values for horizontal pleiotropy 0.232 and 0.435). The effect was confirmed in additional analyses including multivariable Mendelian randomization analyses.
Conclusion
This two-sample Mendelian randomization study showed that genetically determined increased IGF1 levels are causally associated with decreased migraine risk. Future randomized controlled trials are warranted to confirm the benefits of IGF1 administration on migraines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Studies have shown that insulin-like growth factor 1 (IGF1) might play a role in the pathogenesis of several neurological disorders, including migraine. However, the association between IGF1 and migraine is unclear. |
In the present study, we investigate the causal effect of circulating IGF1 levels on migraine risk using the two-sample Mendelian randomization method. |
This two-sample Mendelian randomization study showed that genetically determined increased IGF1 levels are causally associated with decreased migraine risk. |
Future randomized controlled trials are warranted to confirm the benefits of IGF1 administration on migraines. |
Introduction
Insulin-like growth factor 1 (IGF1) is a pleiotropic polypeptide hormone, structurally similar to proinsulin. IGF1 is mainly produced by the liver upon stimulation by growth hormone [1]. In addition, the stimulatory effect of growth hormone is greatly affected by nutritional status and physical activity [2, 3]. Moreover, IGF1 has key roles in regulating cellular proliferation, differentiation, and apoptosis [1, 4], consistent with epidemiological evidence that increased circulating IGF1 is associated with the risk of several cancers [5], as well as cardiometabolic diseases [6, 7]. Similarly, studies have shown that IGF1 might also play a role in the pathogenesis of several neurological disorders, including migraine [8,9,10,11]. Moreover, preclinical studies have demonstrated that IGF1 could be a novel therapeutic target in treating spreading depression, which is widely accepted as the pathophysiological event underlying migraine aura [12]. However, no study has provided epidemiological evidence regarding the association between IGF1 and migraine.
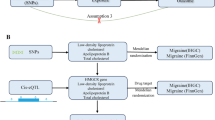
With the increasing availability of summary data from large, genome-wide association studies (GWASs), a new method of two-sample Mendelian randomization (MR) has been widely used to assess causality. Because genetic factors are randomly assigned by nature, two-sample MR can be used to simulate a randomized trial design. Thus, unlike conventional observational study design, this method is not generally susceptible to the reverse causality or confounding [13]. Our study aims to investigate the causal effect of circulating IGF1 levels on migraine risk using the two-sample MR method (Fig. 1). The present study can provide more evidence regarding the potential clinical value of targeting IGF1 for treating migraine.
Design of the present Mendelian randomization study. The three core assumptions for two-sample MR study are as follows: the genetic instrument is associated with the exposure; the instrument genetic instrument does not affect outcome via pathways other than the exposure; and the genetic instrument is not associated with confounders. GWAS genome wide association study, IGF1 Insulin-like growth factor 1, MR Mendelian randomization, SNP single nucleotide polymorphism
Methods
We followed the STROBE-MR guidelines to perform the present study [14]. Ethics approval and informed consent were not required for the present study, as they were obtained in the original studies. The original studies were conducted in compliance with the Declaration of Helsinki.
Selection of Genetic Instruments
We obtained genetic instruments from a GWAS of 363,228 unrelated individuals in the UK Biobank [15]. Most individuals were of European descent (94.3%). First, IGF1 measurements were log-transformed. Then, linear regression was performed by adjusting covariates including the top 40 genotype principal components, as well as age, sex, age × sex, ethnicity, ethnicity × sex, fasting time, assessment center, genotyping batch, estimated sample dilution factor (icosatiles), icosatiles of the time of sampling throughout the day, and month of assessment. The resulting residuals were used to perform the GWAS analysis.
First, the full summary statistics of all single nucleotide polymorphisms (SNPs) were downloaded. We obtained all SNPs associated with IGF1 at a genome-wide significance level of p < 5 × 10−8. Because effect allele frequency was not reported in the summary statistics, we first excluded palindromic SNPs. We then excluded SNPs with multiple alleles and SNPs located around the human leukocyte antigen region (chr6:25–34 Mb) [16, 17, 18]. Finally, we performed a clumping procedure to ensure that the SNPs we used were independent of each other (r2 = 0.01, 10,000 kb). The European 1000 Genome Project v3 reference panel was used as a reference.
