Abstract
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that places a substantial burden on patients, caregivers, and society. The advent of disease-modifying treatments (DMTs) would represent a major advancement in the management of AD, particularly in early AD. It is important to understand the potential value of these therapies to individuals and society.
Methods
A modeling framework was developed to estimate the potential clinical and economic burden of AD in the USA by simulating the impact, relative to that of usual care, of a DMT with hypothesized availability beginning from 2022. The model assessed AD epidemiology, disease progression, and burden of illness from 2020 to 2050. Model outcomes included the total number of Americans with mild cognitive impairment (MCI) due to AD and mild, moderate, or severe AD dementia in community or residential care settings and their associated care costs, including direct medical and non-medical costs for healthcare resource use and indirect costs for caregiving.
Results
A hypothetical DMT was compared to the usual care under different effect scenarios based on delay in onset of AD (1, 3, and 5 years) and DMT uptake (25%, 50%, and 100%). A delay in the onset of AD by 5 years would reduce the prevalence of AD in 2050 by 6%, 12%, and 25%, resulting in savings of $0.783, $1.566, and $3.132 trillion from 2022 to 2050 for the 25%, 50%, and 100% uptake scenarios, respectively.
Conclusion
This analysis demonstrated that DMTs that provide even small delays in the onset of AD can lead to an increase in disease-free years and sizable savings in the cost of care, providing significant benefits to patients, caregivers, and society.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Alzheimer’s disease (AD) places a substantial economic burden on patients and healthcare systems. The need exists to assess the value of potential disease-modifying treatments (DMTs) in patients with early AD. |
The objective of this study was to develop a simulation approach based on AD etiology and pathophysiology to account for the burden of illness following the introduction of a hypothetical DMT, and to assess the patient and societal value of delaying the onset of AD. |
The number of individuals aged 60 years or older with AD is projected to rise by 93% from 2020 to 2050 under the usual care scenario, resulting in a $581 billion increase for the cost of care. |
The introduction of a DMT that delays the onset of AD would provide an immediate and meaningful reduction in cost of care. A 5-year delay in AD onset would reduce the prevalence of AD in 2050 by 25% if all eligible patients are treated, which translates into cumulative savings of $3.132 trillion from 2022 to 2050. |
Our analyses indicated that a hypothetical DMT could shift the distribution of patients to earlier stages of the disease, substantially reducing patient/caregiver burden associated with advanced stages of AD. |
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that accounts for 60–80% of all dementia cases—an estimated 6.2 million people in the USA over the age of 65 have AD [1]. The underlying pathology of the disease develops long before manifestation of symptoms; signs of AD may begin with mild cognitive impairment (MCI) and advance through mild, moderate, and severe stages of dementia. Rates are projected to reach 8.5 million individuals with AD by 2030 and 12.7 million by 2050 in the absence of treatments to slow progression of or cure the disease [1].
AD places a substantial economic burden on patients and healthcare systems. The National Alzheimer’s Project was established in 2011 to leverage US federal programs to “accelerate the development of treatments that would prevent, halt, or reverse the course of Alzheimer’s disease” [2]. The drug development pipeline is crowded with 126 agents in 152 clinical trials; these include amyloid, tau, and neuroinflammation as well as repurposed drugs and potential curative gene therapies [3]. Aducanumab, a monoclonal antibody directed at amyloid beta (Aβ) plaques, received accelerated approval from the US Food and Drug Administration (FDA) in 2021 as the first treatment for patients with early AD [4]. Understanding the potential value of disease-modifying treatments (DMTs) in early AD is of great importance, and burden-of-illness analyses can serve as a starting point for these discussions.
Published studies on the clinical and economic impacts of AD and delaying the disease onset vary widely in methods, ranging from simple prevalence-based approaches to conventional Markov models. The objective of this study was to develop a simulation approach based on AD etiology and pathophysiology to account for the burden of illness following the introduction of a hypothetical DMT, and to assess the patient and societal value of delaying the onset of AD. Scenario and sensitivity analyses were conducted to identify the key model inputs that resulted in the highest cost savings.
