Abstract
Introduction
Fatigue, cognitive impairment, depression, and pain are highly prevalent symptoms in multiple sclerosis (MS). These often co-occur and may be explained by a common etiology. By reviewing existing literature, we aimed to identify potential underlying biological processes implicated in the interconnectivity between these symptoms.
Methods
A literature search was conducted to identify articles reporting research into the biological mechanisms responsible for the manifestation of fatigue, cognitive impairment, depression, and pain in MS. PubMed was used to search for articles published from July 2011 to July 2021. We reviewed and assessed findings from the literature to identify biological processes common to the symptoms of interest.
Results
Of 693 articles identified from the search, 252 were selected following screening of titles and abstracts and assessing reference lists of review articles. Four biological processes linked with two or more of the symptoms of interest were frequently identified from the literature: (1) direct neuroanatomical changes to brain regions linked with symptoms of interest (e.g., thalamic injury associated with cognitive impairment, fatigue, and depression), (2) pro-inflammatory cytokines associated with so-called ‘sickness behavior,’ including manifestation of fatigue, transient cognitive impairment, depression, and pain, (3) dysregulation of monoaminergic pathways leading to depressive symptoms and fatigue, and (4) hyperactivity of the hypothalamic–pituitary-adrenal (HPA) axis as a result of pro-inflammatory cytokines promoting the release of brain noradrenaline, serotonin, and tryptophan, which is associated with symptoms of depression and cognitive impairment.
Conclusion
The co-occurrence of fatigue, cognitive impairment, depression, and pain in MS appears to be associated with a common set of etiological factors, namely neuroanatomical changes, pro-inflammatory cytokines, dysregulation of monoaminergic pathways, and a hyperactive HPA axis. This association of symptoms and biological processes has important implications for disease management strategies and, eventually, could help find a common therapeutic pathway that will impact both inflammation and neuroprotection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Fatigue, cognitive impairment, depression, and pain are highly prevalent symptoms that often co-occur in multiple sclerosis (MS); therefore, these symptoms may be explained by a common etiology. |
By reviewing existing literature, we aimed to identify potential underlying biological processes implicated in the interconnectivity among fatigue, cognitive impairment, depression, and pain symptoms in MS. |
We found that the co-occurrence of fatigue, cognitive impairment, depression, and pain in MS appears to be associated with a common set of underlying etiological factors, namely neuroanatomical changes, pro-inflammatory cytokines, dysregulation of monoaminergic pathways, and a hyperactive hypothalamic-pituitary-adrenal axis. |
These findings have important implications for pharmacological and non-pharmacological disease management strategies as well as for future research activities in MS. |
Introduction
Multiple sclerosis (MS) is a neurological disease characterized by inflammation and degeneration of the central nervous system (CNS) [1]. In 2020, it was estimated to affect 2.8 million people globally [2], with the number expected to increase over time [3, 4]. There are a broad range of symptoms associated with MS, and they manifest differently between patients. However, common symptoms impacting the social and physical functioning of people living with MS (PlwMS) include fatigue [5], cognitive impairment [6], pain [7], and depression [8] and are estimated to affect approximately 37–78% [9], 34–65% [10], 29–86% [11], and 5–59% [12] of patients, respectively. The range of prevalence observed for each symptom likely reflects differences in study populations and methodologies used.
Notably, these symptoms often co-occur, having a significant impact on patients’ lives. Co-occurrence of fatigue, pain, depression, and cognitive complaints has been shown to have a negative association with quality of life (QoL), with worsening symptoms being linked to poorer psychological and physical QoL. A single-factor model was found to explain the associations among these symptoms [13]. Other research suggests that these symptoms cluster in two distinct patterns: an ‘emotional/cognitive’ cluster comprising depression and cognitive impairment and a ‘physical’ cluster that includes pain and fatigue, each of which has been shown to contribute to the prediction of QoL and perceived health status [14, 15]. The cluster of fatigue, depression, and pain has shown a strong negative predictive relationship with physical activity behavior (i.e., the group of PlwMS who reported the worst clustered fatigue, depression, and pain was the least physically active). This relationship was driven predominantly by functional limitations [16].
Regarding the prevalence and relative impact of these clustered symptoms across the disease course, evidence suggests that symptom co-occurrence is evident immediately following diagnosis in the majority of patients, with pain and fatigue reported as the symptoms that most commonly co-occur [17]. The rate of symptom co-occurrence remains relatively stable during the first year post-diagnosis [17]. In older adults, symptom clusters are characterized by a predominance of fatigue, depression, and anxiety symptoms—and associated severe sleep problems [18].
Given that symptom co-occurrence is relatively widespread among PlwMS, it may be that there is a common etiology explaining this phenomenon. Identifying such a unifying pathway (or pathways) driving symptom interconnectivity might help to inform the development of behavioral and pharmacological interventions targeted at alleviating multiple symptoms simultaneously. Furthermore, a better understanding of the interconnected nature (or ‘clustering’) of these symptomatic domains may help to inform symptom assessment practices, including the development of more accurate patient-reported outcome (PRO) measures.
To this end, the objective of the present article was to provide a narrative review of existing literature to identify potential underlying biological processes that may explain the interconnected nature of fatigue, cognitive impairment, depression, and pain in PlwMS.
Methods
The purpose of the literature search was to identify published articles containing the findings of primary research focused on investigating the biological processes implicated in the interconnectivity among fatigue, cognitive impairment, depression, and pain in PlwMS.
PubMed was used to search for all articles published during the 10 years prior to July 16, 2021, using the search terms ‘Multiple Sclerosis AND ((cognition AND fatigue AND depression) OR (cognition AND fatigue AND pain) OR (cognition AND pain AND depression) OR (fatigue AND pain AND depression)).’ Note that as the focus of the search was to identify interconnectivity between multiple symptoms, the search strategy would not necessarily identify articles that described fewer than three of the symptoms listed. Since this is a narrative review, no typical criteria for inclusion and exclusion were predefined. We simply retained papers that reported research related to the topic in question. Importantly, the intention was not to conduct a systematic review of the literature, but to pragmatically search for relatively recent literature on the topic, including previous reviews that capture earlier research not detected during our search.