A total of 431 independent SNPs were included as genetic instruments (Supplementary Table 1). The F statistic for each SNP was calculated using the following formula: beta2/SE2. The values of the F statistics ranged from 29 to 1478. The variance explained by these SNPs was calculated to be 10.49%, using the following formula: 2 × beta2 × MAF × (1 − MAF) [19], where MAF denotes minor allele frequency.
Outcome Datasets
We used two independent GWASs of migraine to perform the MR study. The first GWAS summary statistics were obtained from the last release (release 6) of the FinnGen study [20], which included 10,536 migraine cases and 208,845 controls. A total of 8647 (82.07%) patients were female. The mean age at first migraine event was 40.27 years. Moreover, 4366 cases presented with aura and 3924 cases presented without aura. Migraine was defined by codes from the 8th, 9th, and 10th versions of the International Classification of Disease. In the FinnGen study, mixed-model logistic regression was performed by adjusting for age, sex, the 10 principal components, and genotyping batch.
The second GWAS summary statistics were obtained from a genetic study of 554,569 individuals (28,852 migraine cases) from the Genetic Epidemiology Research in Adult Health and Aging cohort and the UK Biobank cohort [21, 22, 23, 24]. Most individuals were of European descent (92.55%), and the mean age was 57.82 years. Additionally, there were 22,500 female cases and 279,762 female controls. Migraine cases in the Genetic Epidemiology Research in Adult Health and Aging cohort were determined using a migraine probability algorithm based on migraine-specific prescriptions and codes from the 9th and 10th versions of the International Classification of Disease. Meanwhile, most migraine cases in the UK biobank were based on self-reported data. Logistic regression was performed by adjusting for age, sex, and ancestry principal components. Although overlap occurred between this GWAS and the IGF1 GWAS, a recent study showed that two-sample MR can be utilized for a single large dataset from large biobanks, such as the UK biobank [25]. In addition, the calculated results showed the bias due to sample overlap to be negligible (0.68%) on the basis of a sample overlap proportion up to 65.50% using a web tool (https://sb452.shinyapps.io/overlap/) [26].
Harmonizing Exposure and Outcome SNP Effects
Before performing the MR analysis, we harmonized the alleles and effects to ensure each SNP’s effect on the exposure and the outcome corresponds to the same effect allele [27]. For wrong effect alleles (e.g., G/T and T/G), the signs of the SNP-outcome effect and the alleles for outcome were both flipped. In addition, if one study reported the effect on the forward strand and the other on the reverse strand (e.g., G/T and C/A), the outcome alleles were harmonized to match those of the exposure alleles. SNPs with incompatible alleles (e.g., A/G and A/C) between the exposure and the outcome were excluded.
MR Analysis
The Wald ratio method was used to obtain the estimated causal effect of IGF1 on migraine risk based on each SNP [27]. A random-effects inverse variance weighted (IVW) meta-analysis of each Wald ratio estimate was performed to obtain a pooled effect of all SNPs [27]. The estimate from the IVW method was considered as the main MR result. Each SNP was treated as a valid natural experiment for this method. This method allows each SNP to have different mean effects and will return an unbiased estimate if the net-horizontal pleiotropy across all SNPs is balanced [27]. Moreover, horizontal pleiotropy indicates that the instrument SNP can influence the outcome through other pathways. Other robust methods, including MR-Egger, weighted median, and weighted mode analyses, were used to perform sensitivity analyses. Additionally, the MR-pleiotropy residual sum and outlier (MR-PRESSO) method was performed to detect outliers.
Meta-analysis was performed to assess the pooled effects of IGF1 on migraine using IVW estimates from the two outcome datasets [28]. We then performed a meta-analysis to combine the effects (i.e., beta and SE) of genetic instruments on migraine of the two migraine datasets [29, 30]. The resulting effects of SNPs on migraine were then used to perform a two-sample MR analysis.
Previous two-sample MR analysis showed that increased IGF1 levels were causally associated with increased risk of type 2 diabetes [31]. In addition, smoking, diastolic blood pressure, insomnia, and serum calcium were found to be positively associated with migraine risk [32, 33, 34, 35]. Thus, we performed sensitivity analysis by excluding SNPs associated with these traits (p < 5 × 10−8). SNPs associated with body mass index, lipids, and physical activity were also excluded in sensitivity analysis. In addition, multivariable MR analysis was performed by adjusting these traits. The effects of the SNPs on these factors were obtained from the largest GWASs publicly available. For SNPs that were found in the corresponding GWAS, we obtained the data from the OpenGWAS database [27].