Methods
Model Overview
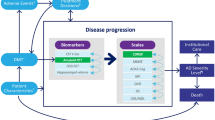
A modeling framework was developed to estimate the economic burden of AD in the USA assuming that an amyloid-targeting treatment will become available in 2022; this hypothetical DMT was compared to the usual care under different scenarios based on delay in the onset of AD (1, 3, and 5 years) and DMT uptake among eligible patients (25%, 50%, and 100%). The model consisted of three modules—epidemiology, disease progression, and burden of illness (Fig. 1)—and assessed the annual burden of illness of AD from 2020 to 2050 (aka analysis interval) under different scenarios. The epidemiology module used the annual incidence rates of MCI due to AD by age group with the projected US population estimates by age group over 60 years old and reported on the total number of new subjects with MCI due to AD each year from 2000 to 2050 (Fig. 1a). The disease progression module leveraged the AD Archimedes Condition Event (AD ACE) disease simulator to track disease progression of incident subjects with MCI due to AD over their lifetime (Fig. 1b).
The AD ACE is a patient-level simulation model that predicts the natural history of individuals from a preclinical disease state through the severe AD stage and estimates potential effects of a DMT on disease progression. The model simulates disease progression based on changes in the underlying AD biomarkers (e.g., measures of Aβ and tau levels) and their connections to clinical presentation of AD, which are measured by various patient-level scales of cognition, behavior, function, and dependence. In early AD, disease progression is measured by interconnected predictive equations derived from longitudinal assessments of clinical and biomarker data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [5]. Biomarkers extracted from the ADNI longitudinal data set were cerebrospinal fluid (CSF) proteins (Aβ1–42 and total-tau) linked to abnormal brain deposits, fluorodeoxyglucose (FDG)–positron emission tomography (PET) linked to reduced brain cell metabolic activity, and one magnetic resonance imaging (MRI) measurement of hippocampal volume linked to brain shrinkage. Full details on the AD ACE model structure, data inputs, and predictive equations have been previously published [6, 7].
As patients progress to more severe stages of AD that the ADNI study does not effectively represent, the AD ACE switches to Assessment of Health Economics in Alzheimer’s Disease II (AHEAD) equations for cognition and behavioral scales to make the model more representative and accurate across all stages of AD [8, 9]. Additionally, the model captured transitions to/between community and institutional care settings as patients progressed to more severe stages of AD. Full details on the AD ACE model structure and equations have been previously published [7, 10,11,12]. The AD ACE was recently used to study the potential long-term health and economic outcomes of lecanemab in patients with early AD.
The disease progression module reported the annual proportions of patients with MCI due to AD, and with mild, moderate, or severe AD; proportions of deceased patients; proportions of patients in community or residential care; as well as the mean annual total costs of community or residential care per patient over lifetime, including direct medical and non-medical costs for healthcare resource use and indirect costs for caregiving. The outcomes from the epidemiology module (i.e., annual MCI incidence) and disease progression module (i.e., annual AD disease stage, mortality, and costs) were used to estimate the total number of Americans with MCI due to AD and mild, moderate, or severe AD in community or residential care settings and their associated costs each year, and subsequently compute the clinical and economic burden of AD during the analysis interval in the burden of illness module (Fig. 1c). A pre-analysis interval from 2000 to 2019 was incorporated in the burden of illness module to gradually accumulate the number of alive patients as they progress in their disease to achieve an accurate estimate of patients with MCI and AD during the analysis interval. The pre-analysis interval was initiated in 2000 as almost all patients with incident MCI from this year were dead before the start of the analysis interval and with minimal impact on burden of illness analyses. The model-predicted AD prevalence in 2020 closely matched the estimates by the Alzheimer’s Association (i.e., 6.1 million individuals in 2020) [1].
Disease Progression and DMT Effect
The natural history of AD progression for patients receiving usual care was modeled using AD ACE based on disease equations developed from longitudinal patient-level ADNI data for early AD [5] and published AHEAD equations for more severe stages of AD [8, 9]. Clinical Dementia Rating scale Sum of Boxes (CDR-SB) thresholds were used to determine patients’ disease severity at baseline and over time in AD ACE (i.e., MCI due to AD < 4.5, mild AD ≥ 4.5 to < 9.5, moderate AD ≥ 9.5 to < 16, and severe AD ≥ 16) [13].