Titles and abstracts of returned articles were screened to identify papers reporting the findings of research into the biological processes linked with the symptoms of interest. Additional relevant articles were identified from the reference lists of a number of review articles returned during the search. The full texts of all relevant articles were then reviewed. For each paper, we used an Excel tracking document to record the respective symptoms and biological processes and the associated key findings. Based on this information, we identified a set of mechanistic groupings under which to categorize findings. These groupings serve to structure the results section in the present article.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. As such, ethics committee approval was not sought.
Results
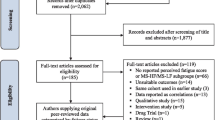
The literature search identified a total of 693 articles. Screening of titles resulted in the exclusion of 406 articles, with a further 212 articles excluded during screening of the abstracts. This resulted in 75 articles, consisting of 46 original articles and 29 review articles. An additional 206 articles were identified from the reference lists of the 29 reviews. This resulted in 252 original articles that were reviewed to extract relevant results that reported biological processes associated with fatigue, cognitive impairment, depression, or pain in MS (Fig. 1).
Following review of the relevant literature, articles were categorized based on one or more symptoms measured (fatigue, cognitive impairment, depression, and pain). In total, 222 articles contained results relevant to one of the symptoms of interest (i.e., associated the measurement of a biological process with the measurement of the symptom of interest), 25 articles studied 2 symptoms, and 5 articles measured ≥ 3 symptoms. Additionally, following categorization of the biological processes associated with the symptoms of interest across all relevant articles, four primary biological processes were identified and named as follows: neuroanatomy, inflammation, monoamines, and the hypothalamic–pituitary-adrenal (HPA) axis. In total, 228 articles contained results relating to neuroanatomy (changes in the structure and function of the CNS assessed using traditional and novel imaging techniques); 9 articles contained results related to inflammation (changes in inflammatory cells, cytokines or other inflammatory mediators in MS); 3 articles contained results related to monoamines (serotonin, dopamine, noradrenaline, and/or associated pathways), and 4 articles contained results related to HPA axis function (changes in HPA axis hormones). Additionally, five studies included results that referred to more than one of the aforementioned biological processes, while three articles referred to processes not specifically captured by the previous categories (myelin oligodendrocyte glycoprotein antibody positivity, vitamin D levels, and resting muscle oxygen consumption in the upper limb; Table 1 and Table S1 in the electronic supplementary material).
Figure 2 provides a graphical representation of the literature search results and potential key biological processes underpinning the interconnectivity among fatigue, cognitive impairment, depression, and pain.
Graphical representation of the literature search results and potential key biological processes underpinning the interconnectivity among fatigue, cognitive impairment, depression, and pain. The potential links between the biological processes and symptoms are color-coded, e.g., a blue circle or line indicates a link with depression. References shown in the colored boxes support the relationships represented in the figure. The dashed gray lines and associated references represent the way in which each of the biological mechanisms may directly impact the others. HPA, hypothalamic-pituitary-adrenal
Neuroanatomy
Structural Brain Changes
Neuroanatomical changes were identified as the primary biological process associated with fatigue, cognitive impairment, depression, and pain, representing > 90% of all articles identified from our search.
Global brain atrophy begins at an early stage of MS and accelerates with disease progression (see [82, 272]). A longitudinal study found an association between progressive brain atrophy during a 2-year period and worsening fatigue over the subsequent 6 years in patients with MS [205], while associations were also seen between whole-brain lesion volume and fatigue [222], and impairments in processing speed [107, 224], and between a decrease in brain volume and depression [236] and impairments in processing speed [107, 224].
A degree of overlap is apparent between the locations of subcortical atrophy in PlwMS and the presence of cognitive impairments, fatigue, and pain. For example, PlwMS who reported fatigue showed greater atrophy in regions involved in the processing of effort and reward, such as the striatum, than those without fatigue. This is consistent with the idea that impairment of cortico-striatal networks contributes to fatigue in MS [181, 183, 185, 187]. However, atrophy of subcortical areas, including the striatum (caudate and putamen), globus pallidus, thalamus, and nucleus accumbens, was also associated with impairments in cognition, particularly processing speed [31, 107, 137]. In addition, changes in gray matter thickness of the caudate, putamen, and thalamus were associated with pain [251].
Furthermore, overlap was evident in the areas of cortex associated with fatigue, cognitive impairment, depression, and pain in PlwMS. Atrophy of frontal and/or parietal cortex was more extensive in individuals reporting fatigue [183, 185, 210] and in those with impairments in verbal learning, spatial learning, attention, conceptual reasoning, and processing speed [32, 115, 159]. Atrophy of frontal cortex, along with atrophy of temporal [246], cingulate, and parietal cortex [228], was associated with depression in PlwMS [228, 232, 246]. Atrophy of frontal and parietal cortex was also associated with pain [251].
Functional Connectivity
Global CNS neurodegeneration, as well as damage to specific structures in the brain and spinal cord, can lead to disruption of neural networks and functional connectivity. The demyelination typical of MS may disrupt communication between the nodes of neural networks. Several articles identified from our literature search support the hypothesis that disruption to this functional connectivity is associated with the four symptoms of interest.
Disruption to the functional connectivity of the default mode network (DMN), which is involved in internally oriented cognitive processes such as ‘mind wandering,’ recall of past events, and simulation of future events [273], is associated with cognitive impairment and depression [65, 85, 139, 164, 241]. Decreased cognitive performance was associated with reduced resting-state network connectivity, including in the DMN, in people with relapsing–remitting MS (RRMS) [65, 105, 274] and progressive forms of MS [139]. By contrast, a group of individuals with early-stage MS showed increased functional connectivity in the DMN despite marked reductions in white matter integrity [85]. As the participants in the latter group were also cognitively impaired relative to controls, Hawellek et al. argued that their findings suggest reduced cognitive efficiency in early-stage MS.