We also performed a reverse two-sample MR analysis to assess the causal effects of migraine on circulating IGF1 levels. Genetic instruments for migraine were obtained from a large GWAS including 102,084 migraine cases [36]. However, we did not include this migraine GWAS as the outcome dataset because the full summary-level data was not publicly available. In addition, the two migraine GWAS datasets included in our study were independent of each other and had enough power to detect a small effect.
We used the TwoSampleMR [27] and MR-PRESSO [37] packages from R (version 3.6.1) to perform all MR analyses. Data harmonizing was performed using the “harmonise_data” function in the TwoSampleMR package. A P value less than 0.05 was considered statistically significant. Meta-analysis of estimates from the IVW method was performed using the meta package from R. Meta-analysis of the two sources of SNP-migraine effects was performed using the METAL tool [38].
Power of MR Test
The power of MR test was calculated using a web tool [39]. The proportion of variance explained by the SNPs for exposure, total sample size of the outcome, and proportion of cases of the outcome were used to calculate the power. The power was calculated on the basis of a type I error rate of 0.05. Additionally, the proportion of variance explained by the SNPs included in the two migraine datasets was 10.40% and 9.96%, respectively. The detectable odds ratio (OR) based on the FinnGen study with an 80% power was less than 0.914 (or greater than 1.087), and that of another study was less than 0.946 (or greater than 1.054).
Results
A total of 425 SNPs, including two proxy SNPs (r2 > 0.8), were found in the migraine GWAS from the FinnGen study (Supplementary Table 2). MR analysis using the IVW method showed that increased IGF1 levels were significantly associated with decreased risk of migraine [OR 0.905, 95% confidence interval (CI) 0.842–0.972, p = 0.006] (Fig. 2 and Supplementary Fig. 1). A significant association was also observed on the basis of the weighted mode method (OR 0.830, 95% CI 0.699–0.986, p = 0.034). Although the MR-Egger and weighted median methods did not find a significant association, no unbalanced horizontal pleiotropy was found by MR-Egger regression (p = 0.232). In addition, the MR-PRESSO method did not find outlier SNPs. Sensitivity analysis by excluding SNPs associated with other factors showed similar results (OR 0.901, 95% CI 0.826–0.982, p = 0.018).
A total of 417 SNPs, including four proxy SNPs (r2 > 0.8), were found in the second migraine GWAS (Supplementary Table 2). MR analysis using the IVW method also showed that increased IGF1 levels were significantly associated with a decreased risk of migraine (OR 0.929, 95% CI 0.882–0.979, p = 0.006) (Fig. 2 and Supplementary Fig. 1). The MR-PRESSO method after excluding two outlier SNPs showed a similar association. Although all other methods did not result in a significant association, no unbalanced horizontal pleiotropy was found by MR-Egger regression (p = 0.435). Additionally, sensitivity analysis by excluding the SNPs associated with other factors showed that the effect decreased (OR 0.944, 95% CI 0.887–1.005, p = 0.069).
The pooled OR and corresponding 95% CI of the two IVW estimates from the outcome GWASs were 0.921 and 0.883–0.960 (p = 0.0001, heterogeneity I2 = 0%). Using the pooled estimates of SNP effects on migraine, MR analysis showed a similar effect of IGF1 on migraine (OR 0.923, 95% CI 0.881–0.967, p = 6.57 × 10−4) (Fig. 3 and Supplementary Fig. 2). Sensitivity analysis by excluding the 129 SNPs associated with other factors showed that the effect decreased, but remained significant (OR 0.932, 95% CI 0.883–0.985, p = 0.012). We also performed sensitivity analysis by excluding the SNPs found in only one migraine dataset and those with evidence of heterogeneity (I2 > 50%) between the two datasets. The effect remained unchanged by using the remaining SNPs (OR 0.917, 95% CI 0.871–0.965, p = 9.39 × 10−4).
Multivariable MR IVW analysis was performed using the pooled migraine dataset by adjusting each other factor alone or all factors together. The results were similar to the effects from the conventional IVW method (Fig. 4).