The treatment effect was modeled on the basis of the key assumption that the effect of a DMT on the clinical outcomes is correlated with the amyloid PET level as a surrogate endpoint [14, 15]. In AD ACE the relationships between biomarkers of disease and clinical outcomes are based on correlations mainly observed in the ADNI data, and disease equations are evaluated repeatedly at subsequent time intervals every 6 months to estimate the AD disease trajectory of patients. An anti-amyloid DMT can be potentially modeled in AD ACE by imposing effects on estimated amyloid PET standardized uptake value ratio (SUVr) outcomes of a simulated patient. In this study, a calibration process was applied to adjust the predicted measures of amyloid PET SUVr at each time interval to slow down the progression of disease and achieve 1-, 3-, and 5-year average delay in onset of AD. Amyloid PET is a predictor in all AD ACE disease equations; therefore, any calibrated reduction in amyloid PET SUVr at a given time interval impacts the prediction of amyloid PET SUVr and other modeled AD biomarkers and scales at later time intervals and consequently the time to onset of AD. Calibration is the process of determining or adjusting parameter values in a model by constraining model output to replicate empirical data within an acceptable range. In this analysis, calibration was performed by comparing model output from different reductions in amyloid PET SUVr in subsequent time intervals to identify the parameter sets that best correspond to desired average delay in onset of AD.
The calibration process was focused on patients with amyloid-β-positive (Aβ+) MCI who were eligible to receive the hypothetical DMT. The AD ACE model was run to calibrate the effects of a hypothetical amyloid-targeting treatment to achieve 1-, 3-, and 5-year delays in onset of AD. A lifetime simulation of 2000 sampled ADNI patients was used during the treatment effect calibration process where treatment discontinuation was not allowed. The ADNI sample population included 526 Aβ+ patients ages 60 or older who received a clinical diagnosis of MCI, which was defined by a score > 24 on the Mini-Mental State Examinations (MMSE) and a global Clinical Dementia Rating scale of 0.5 at baseline. The accumulation of amyloid-β in the brain was measured by PET imaging with 18F-AV-45 (florbetapir), and patients with a baseline mean cortical standardized uptake value ratio (mcSUVR) ≥ 1.1 were considered as Aβ+. To inform the burden-of-illness analysis, the analysis was focused on patients with MCI. Scenarios were run separately for usual care and each of the hypothetical DMT effects and uptake scenarios by sampling 2000 profiles from a cohort of 826 ADNI patients ages 60 or older with MCI. Under the 100% uptake scenario, the hypothetical DMT was initiated immediately for patients who were Aβ+ at baseline and with a delay for those who became Aβ+ by crossing the 18F-AV-45 cutoff value of mcSUVR ≥ 1.1 or progressed to mild AD defined as a CDR-SB ≥ 4.5 during the simulation runs. The DMT was terminated when a patient’s AD reached moderate severity, as measured by a CDR-SB score ≥ 9.5. Other types of treatment discontinuation were not considered in this analysis (e.g., patient decision, adverse events). This economic analysis assumed that the hypothetical DMT will become available in 2022 for eligible patients, hence patients could not access treatment prior to 2022 and a treatment time rule was applied to incident patients entering the model from 2000 to 2021. Each DMT scenario was run 22 times and the timing to DMT initiation lagged from zero to 21 years. For example, patients with incident MCI in 2005 had a minimum time lag of 17 years before treatment initiation, whereas the time lag was at least 7 years for the incident patients in 2015.
Model Inputs
This assessment is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Epidemiology Inputs
Age-stratified incidence of MCI and US Census Bureau projections of the general population [16,17,18] were used in the model to estimate the total number of individuals in the USA with a new diagnosis of MCI for each year from 2000 to 2050. A targeted literature review was conducted to inform the MCI incidence and prevalence rates by age group; these results varied widely across published studies, primarily because of different criteria used to define MCI [19]. A systematic review of population-based studies on age-specific incidence of MCI in the USA, Australia, and Europe indicated substantial uncertainty around the incidence of amnestic and non-amnestic MCI subtypes (e.g., MCI incidence rate ranged from 12.4 to 35.9 per 1000 person-years for the 70- to 74-year-old group) [19]. Data were sparse on MCI incidence rates for individuals ages 60 to 70 years, but limited data were available on MCI prevalence rates [20,21,22].
Age-specific MCI incidence rates from multiple sources were explored in the model and ultimately those rates that resulted in total patients with AD projections that were close to the 2050 projections provided by the Alzheimer’s Association were selected as a benchmark [1]. The amnestic MCI incidence rates from Roberts et al. [23] were considered for age 70 years or older, and rates for the 60- to 70-year-old group were derived from prevalence rates in Petersen et al. [20]. The incidence rates (per 1000 person-years) used in the epidemiology module by age group are shown in Table 1.