However, it is important to consider that while neurodegenerative damage can directly influence symptoms associated with the affected brain structures, compensatory mechanisms may mitigate the impact of this damage via the increased or decreased recruitment of other brain structures. This is known as cortical reorganization (reviewed in [275,276,277]). It is thus possible that the increased functional connectivity observed by Hawellek et al. within the DMN reflects a compensatory process aimed at reducing cognitive impairment. Likewise, increased DMN resting-state connectivity within the periphery of the posterior cingulate cortex was suggested to have a compensatory role in cognitively impaired individuals with RRMS [278].
Task-based functional magnetic resonance imaging (MRI) studies in MS have provided further evidence of cortical reorganization by showing that on certain cognitive function measures, PlwMS can perform comparably to healthy controls while recruiting different cortical and subcortical structures and demonstrating different brain activation patterns [27, 124, 125, 131, 220]. Patients with clinically isolated syndrome (CIS) who improved their performance on a test of working memory and processing speed over a 12-month interval showed increased activation of the right lateral prefrontal cortex (PFC) over the same period, suggesting that this may be a compensatory response [28]. Parry and colleagues proposed that medial PFC recruitment may be a form of adaptive plasticity that compensates for deficits related to reduced right PFC activity in MS [124]. At baseline, PlwMS performing a counting Stroop task showed stronger activation of left medial PFC, but weaker activation of right PFC, than healthy controls. However, after taking a central cholinesterase inhibitor, rivastigmine, this difference disappeared [124]. Furthermore, while patients with clinically definite MS and CIS did not differ significantly from healthy controls in performance on the Paced Visual Serial Addition Task, individuals with clinically definite MS showed increased activation of the hippocampus and parahippocampal area compared with patients with CIS, which may have indicated a need in the patients with clinically definite MS to bolster their working memory capacity via recruitment of these additional brain regions [131].
The capacity for compensation is, however, finite. PlwMS who showed minor impairment on attentional tasks relative to healthy controls displayed increased and additional activation of various brain areas, mainly in frontal cortex and posterior parietal cortex, that were not activated in healthy controls [125]. However, this additional activation was not observed in PlwMS whose performance was severely impaired, suggesting that in individuals with severe cognitive impairment the capacity to compensate by recruiting additional brain areas may have been exhausted [125].
In addition to compensatory mechanisms, PlwMS may have a level of reserve capacity that offers some protection from the symptomatic changes associated with the disease. Greater intellectual enrichment (based on vocabulary knowledge) in PlwMS was associated with less deactivation of the DMN and with less recruitment of the PFC during both low (0-Back and 1-Back tasks) and high (2-Back task) cognitive demands, with a better cognitive performance during the high-demand tasks also associated with greater intellectual enrichment [164]. The authors proposed that the DMN and PFC provide a link between intellectual enrichment and cognitive status in PlwMS, with greater maintenance of the DMN and lower need for prefrontal recruitment representing a basis of cognitive reserve [164]. Taken together, these results suggest that damage to the DMN may contribute to cognitive decline, while intellectual enrichment may provide protection against the cognitive impacts of the disease. Given that disruption to the DMN is linked with both cognitive decline and depression, it is interesting to postulate that intellectual enrichment might also protect against depression in PlwMS.
Disrupted functional connectivity is also associated with fatigue and depression [85, 141, 194, 200, 219, 235, 240]. Fatigue severity in PlwMS has been negatively correlated with functional connectivity of certain brain regions, while positively correlated with connectivity between other regions [194, 200]. In people with RRMS, fatigue severity was negatively correlated with functional connectivity between the basal ganglia and frontal and parietal cortex (independent of age, sex, and disease severity), but positively correlated with functional connectivity between caudate nucleus and motor cortex [194]. No association between fatigue severity and basal ganglia functional connectivity was observed in healthy controls, suggesting that this phenomenon was specific to PlwMS [194]. Investigating the striatal subdivisions, Jaeger and colleagues found that fatigue severity negatively correlated with functional connectivity of the caudate nucleus and ventral striatum with the sensorimotor cortex (SMC), while positively correlating with functional connectivity of the dorsolateral PFC with the rostral inferior parietal gyrus and the SMC [200]. In individuals with early-onset MS (onset < 30 years of age), fatigue severity negatively correlated with functional connectivity between the left insula and posterior cingulate gyrus and between the right thalamus and left parietal operculum [219]. The authors of the latter study reported increased connectivity between the right thalamus and right precentral gyrus and between the left hippocampus and left precentral gyrus in PlwMS reporting fatigue versus those without fatigue, although functional connectivity in these regions did not correlate with fatigue severity [219].
There is evidence that increased functional connectivity may be a compensatory mechanism for countering fatigue in PlwMS. For example, after performing a mentally challenging task, PlwMS with cognitive fatigue showed increasingly stronger resting-state functional connectivity between the left superior frontal gyrus and occipital, frontal, and temporal areas than healthy controls and PlwMS without cognitive fatigue [211]. Similarly, when performing a motor activity after completing a cognitive task, PlwMS with fatigue recruited significantly more of their brain than they had when doing the same motor activity prior to the cognitive task; this was not observed in healthy controls [220]. Notably, the increased brain activity required to perform mental or physical activities has also been proposed to potentially contribute to the fatigue observed in MS [279].
Disrupted activity in the limbic system is associated with levels of depression in PlwMS [235, 240]. People with MS and comorbid major depressive disorder (MDD) exhibited increased local path length in the right hippocampus and right amygdala compared with PlwMS without MDD and with healthy controls [235]. No significant difference was observed in the local path length between healthy controls and PlwMS without MDD [235]. Further analyses showed that, compared with the healthy controls and those with MS but no MDD, PlwMS with MDD had an increased shortest distance between the right hippocampus/right amygdala and a series of frontal and prefrontal cortical regions, including the dorsolateral PFC [235], which is thought to play a key role in the pathophysiology of depression [280]. A large study in PlwMS (n = 77) demonstrated that depression scores negatively correlated with functional connectivity of both the hippocampus and amygdala with the dorsolateral PFC, providing further support for a role of these regions in mediating depression in PlwMS [240].