Effects of IGF1 on migraine risk with confounders adjusted. ORs indicate the effect of IGF1 on migraine risk when each/all confounders were adjusted using multivariable MR method. HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, CI confidence interval, MR Mendelian randomization, OR odds ratio
MR analysis using GWAS data of migraines with an aura in the FinnGen study showed that IGF1 was not associated with migraine risk (OR 0.967, 95% CI 0.873–1.070, p = 0.512) (Fig. 5 and Supplementary Fig. 3). However, MR analysis using the GWAS data of migraines without an aura in the FinnGen study showed that increased IGF1 levels were significantly associated with a decreased risk of migraine (OR 0.896, 95% CI 0.805–0.998, p = 0.047).
Reverse MR IVW analysis showed that migraine was not associated with IGF1 levels (beta − 0.004, 95% CI − 0.033 to 0.025, p = 0.765) (Supplementary Fig. 4). Although the weighted median and weighted mode methods showed that migraine was associated with increased IGF1 levels, no unbalanced horizontal pleiotropy was found by MR-Egger regression (p = 0.378).
Discussion
Using two-sample MR, this study showed that genetically determined increased IGF1 levels might be causally associated with a decreased risk of migraine. The effect was confirmed in two independent large migraine datasets and in additional analyses, including multivariable MR analysis. Although the effect was not confirmed in migraines with an aura, the sample size of this subset was relatively small.
Previous studies have shown that IGF1 may have neuroprotective effects on neurological conditions, such as brain trauma and neurodegenerative diseases, by stimulating protein synthesis in neurons, glia, oligodendrocytes, and Schwann cells; improving neuronal survival; and inhibiting apoptosis [3, 40, 41, 42]. Furthermore, preclinical studies have demonstrated that IGF1 might be a novel therapeutic target against migraine [11]. Preclinical in vitro studies have shown that IGF1 could mitigate spreading depression by increasing endogenous antioxidants and decreasing oxidative stress [43, 44]. This idea is partially supported by a finding from another study that the accumulation of reactive oxygen species may be a key mechanism of cortical spreading depression initiation [45]. Cortical spreading depression may in turn induce oxidative stress in the trigeminal nociceptive system [46]. Together, these findings suggest that IGF1 may be able to inhibit oxidative stress and cortical spreading depression [11]. The effect of IGF1 on spreading depression and oxidative stress has been further confirmed by in vivo studies [47, 48].
Calcitonin gene-related peptide, a mediator of migraine pain, plays an important role in migraine pathophysiology. Drugs that target the ligands and receptors of calcitonin gene-related peptide have been shown to be effective and safe for those with migraines [49, 50]. Moreover, in vivo studies have demonstrated that nasal administration of IGF1 could mitigate spreading depression and reduce trigeminal ganglion oxidative stress and calcitonin gene-related peptide levels [48, 51]. In addition, intranasal treatment with IGF1 showed no aberrant effects on blood glucose levels, nasal mucosa, or serum markers of toxicity [47].
IGF1 plays a major part in the regulation of body composition and glucose metabolism [52, 53]. On the other hand, insulin resistance and its comorbidities of obesity might have a biological association with migraine headache [54, 55]. Therefore, low IGF1 levels in migraineurs might be a marker or a consequence of abnormal glucose metabolism and obesity. Although our MR analyses showed the effect of IGF1 on migraine was independent of type 2 diabetes and body mass index, the role of abnormal glucose metabolism and obesity should be considered in future studies when investigating the effect of IGF1 on migraine.
Increased circulating IGF1 levels have been shown to be associated with harmful effects on non-neurological diseases. A two-sample MR study investigated the associations between genetically predicted circulating IGF1 levels and glycemic traits, lipids, blood pressure, body composition, and cardiometabolic diseases, including cardiovascular disease [31]. The results showed that increased IGF1 levels may be causally associated with an increased risk of type 2 diabetes. This association was supported by a randomized trial including 330 patients with amyotrophic lateral sclerosis [56]. The trial showed that a 2-year treatment period with subcutaneously administered IGF1 was associated with an increased risk of presumed or documented hypoglycemia (12.6% vs. 5.5%, p = 0.034). Additionally, recent MR studies showed that IGF1 levels were positively associated with colorectal cancer risk and negatively associated with renal cell carcinoma and bladder cancer risk [57, 58, 59]. Because migraine is a long-term condition, the long-term adverse effects of IGF1 treatment, especially those on cancer development, should be considered. Thus, intranasal treatment might be a more promising administration method than subcutaneous injection.