Clinical Inputs
Clinical data inputs included AD progression, death, rate of institutionalization, and the assumptions around the hypothetical DMT efficacy and its initiation/stopping rules. Disease progression through the AD continuum was governed by the disease equations in the AD ACE (i.e., the evolution of AD biomarkers and various relevant patient-level scales of cognition, behavior, function, and dependence) [6]. Mortality was modeled on the basis of the death rates from the general US population, weighted by hazard ratios (HR) for each AD severity level [24] (HR = 2.92, mild; HR = 3.85, moderate; and HR = 9.52, severe). Patients were subject to an annual risk of institutionalization that varied over disease severity with 2.6% for mild AD, 5.5% for moderate AD, and 8.1% for severe AD [25].
Cost Inputs
The analysis considered the costs of community-based and nursing home care for people with AD dementia. Current US literature lacks estimates for these costs across the full disease continuum, so inputs from multiple sources were considered and combined as needed. The GERAS-US study [26] results were used to inform the community-based care costs for patients with MCI and mild AD. This prospective, longitudinal cohort study was adapted from the GERAS I study [27]; it assessed the cross-sectional total societal costs associated with patients and study partners in MCI and mild AD, including direct medical and non-medical costs for healthcare resource use and indirect costs for caregiving. GERAS I was conducted in multiple European countries and reported on community-based costs for patients with mild, moderate, and severe AD dementia; the study indicated that increasing disease severity was associated with increased costs. The mean monthly costs for patients with moderate and severe AD dementia were not reported in GERAS-US. Therefore, the mean relative ratio between estimated costs for mild AD vs. moderate and severe AD from GERAS I for three European countries was computed and applied to the community-based care costs for mild AD from GERAS-US to approximate those mean monthly costs in the USA. The computed mean relative ratios for mild-to-moderate AD (1.4) and mild-to-severe AD (2.0) were well aligned with the findings from Leon et al. [28] and Small et al. [10], cited in the Alzheimer’s Association model [29], on relative change in average cost of care by disease stage. Table 1 provides the breakdown of the cost categories used in the model for community-based care where disease severity was defined by the MMSE scale. Half of the MCI costs provided by GERAS-US was considered in this economic analysis as the MCI incidence rates used in this model were informed from population-based studies, which include patients who were not necessarily seeking access to healthcare in the very early stages of disease.
The residential care cost was informed by Genworth’s Cost of Care Survey tool [30]. The 2020 monthly median cost for a private/semi-private room in a nursing home facility in the USA was $8175. The model does not adjust for disease stage for nursing home costs since the cost is usually the same for all patients. The costs of the hypothetical DMT and diagnostics (e.g., PET scans) to determine amyloid count were not considered in the analysis because of uncertainties around requirements and potential variations in reimbursement policies.
All per capita costs derived from GERAS-US were inflated to 2020 US dollars. For this analysis it was decided not to increase estimated cost results over time to reflect the impact of general inflation. Healthcare inflation rates reported by the Consumer Price Index [31] approximated the general inflation rate, so there was no need to inflate the resulting total cost figures for each year through 2050 to capture excess cost growth.
Model Outcomes
The model estimated the economic effects of delay in the onset of AD under different hypothetical DMT effect and uptake scenarios. The total number of patients with prevalent MCI and AD were projected annually from 2020 to 2050, along with the proportion of patients in each AD severity stage. The overall direct and indirect cost breakdowns by location of care and by patient or caregiver were calculated and reported over time, as well as 10- and 25-year cost savings from the initiation of the DMT.
Results
Base-Case Analyses
The projected number of people in the USA age 60 or older with MCI due to AD and AD (mild, moderate, and severe) from 2020 to 2050 is shown in Fig. 2 for all effect scenarios. There was no difference in the prevalence of patients with MCI and AD until 2022 when the hypothetical DMT becomes available. In the absence of a DMT, the number of individuals with MCI and AD increased by 7.68 million and 5.72 million by 2050, respectively. Delaying the onset of AD by 1, 3, and 5 years with a DMT resulted in a reduction of AD prevalence by 0.56 million, 1.78 million, and 2.94 million by 2050, respectively. In contrast, prevalence of MCI increased under the three DMT effect scenarios by 1.56 million, 4.72 million, and 7.35 million by 2050, since the time to AD was delayed and more individuals stayed longer in the MCI stage. In the two reduced uptake scenarios (25% and 50%) for a 5-year delay in onset of AD, the prevalence of MCI increased by 1.84 million and 3.67 million, and the prevalence of AD reduced by 0.74 million, and 1.47 million, respectively (Fig. 3).