Inflammation
Pro-inflammatory cytokines released from lymphocytes that have infiltrated the CNS of PlwMS contribute to the inflammatory signaling pathways at the heart of MS pathogenesis [20, 21]. However, they also act directly on the brain to produce so-called “sickness behaviors,” including symptoms such as fatigue, depression, pain, and transient cognitive decline [281]. It is unsurprising, therefore, that these symptoms are commonly experienced by PlwMS, given the inflammatory component of the disease, particularly in its early stages. We identified several articles that demonstrate a relationship among inflammation, depression, and fatigue.
In a prospective study of peripheral immune cells in individuals with progressive MS (n = 13), the times of greatest depression (based on Center for Epidemiological Studies Depression [CES-D] score) were associated with fewer CD8+ T cells and a higher CD4+/CD8+ T cell ratio than the times of least depression [260]. The participants were enrolled in a placebo-controlled trial of cyclosporine, where 6 of the 13 individuals received cyclosporine and the others received placebo. In the placebo group, the number and percentage of CD4+ T cells were higher at the times of greatest psychological distress versus the times of least depression [260].
There were no differences in absolute numbers of CD4+ and CD8+ T cells between individuals with RRMS and comorbid MDD (n = 10) and those with RRMS without MDD (n = 34) [257]. However, differences in T cell function were observed, with more tumor necrosis factor (TNF)-α- and interferon (IFN)-γ-producing CD8+ T cells detected in the comorbid MDD group—a trend that was retained when the authors controlled for Expanded Disability Status Scale score or disease-modifying therapy (DMT) [257]. The frequency of CD8+ T cells did not predict depression severity but was a significant predictor of fatigue scores [257]. This latter finding was consistent with that of a prior study, where levels of the pro-inflammatory cytokines TNF-α- and IFN-γ were significantly greater (both p = 0.01) in whole blood samples from PlwMS with fatigue (n = 15; Fatigue Severity Scale [FSS] mean scores ≥ 5) than PlwMS without fatigue (n = 15; FSS mean scores < 4) [253].
In samples of cerebrospinal fluid (CSF) from people with RRMS (n = 47), levels of interleukin (IL)-6 (but not IL-8) positively correlated with self-rated depression and fatigue symptoms (p < 0.05) [255]. It could not be determined whether these associations were specific to MS as the study did not include healthy controls. Using a multinominal logistic regression analysis, Kallaur and colleagues examined immune inflammatory markers in healthy controls (n = 249), PlwMS without MDD (n = 108; Hospital Anxiety and Depression Scale [HADS] depression subscale score ≤ 8), and PlwMS with MDD (n = 42; HADS depression subscale score > 8) [262]. Serum IL-6 levels were the highest in individuals with MS and comorbid MDD [262]. These data in PlwMS are consistent with previous reports that IL-6 is an inflammatory marker associated with depression in the general population [282, 283]. Of note, serum levels of the T helper 2 (Th2) cytokine IL-4 were higher in PlwMS without MDD than in healthy controls, but significantly lower in PlwMS with MDD than in those without MDD. According to the authors, the latter finding may indicate that depression in PlwMS is related to increased peripheral immune-inflammatory potential [262].
In the articles we identified, there was limited information on the relationship between inflammation and cognitive impairment. In a cohort of individuals diagnosed with either RRMS or secondary progressive MS, greater levels of depression and fatigue were detected in participants classified as cognitively impaired (n = 25) than in those classified as cognitively preserved (n = 25) [254]; notably, the percentage of IFN-γ-producing CD4+ and CD8+ T cells was higher in the cognitively preserved group. The latter finding led the authors to conclude that inflammation might not contribute to cognitive dysfunction during remission in MS; however, they also acknowledged that measuring intracellular IFN-γ may not reflect the circulating levels of IFN-γ [254]. The participants were not on steroid therapy or taking psychoactive drugs or antidepressants, but it was not clear if they were receiving any other treatments that could have influenced the results of the study. For example, IFN-β therapy has been associated with lower levels of IFN-γ-producing CD8+ T cells [257].
The mechanistic role of inflammation in the symptoms of interest is further supported by evidence of worsening symptoms during MS relapses (i.e., periods of acute inflammatory activity) [284, 285], although we acknowledge that structural changes occurring during MS relapses may also contribute to symptom worsening at this time. While the exact mechanisms of inflammatory-mediated symptom manifestation are not fully understood, it is likely a combined result of acute inflammation, chronic inflammation, and immune-mediated neurodegeneration affecting specific regions of the CNS, as well as immune-mediated alterations to monoaminergic pathways and activation of the HPA axis, both of which are discussed in more detail below.
Monoamines
Monoamine neurotransmission plays an important role in mood regulation [286], and disruption of the associated monoaminergic pathways has been implicated in the manifestation of classic MDD symptoms, namely low mood and changes in appetite, pain responses, and sleep [287]. It has been proposed that in PlwMS, fatigue may result from an imbalance in dopamine secondary to abnormalities in, and disruption of communication between, the frontal and striatal regions of the brain, which are heavily innervated by dopaminergic neurons. This hypothesis is supported by evidence of the successful treatment of fatigue in MS and other disorders (e.g., Parkinson’s disease, chronic fatigue syndrome, cancer) using dopaminergic psychostimulants and other modulators of dopamine [288].
We identified three articles that explored the relationship between monoamines and the symptoms of fatigue and depression in PlwMS. In a study assessing the effect of the wakefulness-promoting drug modafinil on fatigue in MS, Niepel and colleagues found that PlwMS with fatigue (n = 17) had reduced autonomic function (assessed by diastolic blood pressure) and reduced levels of alertness (assessed by several subjective and objective measures) at baseline compared with PlwMS who did not have fatigue (n = 9) and with age- and sex-matched healthy volunteers (n = 9). However, there was no difference between PlwMS with fatigue and either PlwMS without fatigue or healthy volunteers in other measures of autonomic function (systolic blood pressure, heart rate, pupil diameter). The authors hypothesized that PlwMS with fatigue might be more sensitive to the alerting and sympathetic-activating effects of modafinil than PlwMS without fatigue or healthy controls, but found no difference among the three groups in the magnitude of increases in alertness and sympathetic measures following a single 200-mg dose of modafinil. Regardless, the authors postulated that the role of monoamines in fatigue in MS is supported by the anti-fatigue effect of modafinil and that this is likely achieved by stimulation (via the dopaminergic system) of the wakefulness-promoting noradrenergic locus coeruleus nucleus, which is damaged in MS [267].