This study had several limitations. First, the sample sizes for the migraine subtypes were relatively small. Thus, the migraine subtypes should be interpreted cautiously because of the insufficient power and the possibility of bias. Second, women experienced a higher prevalence of migraine than men, and the efficacy of IGF1 therapy may be related to the plasma level of sex hormones [3]. However, sex-stratified analysis was not performed in the present study because the individual-level data were not available. Third, most migraine cases in the UK biobank were based on self-reported data, which may lead to migraine misclassification. Although migraine misclassification might bias the MR analyses results, the effect of IGF1 on migraine shows consistently across two migraine datasets. Finally, nearly all of the included individuals were of European descent, and the effects may not be generalizable to other populations.
Conclusions
This two-sample MR study showed that genetically determined increased IGF1 levels might be causally associated with decreased risk of migraine. This effect was confirmed in two large, independent migraine datasets and in additional analyses including multivariable MR. Future randomized controlled trials are warranted to confirm the benefits of IGF1 administration for migraines. In addition, future studies should be performed to determine IGF1 levels during and between migraine attacks, its association with oxidative stress markers and calcitonin gene-related peptide levels, and the role of IGF1 in migraine pathogenesis.
References
Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4(7):505–18.
Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15(1):80–101.
Bianchi VE, Locatelli V, Rizzi L. Neurotrophic and neuroregenerative effects of GH/IGF1. Int J Mol Sci. 2017;18(11):2441.
Bailes J, Soloviev M. Insulin-like growth factor-1 (IGF-1) and its monitoring in medical diagnostic and in sports. Biomolecules. 2021;11(2):217.
Knuppel A, Fensom GK, Watts EL, et al. Circulating insulin-like growth factor-I concentrations and risk of 30 cancers: prospective analyses in UK biobank. Can Res. 2020;80(18):4014–21.
Geng T, Wang M, Li X, et al. Birth weight modifies the relation between adulthood levels of insulin-like growth factor-1 and type 2 diabetes: a prospective cohort study. BMJ Open Diabetes Res Care. 2021;9(1):000.
Carlzon D, Svensson J, Petzold M, et al. Both low and high serum IGF-1 levels associate with increased risk of cardiovascular events in elderly men. J Clin Endocrinol Metab. 2014;99(11):E2308–16.
Aldhaleei WA, Bhagavathula AS, Alshehhi F. Dyke-Davidoff-Masson syndrome presenting as recurrent chronic headache in the late adult life. Brain Circ. 2020;6(2):123–5.
Bibollet-Bahena O, Cui QL, Almazan G. The insulin-like growth factor-1 axis and its potential as a therapeutic target in central nervous system (CNS) disorders. Cent Nerv Syst Agents Med Chem. 2009;9(2):95–109.
Zappa Villar MF, Lopez Hanotte J, Crespo R, Pardo J, Reggiani PC. Insulin-like growth factor 1 gene transfer for sporadic Alzheimer’s disease: new evidence for trophic factor mediated hippocampal neuronal and synaptic recovery-based behavior improvement. Hippocampus. 2021;31(10):1137–53.
Chen SP, Ayata C. Novel therapeutic targets against spreading depression. Headache. 2017;57(9):1340–58.
Chen SP, Ayata C. Spreading depression in primary and secondary headache disorders. Curr Pain Headache Rep. 2016;20(7):44.
Smith GD, Ebrahim S. “Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22.
Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614–21.
Sinnott-Armstrong N, Tanigawa Y, Amar D, et al. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat Genet. 2021;53(2):185–94.
Zhou Y, Qian X, Liu Z, et al. Coagulation factors and the incidence of COVID-19 severity: Mendelian randomization analyses and supporting evidence. Signal Transduct Target Ther. 2021;6(1):222.
Cupido AJ, Kraaijenhof JM, Burgess S, Asselbergs FW, Hovingh GK, Gill D. Genetically predicted neutrophil-to-lymphocyte ratio and coronary artery disease: evidence from mendelian randomization. Circ Genom Precis Med. 2022;15(1): e003553.
Taylor-Bateman V, Gill D, Georgakis M, Malik R, Munroe P, Traylor M. Cardiovascular risk factors and MRI markers of cerebral small vessel disease: a Mendelian randomization study. Neurology. 2021;98:343–51.