The proportions of patients in the usual care scenario changed very little from 2020 to 2050 for mild (65–63%), moderate (25–26%), and severe (10–11%) AD. In contrast, the patient numbers were redistributed under the three hypothetical DMT effect scenarios, showing a pattern of increasing patient concentration toward less severe disease stages. For example, the model estimated 74% of patients were at mild AD stage, 20% at moderate AD stage, and 6% at severe AD stage by 2050, assuming a DMT effect with 5-year delay in AD onset (Fig. 4). Correspondingly, the number of individuals requiring institutional care decreased from 0.61 million to 0.55 million by 2050 under this scenario.
The total costs of care by location and cost type under usual care and three DMT effect scenarios from 2020 to 2050 is presented in Fig. 5. Informal caregiving and patient healthcare were the top two cost categories, comprising approximately 40% and 30% of the overall costs, respectively. In 2050, the annual cost of care decreased by 2%, 6%, and 10% compared to the usual care scenario under the 1-, 3-, and 5-year-delay DMT effect scenarios, respectively.
The results of total cost savings over 10 and 25 years for the three DMT effect scenarios and reduced uptake (25%, 50%) versus full uptake for a 5-year-delay DMT effect scenario are displayed in Fig. 6. Starting with the introduction of the DMT in 2022, the 1-, 3-, and 5-year-delay DMT effect scenarios resulted in total cost savings of $0.233 trillion, $0.666 trillion, and $0.994 trillion over a 10-year period and $0.585 trillion, $1.757 trillion, and $2.764 trillion over a 25-year period, respectively. Similarly, the 25%, 50%, and full uptake scenarios resulted in total cost savings of $0.249 trillion, $0.497 trillion, and $0.994 trillion over a 10-year period and $0.691 trillion, $1.382 trillion, and $2.764 trillion over a 25-year period, respectively. Although these calculations do not account for the cost of treatment, the economic gain helps present the value of the hypothetical treatment.
Results from the AD ACE simulations indicated that patients with Aβ+ MCI who received the hypothetical DMT and experienced 1-, 3-, and 5-year delay in AD onset achieved on average 0.36, 1.03, and 1.62 more years of life and their AD prevalence over lifetime reduced from 81% to 77%, 70%, and 63%, respectively.
Sensitivity Analyses
The results of the sensitivity analyses of the key model parameters on the DMT effect scenario with a 5-year delay are displayed in the tornado diagram in Fig. 7. The analysis focused on the impact of ± 20% change in parameters on the 25-year cost-savings results. The parameters varied in sensitivity analyses were costs of care by AD severity stage, HR for mortality by AD severity stage, probability of institutionalization, and MCI incidence rate. MCI incidence rate, cost of care for severe AD stage, MCI stage, and moderate AD stage resulted in the most variation in the 25-year cost savings. Increasing/decreasing MCI incidence or cost of care associated with severe AD by 20% resulted in near 20% more/less cost savings over 25 years. The probability of institutionalization, cost of care for mild AD stage, and HR for mortality in mild AD were the least influential on results. Increasing and decreasing institutionalization rate by 20% were only associated with − 0.4% and − 0.4% difference from the base case, respectively.
Discussion
Alzheimer’s disease is a progressive, neurodegenerative disease and represents a major global health crisis with a large and rapidly growing burden on patients, caregivers, and healthcare system. There is an urgent need for effective DMT strategies targeting the fundamental pathophysiology of the disease that interrupt or slow the disease progression, improve patient health outcomes, and reduce economic burden. It is equally important to assess and communicate potential clinical benefits of these strategies beyond clinical trial endpoints and into clinical practice objectively and appropriately. This is more challenging for treatment strategies that are designed to treat earlier, asymptomatic, or minimally symptomatic AD stages.
In this assessment, the number of individuals aged 60 years or older with AD is projected to rise by 93% from 2020 to 2050 under the usual care scenario, resulting in a $581 billion increase for the cost of care, which is mainly attributed to informal caregiving. The introduction of a DMT that delays the onset of AD would provide an immediate and meaningful reduction in these costs. A 5-year delay in AD onset would reduce the prevalence of AD in 2050 by 25%, which translates into a cumulative savings of $3.132 trillion from 2022 to 2050. These analyses also indicated that a hypothetical DMT could shift the distribution of patients to earlier stages of the disease, substantially reducing patient/caregiver burden associated with advanced stages of AD.