The role of impaired serotonergic neurotransmission in depression is well established [269]. The kynurenine pathway is the primary non-protein route of metabolism for tryptophan (the precursor of serotonin) and is known to be disrupted in the inflammatory milieu characteristic of MS [269, 289]. Aeinehband et al. used liquid chromatography-mass spectrometry to determine levels of tryptophan, kynurenine, kynurenic acid, and quinolinic acid in CSF samples from 48 individuals with RRMS. Of the 48 participants, 12 fulfilled criteria for depression based on clinical rating scales. A modest correlation was found between low levels of tryptophan and psychiatrist ratings of depression. However, the predictive value of this measure was limited, since low levels of tryptophan were also detected in some non-depressed PlwMS [289].
Fatigue, depression, and pain are all associated with signs of anhedonia, such as reduced motivation and a lack of positive affect, and with overlapping structural and functional alterations in areas of the brain involved in reward processing [270]. Dysfunctional reward processing may be a functional mechanism common to fatigue, depression, and pain in PlwMS since monoaminergic neurotransmission plays a key role in reward processing and is disrupted by neuroinflammation [270, 290]. Pardini and colleagues examined the relationship between reward-related cognition and fatigue in MS by assessing fatigue values among PlwMS at baseline and after 3 months of therapy with escitalopram or bupropion and determining correlation with these and patients’ reward perception [268, 291]. Reward responsiveness was reduced in PlwMS who had fatigue compared with PlwMS who did not have fatigue. It should be noted that the investigators excluded individuals with depression from the study to control for possible confounding factors; these findings are therefore applicable only to this selected population [268, 291]. The authors concluded that the more marked response to bupropion (a dopamine/noradrenaline-enhancing drug) in individuals presenting with lower activation scale reward-responsiveness behavioral (BAS-RR) scores (i.e., with reduced dopaminergic tone) supported the theory that reward perception could underpin fatigue in PlwMS. However, in subjects with higher BAS-RR scores (i.e., with higher prefrontal dopaminergic tone), both escitalopram and bupropion alleviated fatigue, indicating that other mechanisms may also be at play in MS-related fatigue.
Recent studies that examined the structural and functional alterations in regions of the brain associated with monoaminergic neurotransmission further implicate the monoaminergic pathways in MS-related fatigue [292, 293]. Carandini and colleagues identified damage to several of the monoaminergic fiber tracts projecting from the brainstem nuclei in individuals with RRMS (n = 68), but not in healthy controls (n = 34) [292]. Moreover, the damage to the brainstem monoaminergic nuclei tracts was moderately associated with worsening cognitive fatigue (assessed by the Modified Fatigue Impact Scale cognitive subscale [MFIS-Cog]). In PlwMS classed as highly fatigued (MFIS-Cog > 15; n = 26), noradrenaline transporter (NAT)-enriched functional connectivity was significantly lower in several frontal and prefrontal areas than in PlwMS in the mildly fatigued group (MFIS-Cog ≤ 15; n = 29) [293]. Furthermore, the NAT-enriched functional connectivity values negatively correlated with the MFIS-Cog scores [293]. No between-group differences in dopamine transporter- or serotonin transporter-enriched functional connectivity were observed [293]. The authors acknowledged that their results may appear to disagree with a role for dopamine in fatigue in MS, but noted that these data do not exclude a contribution of dopamine to the changes in NAT-related functional connectivity.
Hypothalamic–pituitary-adrenal axis
The HPA axis is the primary system responsible for the synthesis, release, and diurnal control of stress hormones [294]. Evidence suggests that communication between the immune system and the HPA axis is disrupted in MS and that HPA axis hyperactivity is implicated in neurodegeneration [295]. Notably, HPA axis dysregulation is also associated with depression [296] and, possibly, chronic fatigue [297]. Our literature search identified several studies linking stress hormones (particularly cortisol) with depression and fatigue in MS [230, 233, 253, 257, 263, 264, 266]. In their cross-sectional study of 23 PlwMS (RRMS subtype) and 50 age- and sex-matched controls, Fassbender et al. found that the former group had higher depression and anxiety scale scores and a failure of suppression of cortisol release after dexamethasone pretreatment. The authors concluded that affective symptoms and dysfunction of the HPA system were associated with laboratory (cell counts) and neuroradiological (gadolinium enhancement of MS plaques) indicators of cerebral inflammation, which suggested that affective and neuroendocrinological disorders in MS are causally linked with inflammatory brain injury [230]. In their cross-sectional study, Gold et al. found that PlwMS with comorbid MDD (n = 8) had normal morning salivary cortisol levels but elevated evening salivary cortisol levels, resulting in a flattened slope, providing further evidence for a role of HPA axis hyperactivity in major depression in MS [233]. Gold et al. assessed the volume of hippocampal subregions, diurnal salivary cortisol, and depression in 29 PlwMS (RRMS subtype) and 20 matched healthy controls. PlwMS had smaller hippocampal volumes than the controls, particularly in the cornu ammonis (CA) 1 and subiculum subregions. In addition, PlwMS who had depressive symptoms had smaller CA2-CA3 and dentate gyrus (CA23DG) volumes and higher cortisol levels than the controls. In the MS group, CA23DG volume was correlated with depressive symptoms and cortisol levels. The authors concluded that this provided in vivo evidence for the selective association of smaller hippocampal CA23DG subregion volumes with cortisol hypersecretion and depressive symptoms in MS. A distinction between severity of depression and cortisol response was identified by Kern et al. These authors postulated that a hyperactive HPA axis is primarily present in PlwMS expressing moderately elevated depressive symptoms and that the cortisol-awakening response does not differ significantly between PlwMS with less severe depression and healthy controls [266].