Park JH, Wacholder S, Gail MH, et al. Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet. 2010;42(7):570–5.
FinnGen. Documentation of R6 release. 2022. https://finngen.gitbook.io/documentation/. Accessed 15 Feb 2022.
Choquet H, Yin J, Jacobson AS, et al. New and sex-specific migraine susceptibility loci identified from a multiethnic genome-wide meta-analysis. Commun Biol. 2021;4(1):864.
Banda Y, Kvale MN, Hoffmann TJ, et al. Characterizing race/ethnicity and genetic ancestry for 100,000 subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort. Genetics. 2015;200(4):1285–95.
Kvale MN, Hesselson S, Hoffmann TJ, et al. Genotyping informatics and quality control for 100,000 subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort. Genetics. 2015;200(4):1051–60.
Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3): e1001779.
Minelli C, Del Greco MF, van der Plaat DA, Bowden J, Sheehan NA, Thompson J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol. 2021;50(5):1651–9.
Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40(7):597–608.
Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018. https://doi.org/10.7554/eLife.34408.
Zhang Z, Wang M, Yuan S, Cai H, Zhu S-G, Liu X. Genetically predicted coffee consumption and risk of Alzheimer’s disease and stroke. J Alzheimer’s Dis. 2021;83(4):1815–23.
Pistis G, Milaneschi Y, Vandeleur CL, et al. Obesity and atypical depression symptoms: findings from Mendelian randomization in two European cohorts. Transl Psychiatry. 2021;11(1):96.
Howell AE, Robinson JW, Wootton RE, et al. Testing for causality between systematically identified risk factors and glioma: a Mendelian randomization study. BMC Cancer. 2020;20(1):508.
Larsson SC, Michaelsson K, Burgess S. IGF-1 and cardiometabolic diseases: a Mendelian randomisation study. Diabetologia. 2020;63(9):1775–82.
Yuan S, Daghlas I, Larsson SC. Alcohol, coffee consumption, and smoking in relation to migraine: a bidirectional Mendelian randomization study. Pain. 2022;163(2):e342–8.
Guo Y, Rist PM, Daghlas I, et al. A genome-wide cross-phenotype meta-analysis of the association of blood pressure with migraine. Nature Commun. 2020;11(1):3368.
Daghlas I, Vgontzas A, Guo Y, Chasman DI, International Headache Genetics Consortium, Saxena R. Habitual sleep disturbances and migraine: a Mendelian randomization study. Ann Clin Transl Neurol. 2020;7(12):2370–80.
Yin P, Anttila V, Siewert KM, Palotie A, Davey Smith G, Voight BF. Serum calcium and risk of migraine: a Mendelian randomization study. Hum Mol Genet. 2017;26(4):820–8.
Hautakangas H, Winsvold BS, Ruotsalainen SE, et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat Genet. 2022;54(2):152–60.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–1.
Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497–501.
Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21(15):5678–84.
Carlson SW, Saatman KE. Central infusion of insulin-like growth factor-1 increases hippocampal neurogenesis and improves neurobehavioral function after traumatic brain injury. J Neurotrauma. 2018;35(13):1467–80.
Shandilya A, Mehan S. Dysregulation of IGF-1/GLP-1 signaling in the progression of ALS: potential target activators and influences on neurological dysfunctions. Neurol Sci. 2021;42(8):3145–66.
Grinberg YY, van Drongelen W, Kraig RP. Insulin-like growth factor-1 lowers spreading depression susceptibility and reduces oxidative stress. J Neurochem. 2012;122(1):221–9.
Grinberg YY, Dibbern ME, Levasseur VA, Kraig RP. Insulin-like growth factor-1 abrogates microglial oxidative stress and TNF-alpha responses to spreading depression. J Neurochem. 2013;126(5):662–72.
Malkov A, Ivanov AI, Popova I, et al. Reactive oxygen species initiate a metabolic collapse in hippocampal slices: potential trigger of cortical spreading depression. J Cereb Blood Flow Metab. 2014;34(9):1540–9.
Shatillo A, Koroleva K, Giniatullina R, et al. Cortical spreading depression induces oxidative stress in the trigeminal nociceptive system. Neuroscience. 2013;253:341–9.
Grinberg YY, Zitzow LA, Kraig RP. Intranasally administered IGF-1 inhibits spreading depression in vivo. Brain Res. 2017;1677:47–57.