Different DMT uptake scenarios were explored. Results indicated that a higher uptake of a DMT that delays the onset of AD by 5 years would lead to fewer patients living with AD in 2050 and more cost savings over a 10-year and 25-year period.
According to the results of sensitivity analyses, a decrease in MCI cost of care or the hazard of death, or an increase in the AD cost of care in any stage would lead to additional cost savings under the 5-year-delay DMT effect scenario. The observed trends were mainly derived from the increased number of patients with MCI and the shifting of patients to less severe AD stages under DMT effect scenarios. A higher impact on savings was observed with costs associated with more severe AD stages as such costs are higher as compared to less severe stages, which in turn affects the absolute cost offsets between usual care and DMT effect scenarios. The incidence of patients with MCI also had a big impact on the projected cost savings.
Previous economic models mainly relied on Markov models to assess the impact of the delay in the onset of AD. The 2021 Alzheimer’s Association’s analysis explored the impact of a new DMT introduced in 2025 that delays disease onset by 5 years [32] based on a Markov model developed by the Lewin Group [33]. Zissimopoulos and colleagues [34] used the same effect scenarios (i.e., 1, 3, and 5 years) considered in this study, but employed a Markov process and used the Future Elderly Model to simulate the cognitive state and AD status of the cohort population. Their analysis focused on an older population (≥ 70 years vs. ≥ 60 years in the current analysis) but generally reported larger impacts due to the delay in the onset of AD than what was seen in this analysis. The differences may be related to the approaches that were used to estimate the underlying mechanisms of disease progression. Other published estimates of the burden of AD used more conventional, non-simulation-based approaches. For example, the analysis presented to Alzheimer’s Australia by Access Economics 2004 [35] utilized the formulae from Brookmeyer et al. [36] to predict prevalence of AD. The overall cost estimates from this model under usual care and DMT scenarios differed slightly from the estimates in the analysis by the 2021 Alzheimer’s Association. This was mainly due to differences in the projected number of patients with AD between the two models, the model structures, and how the DMT effect is linked to disease progression, and the difference in cost components and input sources considered to inform the cost of care. That analysis also reported that most patients with prevalent AD are in the severe stage unlike other published literature [37] and the current analysis, which indicated most patients are in the mild AD stage.
The key strengths of this economic analysis are use of a simulation to model disease progression and better account for the heterogeneity in patient population; leveraging an epidemiology module to achieve a robust estimate of patients with prevalent MCI and AD; reporting the impact of a hypothetical DMT on the prevalence of patients with MCI, which is unique to this analysis, along with AD population; and incorporating a newly published study to inform the cost inputs. This model is primarily based on a previously published AD ACE model to simulate the progression of disease, so all limitations and assumptions associated with that model hold in this analysis. Although explored DMTs were all hypothetical, relevant, published outcomes from recent amyloid-targeting AD trials were used to calibrate more realistic effect scenarios. The treatment effect was modeled by calibrating the reduction in amyloid level to achieve different delays in onset of AD which can result in estimation bias of important measures such as costs over lifetime. Accurate estimates of PET SUVr and adequate trial duration to capture long-term DMT effects are required to model and evaluate the potential impacts of DMTs.
This assessment used a modeling framework that consisted of three modules, namely epidemiology, disease progression, and burden of illness. At the core is a model of human physiology that helps describe the pertinent aspects of physiology, pathophysiology, clinical manifestation and severity (i.e., signs and symptoms), effects of treatments, and occurrence of health outcomes. Future research can use the modeling infrastructure to assess health system resources (e.g., personnel, facilities, equipment, and costs) and level of investment needed for better patient management in AD. The modeling infrastructure may also be used to understand the costs and value of early detection, diagnosis, and treatment of patients with early AD. The findings are important to inform different stakeholders about the value of AD prevention, early diagnosis, and treatment.