Regarding fatigue in MS, Gottschalk et al. found that PlwMS (RRMS subtype) with fatigue had significantly higher adrenocorticotropin levels in the combined dexamethasone/corticotropin-releasing hormone (Dex-CRH) test than PlwMS without fatigue, indicating that the former exhibit higher HPA axis activity [263]. Powell et al. noted a similar association between HPA axis activity and recalled fatigue (but not same-day fatigue) in individuals with RRMS [264]. In contrast, Heesen et al. found that fatigue was not significantly correlated with any parameter of the Dex-CRH test [254]. This inconsistency may potentially be explained by differences in disease subtype between the studies by Gottschalk/Powell and Heesen and the fact that MS treatment known to affect cytokines (which influence HPA activity) was not an exclusion criterion in the Heesen et al. study, nor was there categorization of participants into those with and without fatigue.
One explanation for the perturbations of the HPA axis identified by several research groups is modulation of HPA axis activity in an inflammatory environment. Specifically, it has been shown that pro-inflammatory cytokines, such as IL-1 and IL-6, can activate the HPA axis by promoting the release of brain noradrenaline, serotonin, and tryptophan [271], which, in turn, may contribute to the development of certain symptoms in MS (e.g., depression and fatigue).
Discussion
We identified neuroanatomical changes, inflammation, monoaminergic pathway disruption, and dysregulation of the HPA axis as the common processes associated with the manifestation of fatigue, cognitive impairment, depression, and pain in MS. In addition, we have described the overlap between the areas of the brain which are subject to structural and functional changes associated with these frequently occurring MS symptoms. Some of this overlap may arise because lesions in MS often occur in highly connected deep gray matter ‘hubs,’ such as the thalamus, whereby the ripple effects of such lesions can affect many other brain circuits. Notably, in PlwMS, areas of the brain can exhibit increased connectivity with other regions. For example, functional connectivity between the thalamus and other areas typically increases rather than decreases, suggesting that the thalamus may play a more central role within brain networks in PlwMS than in healthy controls [298, 299].
The interpretation of functional brain changes in MS is complicated by the difficulty in distinguishing between changes that are a direct effect of the disease process vis-à-vis compensatory changes as the brain attempts to avoid and overcome loss of function. The occurrence of compensatory changes may vary between individuals and over time, with the capacity for compensatory change diminishing as the disease progresses [125]. A further complication in interpreting measures of functional connectivity is that this is not a static property of brain networks, but rather one that changes dynamically, even during a resting-state functional MRI scan [300]. It is thus possible that methodological differences between studies may influence the changes in functional connectivity observed.
Studying common pathways underlying core symptoms in MS can provide additional insights into the mechanisms of disease pathogenesis and help to gain a better understanding of the symptoms experienced by people living with MS. Fatigue, depression, pain, and cognitive impairment are relatively common symptoms with which many patients present, and as such physicians may not always immediately associate them with MS. By highlighting their frequent co-occurrence and shared pathogenesis, our study suggests that it is worth investigating the possibility of MS in patients who present with these symptoms, particularly in combination, and especially when other risk factors are present.
Our findings also raise important considerations related to the measurement and monitoring of symptoms in PlwMS, as well as disease phenotyping. The ability to precisely measure the biological pathways we have identified, and predict the types and severity of consequent symptoms, would have important implications for intervention strategies. Such capabilities would also be invaluable in helping newly diagnosed patients to gain an insight into what they might expect from their particular disease phenotype. Current imaging methods typically available to most neurologists may not be suitable for detecting the nuanced and subtle features required to gain such clinical insights. For example, traditional MRI modalities may not be sensitive enough to detect thalamic changes or changes to structures implicated in monoaminergic pathway disruption; however, more advanced MRI techniques such as susceptibility-weighted imaging, functional MRI, diffusion tensor imaging, and magnetic resonance spectroscopy could be used to assess these types of pathology [301, 302]. Alternative measurement approaches that promise utility in this regard include assessing the local connectivity of different structures by assessing the whole brain as one network using path lengths and clustering coefficients or by using magneto-/electroencephalography to assess the brain in a non-static way (i.e., microstates) [303].
Identifying common pathways underlying multiple symptoms could have important implications for pharmacological and non-pharmacological disease management. Most DMTs approved for use in MS exert their therapeutic effects via anti-inflammatory mechanisms [304]. Beyond the approved MS DMTs, additional symptomatic treatments include drugs that enhance monoamine neurotransmission and can treat symptoms with an anhedonic component, i.e., pain, fatigue, and depression [270], and drugs that can reduce cognitive dysfunction [305,306,307] and fatigue [305, 308]. There are also several non-pharmacological interventions with the potential to alleviate fatigue, cognitive impairment, depression, and pain by impacting the common pathways identified, e.g., sun exposure [309], diet [310], cognitive behavioral therapy [270, 311, 312], and exercise [313, 314]. Cognitive behavioral therapy may positively influence levels of inflammatory markers in addition to alleviating the anhedonic symptoms themselves [270, 311, 312]. Exercise, now viewed as a cornerstone in MS management, is an effective symptomatic treatment [313], exerting positive effects on the mechanistic pathways identified in our review [314].
Having demonstrated that several different symptomatic treatment strategies are available, it is interesting to note that in a sample of 35,755 PlwMS, 25.5%, 26.1%, 65.2%, and 73.0% had received neither pharmacological nor non-pharmacological therapy for depression, pain, fatigue, and cognitive dysfunction, respectively [316], suggesting that symptomatic therapy may be an unmet need in MS. Given that these symptoms often remain untreated or incompletely treated, successfully targeting any or all of them could greatly enhance patients’ QoL. As our understanding of the biological pathways leading to, and connecting, symptoms of interest increases, it may become possible to identify potential novel therapeutic and non-pharmacological strategies that target several of these symptoms at once. Future therapeutics that could plausibly alleviate multiple symptoms simultaneously include neuro-restorative agents [301], kynurenine metabolites and analogues [317], and agents that regulate the HPA axis.