Won L, Kraig RP. Insulin-like growth factor-1 inhibits nitroglycerin-induced trigeminal activation of oxidative stress, calcitonin gene-related peptide and c-Fos expression. Neurosci Lett. 2021;751: 135809.
Tepper SJ. Anti-calcitonin gene-related peptide (CGRP) therapies: update on a previous review after the American Headache Society 60th Scientific Meeting, San Francisco, June 2018. Headache. 2018;58(Suppl 3):276–90.
de Vries T, Villalon CM, MaassenVanDenBrink A. Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. Pharmacol Ther. 2020;211: 107528.
Won L, Kraig RP. Insulin-like growth factor-1 inhibits spreading depression-induced trigeminal calcitonin gene related peptide, oxidative stress & neuronal activation in rat. Brain Res. 2020;1732: 146673.
Teppala S, Shankar A. Association between serum IGF-1 and diabetes among US adults. Diabetes Care. 2010;33(10):2257–9.
Berryman DE, Glad CAM, List EO, Johannsson G. The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2013;9(6):346–56.
Islam MR, Nyholt DR. Glucose-related traits and risk of migraine-a potential mechanism and treatment consideration. Genes (Basel). 2022;13(5):730.
Del Moro L, Rota E, Pirovano E, Rainero I. Migraine, brain glucose metabolism and the “neuroenergetic” hypothesis: a scoping review. J Pain. 2022. https://doi.org/10.1016/j.jpain.2022.02.006.
Sorenson EJ, Windbank AJ, Mandrekar JN, et al. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology. 2008;71(22):1770–5.
Larsson SC, Carter P, Vithayathil M, Kar S, Mason AM, Burgess S. Insulin-like growth factor-1 and site-specific cancers: a Mendelian randomization study. Cancer Med. 2020;9(18):6836–42.
Chen M, Tsai CW, Chang WS, et al. High circulating insulin-like growth factor-1 reduces the risk of renal cell carcinoma: a Mendelian randomization study. Carcinogenesis. 2021;42(6):826–30.
Tsai CW, Chang WS, Xu Y, Huang M, Bau DT, Gu J. Associations of genetically predicted circulating insulin-like growth factor-1 and insulin-like growth factor binding protein-3 with bladder cancer risk. Mol Carcinog. 2021;60(11):726–33.
Acknowledgements
We want to acknowledge the participants and investigators of the FinnGen study, the Genetic Epidemiology Research in Adult Health and Aging cohort, and the UK Biobank cohort. We also want to acknowledge the participants and investigators of other studies.
Funding
This study was supported by the Science and technology department of Jilin province (20180623052TC), the Jilin Provincial Key Laboratory (20190901005JC) to Yi Yang. Yi Yang funded the journal's Rapid Service Fee.
Author Contributions
Conceptualization: Yi Yang and Zhen-Ni Guo; Methodology: Peng-Peng Niu; Writing - original draft preparation: Reziya Abuduxukuer; Writing - review and editing: Reziya Abuduxukuer, Peng-Peng Niu, Yu-Ming Xu, and Zhen-Ni Guo; Funding acquisition: Yi Yang.
Disclosures
Reziya Abuduxukuer, Peng-Peng Niu, Zhen-Ni Guo, Yu-Ming Xu, and Yi Yang have nothing to disclose.
Compliance with Ethics Guidelines
We followed the STROBE-MR guidelines to perform the present study. Ethics approval and informed consent were not required for the present study, as they were obtained in the original studies. The original studies were conducted in compliance with the Declaration of Helsinki.
Data Availability
Summary-level data of IGF1 was downloaded from https://doi.org/10.35092/yhjc.12355382. The download link for summary-level data of migraine from the FinnGen study can be obtained from https://finngen.gitbook.io/documentation/. The summary-level data of migraine generated by Choquet et al. was downloaded from the NHGRI-EBI GWAS Catalog (https://www.ebi.ac.uk/gwas/downloads/summary-statistics) with a study accession number of GCST90000016.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Abuduxukuer, R., Niu, PP., Guo, ZN. et al. Circulating Insulin-Like Growth Factor 1 Levels and Migraine Risk: A Mendelian Randomization Study. Neurol Ther 11, 1677–1689 (2022). https://doi.org/10.1007/s40120-022-00398-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00398-w