Conclusions
This study focused on evaluating the potential impact of DMTs on burden and trajectory of AD. The study findings can help formulate and prioritize healthcare policies and allocate healthcare resources in accordance with budget constraints to achieve policy efficiency. The AD population and the associated economic burden will continue to grow significantly over the coming decades without effective treatment strategies that interrupt or slow disease progression. This analysis demonstrated that therapeutic options that provide even small delays in the onset of AD can lead to an increase in disease-free years and sizable savings in the cost of care, providing significant benefits to patients, their caregivers, and society.
References
Association A. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17:327–406.
National Alzheimer’s Project Act (NAPA). Draft national plan to address Alzheimer’s disease. https://aspe.hhs.gov/sites/default/files/documents/66904c18bb1f0843c3c113d7099e98c1/napa-national-plan-2021-update.pdf. Accessed 2 June 2021.
Cummings J, Lee G, Zhong K, Fonseca J, Taghva K. Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dement (N Y). 2021;7(1):e12179.
Mahase E. FDA approves controversial Alzheimer’s drug despite uncertainty over effectiveness. BMJ. 2021;373:n1462.
Alzheimer’s disease neuroimaging initiative. Home page 2017. http://adni.loni.usc.edu/. Accessed 2 June 2021.
Kansal AR, Tafazzoli A, Ishak KJ, Krotneva S. Alzheimer’s disease archimedes condition-event simulator: development and validation. Alzheimers Dement (N Y). 2018;4:76–88.
Tafazzoli A, Weng J, Sutton K, et al., editors. Validating simulated cognition trajectories based on ADNI against trajectories from the National Alzheimer’s Coordinating Center (NACC) dataset. In: 11th Clinical Trial on Alzheimer’s Disease (CTAD); 2018; Barcelona, Spain.
Getsios D, Blume S, Ishak KJ, Maclaine GD. Cost effectiveness of donepezil in the treatment of mild to moderate Alzheimer’s disease: a UK evaluation using discrete-event simulation. Pharmacoeconomics. 2010;28(5):411–27.
Guo S, Getsios D, Revankar N, et al. Evaluating disease-modifying agents: a simulation framework for Alzheimer’s disease. Pharmacoeconomics. 2014;32(11):1129–39.
Small GW, McDonnell DD, Brooks RL, Papadopoulos G. The impact of symptom severity on the cost of Alzheimer’s disease. J Am Geriatr Soc. 2002;50(2):321–7.
Tahami Monfared AA, Tafazzoli A, Ye W, Chavan A, Zhang Q. Long-term health outcomes of lecanemab in patients with early Alzheimer’s disease using simulation modeling. Neurol Ther. 2022;11(2):863–80.
Tahami Monfared AA, Tafazzoli A, Chavan A, Ye W, Zhang Q. The potential economic value of lecanemab in patients with early Alzheimer's disease using simulation modeling. Neurol Ther. 2022;11(3):1285–1307.
O’Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s Research Consortium study. Arch Neurol. 2008;65(8):1091–5.
Avgerinos KI, Ferrucci L, Kapogiannis D. Effects of monoclonal antibodies against amyloid-beta on clinical and biomarker outcomes and adverse event risks: a systematic review and meta-analysis of phase III RCTs in Alzheimer’s disease. Ageing Res Rev. 2021;68: 101339.
Fletcher E, Filshtein TJ, Harvey D, Renaud A, Mungas D, DeCarli C. Staging of amyloid beta, t-tau, regional atrophy rates, and cognitive change in a nondemented cohort: results of serial mediation analyses. Alzheimers Dement (Amst). 2018;10:382–93.
Howden LM, Meyer JA. Age and sex composition: 2010: United States Census Bureau; 2011. https://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf. Accessed 2 June 2021.
United States Census Bureau. 2017 national population projections tables: main series 2017. https://www.census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html. Accessed 2 June 2021.
U.S. Census Bureau Population Division. Projected 5-year age groups and sex composition of the population (np2017-t3). September 2018. https://www2.census.gov/programs-surveys/popproj/tables/2017/2017-summary-tables/np2017-t3.xlsx. Accessed 2 June 2021.
Sachdev PS, Lipnicki DM, Kochan NA, et al. The prevalence of mild cognitive impairment in diverse geographical and ethnocultural regions: the COSMIC collaboration. PLoS ONE. 2015;10(11):e0142388.
Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–35.
Lang L, Clifford A, Wei L, et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ Open. 2017;7(2):e011146.
Ganguli M, Chang CC, Snitz BE, Saxton JA, Vanderbilt J, Lee CW. Prevalence of mild cognitive impairment by multiple classifications: the Monongahela-Youghiogheny Healthy Aging Team (MYHAT) project. Am J Geriatr Psychiatry. 2010;18(8):674–83.