Our investigation into the biological processes involved in symptom interconnectivity provides a foundation for future research, which could investigate (1) sub-groups of PlwMS who experience some, none, or all of the symptoms in question to identify biological differences between these groups, (2) the causal relationship between the biological processes and symptoms discussed here, (3) the effectiveness of pharmacological and non-pharmacological interventions to assess their impact on multiple symptoms simultaneously, and (4) longitudinal relationships between structural brain changes and symptoms. Importantly, the measurement of symptoms (i.e., PROs) should be carried out using instruments that have been developed in line with best practice guidelines so that relationships between objectively assessed biological variables are being tested against the most accurate available measures of patient outcomes.
Limitations of our review include that the literature search was restricted to the previous 10 years, that a single database was used for the search, and that we did not conduct a strict systematic search with clearly predefined inclusion and exclusion criteria. However, because we included studies from the reference lists of previous systematic review articles identified during our search, we captured relevant studies published before our cutoff date. Some of the studies had a small sample size, and there was also a paucity of articles focused on the symptom of pain, suggesting that this may be another important area for future research efforts. We acknowledge that due to the search strategy we employed, which used the symptoms of interest in groups of three, potentially informative papers that reported the etiology of individual symptoms may not have been captured. We also used broad terms for each symptom, e.g., we did not discriminate between different types of pain, which we appreciate could have different causes. It is important to note that correlation is not equivalent to causality, with further research necessary to determine the relationship between the biological processes and symptoms covered in this review. Finally, a limitation that has implications not only for the present article, but also for the field of MS symptomology more broadly, is the difficulty to accurately measure and discriminate between these often similar and overlapping symptoms. For example, depression typically presents with fatigue and fatigue can easily be misdiagnosed as depression. As such, to capture these symptomatic domains accurately, the target symptoms must be measured independently using instruments based on robust symptom definitions, with items derived from a sound conceptualization of the underlying construct.
Conclusions
The co-occurrence of fatigue, cognitive impairment, depression, and pain experienced by PlwMS appears to be associated with a common set of underlying etiological factors, namely neuroanatomical changes, pro-inflammatory cytokines, dysregulation of monoaminergic pathways, and a hyperactive HPA axis. These findings help to advance our understanding of the interconnected nature of MS symptoms, have implications for pharmacological and non-pharmacological disease management strategies, and highlight important topics for future research that might facilitate the development of approaches that target both the inflammatory and neurodegenerative components of MS.
References
Tremlett H, Zhao Y, Rieckmann P, Hutchinson M. New perspectives in the natural history of multiple sclerosis. Neurology. 2010;74:2004–15.
Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26:1816–21.
Amankwah N, Marrie RA, Bancej C, et al. Multiple sclerosis in Canada 2011–2031: results of a microsimulation modelling study of epidemiological and economic impacts. Health Promot Chronic Dis Prev Can. 2017;37:37–48.
Amini P, Almasi-Hashiani A, Sahraian MA, Najafi M, Eskandarieh S. Multiple sclerosis projection in Tehran, Iran using Bayesian structural time series. BMC Neurol. 2021;21:1–6.
Krupp LB, Serafin DJ, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010;10:1437–47.
Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–51.
Hirsh AT, Turner AP, Ehde DM, Haselkorn JK. Prevalence and impact of pain in multiple sclerosis: physical and psychologic contributors. Arch Phys Med Rehabil. 2009;90:646–51.
Feinstein A, Magalhaes S, Richard JF, Audet B, Moore C. The link between multiple sclerosis and depression. Nat Rev Neurol. 2014;10:507–17.
Ramirez A, Keenan A, Kalau O, Worthington E, Cohen L, Singh S. Prevalence and burden of multiple sclerosis-related fatigue: a systematic literature review. BMC Neurol. 2021;21:468.
Cortese R, Carotenuto A, Di Filippo M, Lanzillo R. Editorial: Cognition in multiple sclerosis. Front Neurol. 2021;12:751687.
O’Connor A, Schwid S, Herrmann D, Markman J, Dworkin R. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137:96–111.
Marrie R, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler. 2015;21:305–17.
Motl RW, Suh Y, Weikert M. Symptom cluster and quality of life in multiple sclerosis. J Pain Symptom Manag. 2010;39:1025–32.
Shahrbanian S, Duquette P, Kuspinar A, Mayo NE. Contribution of symptom clusters to multiple sclerosis consequences. Qual Life Res. 2015;24:617–29.
Podda J, Ponzio M, Pedulla L, et al. Predominant cognitive phenotypes in multiple sclerosis: insights from patient-centered outcomes. Mult Scler Relat Disord. 2021;51: 102919.
Motl RW, McAuley E. Symptom cluster as a predictor of physical activity in multiple sclerosis: preliminary evidence. J Pain Symptom Manag. 2009;38:270–80.
Valentine TR, Alschuler KN, Ehde DM, Kratz AL. Prevalence, co-occurrence, and trajectories of pain, fatigue, depression, and anxiety in the year following multiple sclerosis diagnosis. Mult Scler. 2022;28:620–31.
Silveira SL, Cederberg KLJ, Jeng B, et al. Symptom clusters and quality of life in persons with multiple sclerosis across the lifespan. Qual Life Res. 2021;30:1061–71.
Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol. 2019;26:27–40.
Tafti D, Ehsan M, Xixis KL. Multiple sclerosis. Treasure Island: StatPearls; 2021.
Wang K, Song F, Fernandez-Escobar A, Luo G, Wang JH, Sun Y. The properties of cytokines in multiple sclerosis: pros and cons. Am J Med Sci. 2018;356:552–60.
Achiron A, Chapman J, Tal S, Bercovich E, Gil H, Achiron A. Superior temporal gyrus thickness correlates with cognitive performance in multiple sclerosis. Brain Struct Funct. 2013;218:943–50.
Amato MP, Hakiki B, Goretti B, et al. Association of MRI metrics and cognitive impairment in radiologically isolated syndromes. Neurology. 2012;78:309–14.
Arnett PA, Rao SM, Bernardin L, Grafman J, Yetkin FZ, Lobeck L. Relationship between frontal lobe lesions and Wisconsin Card Sorting Test performance in patients with multiple sclerosis. Neurology. 1994;44:420–5.