Roberts RO, Geda YE, Knopman DS, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78(5):342–51.
Andersen K, Lolk A, Martinussen T, Kragh-Sorensen P. Very mild to severe dementia and mortality: a 14-year follow-up—the Odense study. Dement Geriatr Cogn Disord. 2010;29(1):61–7.
Green C, Handels R, Gustavsson A, et al. Assessing cost-effectiveness of early intervention in Alzheimer’s disease: an open-source modeling framework. Alzheimers Dement. 2019;15(10):1309–21.
Robinson RL, Rentz DM, Andrews JS, et al. Costs of early stage Alzheimer’s disease in the United States: cross-sectional analysis of a prospective cohort study (GERAS-US)1. J Alzheimers Dis. 2020;75(2):437–50.
Wimo A, Reed CC, Dodel R, et al. The GERAS Study: a prospective observational study of costs and resource use in community dwellers with Alzheimer’s disease in three European countries–study design and baseline findings. J Alzheimers Dis. 2013;36(2):385–99.
Leon J, Cheng CK, Neumann PJ. Alzheimer’s disease care: costs and potential savings. Health Aff (Millwood). 1998;17(6):206–16.
Alzeimer's Association. Trajectory report. https://www.alz.org/help-support/resources/publications/trajectory_report. Accessed 2 June 2021.
Genworth. Cost of Care Survey 2021. https://www.genworth.com/aging-and-you/finances/cost-of-care.html. Accessed 2 June 2021.
US Bureau of Labor Statistics. 12-month percentage change, Consumer Price Index, selected categories 2021. https://www.bls.gov/charts/consumer-price-index/consumer-price-index-by-category-line-chart.htm. Accessed 2 June 2021.
Alzeimer's Association. Changing the trajectory of Alzheimer’s disease: how a treatment by 2025 saves lives and dollars 2015. https://www.alz.org/media/Documents/changing-the-trajectory-r.pdf. Accessed 2 June 2021.
The Lewin Group. Saving lives, saving money: dividends for Americans from investing in Alzheimer’s research 2004. http://www.lewin.com/~/media/Lewin/Site_Sections/Publications/2867.pdf. Accessed 2 June 2021.
Zissimopoulos J, Crimmins E, St. Clair P. The value of delaying Alzheimer’s disease onset. Forum Health Econ Policy. 2014;18(1):25–39.
Access Economics PTY Limited. Delaying the onset of Alzeimer's disease: projections and issues 2004. https://www.dementia.org.au/sites/default/files/20040820_Nat_AE_DelayOnsetADProjIssues.pdf. Accessed 2 June 2021.
Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337–42.
Yuan J, Maserejian N, Liu Y, et al. Severity distribution of Alzheimer’s disease dementia and mild cognitive impairment in the Framingham Heart Study. J Alzheimers Dis . 2021;79(2):807–817.
Acknowledgements
Funding
This study and the journal’s Rapid Service fee were funded by Eisai Inc.
Medical Writing, Editorial, and Other Assistance
The authors would like to acknowledge Evidera’s editor, Colleen Dumont, for proofreading and editorial services funded by Eisai Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Amir Abbas Tahami Monfared and Ali Tafazzoli conceptualized the study and were responsible for its overall direction and planning, with input from Quanwu Zhang. Ali Tafazzoli, Weicheng Ye, Ameya Chavan, and Kristen A. Deger developed the model and performed the analyses.
Disclosures
Weicheng Ye, Ameya Chavan, and Kristen A. Deger are current employees of Evidera, a healthcare research firm that provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. Ali Tafazzoli is a former employee of Evidera. Evidera received funding from Eisai Inc. to conduct the study and develop this manuscript. Amir Abbas Tahami Monfared, Ali Tafazzoli, and Quanwu Zhang are employees of Eisai Inc.
Compliance with Ethics Guidelines
This assessment is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data used for this study are provided in the manuscript. Additional details are available from the corresponding author on request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tahami Monfared, A.A., Tafazzoli, A., Ye, W. et al. A Simulation Model to Evaluate the Potential Impact of Disease-Modifying Treatments on Burden of Illness in Alzheimer’s Disease. Neurol Ther 11, 1609–1623 (2022). https://doi.org/10.1007/s40120-022-00393-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00393-1