Au Duong MV, Boulanouar K, Audoin B, et al. Modulation of effective connectivity inside the working memory network in patients at the earliest stage of multiple sclerosis. Neuroimage. 2005;24:533–8.
Audoin B, Au Duong MV, Ranjeva JP, et al. Magnetic resonance study of the influence of tissue damage and cortical reorganization on PASAT performance at the earliest stage of multiple sclerosis. Hum Brain Mapp. 2005;24:216–28.
Audoin B, Ibarrola D, Ranjeva JP, et al. Compensatory cortical activation observed by fMRI during a cognitive task at the earliest stage of MS. Hum Brain Mapp. 2003;20:51–8.
Audoin B, Reuter F, Duong MV, et al. Efficiency of cognitive control recruitment in the very early stage of multiple sclerosis: a one-year fMRI follow-up study. Mult Scler. 2008;14:786–92.
Bagnato F, Salman Z, Kane R, et al. T1 cortical hypointensities and their association with cognitive disability in multiple sclerosis. Mult Scler. 2010;16:1203–12.
Bakirtzis C, Nikolaidis I, Boziki MK, et al. Cognitive fatigability is independent of subjective cognitive fatigue and mood in multiple sclerosis. Cogn Behav Neurol. 2020;33:113–21.
Batista S, Zivadinov R, Hoogs M, et al. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol. 2012;259:139–46.
Benedict RH, Bakshi R, Simon JH, Priore R, Miller C, Munschauer F. Frontal cortex atrophy predicts cognitive impairment in multiple sclerosis. J Neuropsychiatry Clin Neurosci. 2002;14:44–51.
Benedict RH, Bruce J, Dwyer MG, et al. Diffusion-weighted imaging predicts cognitive impairment in multiple sclerosis. Mult Scler. 2007;13:722–30.
Benedict RH, Bruce JM, Dwyer MG, et al. Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol. 2006;63:1301–6.
Benedict RH, Hulst HE, Bergsland N, et al. Clinical significance of atrophy and white matter mean diffusivity within the thalamus of multiple sclerosis patients. Mult Scler. 2013;19:1478–84.
Benedict RH, Weinstock-Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R. Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol. 2004;61:226–30.
Benedict RH, Zivadinov R, Carone DA, et al. Regional lobar atrophy predicts memory impairment in multiple sclerosis. AJNR Am J Neuroradiol. 2005;26:1824–31.
Benedict RHB, Carone DA, Bakshi R. Correlating brain atrophy with cognitive dysfunction, mood disturbances, and personality disorder in multiple sclerosis. J Neuroimaging. 2004;14:36s–45s.
Berg D, Maurer M, Warmuth-Metz M, Rieckmann P, Becker G. The correlation between ventricular diameter measured by transcranial sonography and clinical disability and cognitive dysfunction in patients with multiple sclerosis. Arch Neurol. 2000;57:1289–92.
Bergsland N, Benedict RHB, Dwyer MG, et al. Thalamic nuclei volumes and their relationships to neuroperformance in multiple sclerosis: a cross-sectional structural MRI study. J Magn Reson Imaging. 2021;53:731–9.
Bergsland N, Schweser F, Dwyer MG, Weinstock-Guttman B, Benedict RHB, Zivadinov R. Thalamic white matter in multiple sclerosis: a combined diffusion-tensor imaging and quantitative susceptibility mapping study. Hum Brain Mapp. 2018;39:4007–17.
Bergsland N, Zivadinov R, Dwyer MG, Weinstock-Guttman B, Benedict RH. Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Mult Scler. 2016;22:1327–36.
Bermel RA, Bakshi R, Tjoa C, Puli SR, Jacobs L. Bicaudate ratio as a magnetic resonance imaging marker of brain atrophy in multiple sclerosis. Arch Neurol. 2002;59:275–80.
Bester M, Lazar M, Petracca M, et al. Tract-specific white matter correlates of fatigue and cognitive impairment in benign multiple sclerosis. J Neurol Sci. 2013;330:61–6.
Bethune A, Tipu V, Sled JG, et al. Diffusion tensor imaging and cognitive speed in children with multiple sclerosis. J Neurol Sci. 2011;309:68–74.
Bisecco A, Capuano R, Caiazzo G, et al. Regional changes in thalamic shape and volume are related to cognitive performance in multiple sclerosis. Mult Scler. 2021;27:134–8.
Bobholz JA, Rao SM, Lobeck L, et al. fMRI study of episodic memory in relapsing-remitting MS: correlation with T2 lesion volume. Neurology. 2006;67:1640–5.
Bomboi G, Ikonomidou VN, Pellegrini S, et al. Quality and quantity of diffuse and focal white matter disease and cognitive disability of patients with multiple sclerosis. J Neuroimaging. 2011;21:e57-63.
Bonavita S, Sacco R, Esposito S, et al. Default mode network changes in multiple sclerosis: a link between depression and cognitive impairment? Eur J Neurol. 2017;24:27–36.
Bonnet MC, Allard M, Dilharreguy B, Deloire M, Petry KG, Brochet B. Cognitive compensation failure in multiple sclerosis. Neurology. 2010;75:1241–8.
Bonzano L, Pardini M, Mancardi GL, Pizzorno M, Roccatagliata L. Structural connectivity influences brain activation during PVSAT in multiple sclerosis. Neuroimage. 2009;44:9–15.
Brass SD, Benedict RH, Weinstock-Guttman B, Munschauer F, Bakshi R. Cognitive impairment is associated with subcortical magnetic resonance imaging grey matter T2 hypointensity in multiple sclerosis. Mult Scler. 2006;12:437–44.
Cader S, Cifelli A, Abu-Omar Y, Palace J, Matthews PM. Reduced brain functional reserve and altered functional connectivity in patients with multiple sclerosis. Brain. 2006;129:527–37.
Calabrese M, Agosta F, Rinaldi F, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol. 2009;66:1144–50.
Calabrese M, Poretto V, Favaretto A, et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain. 2012;135:2952–61.
Calabrese M, Rinaldi F, Mattisi I, et al. Widespread cortical thinning characterizes patients with MS with mild cognitive impairment. Neurology. 2010;74:321–8.
Camp SJ, Stevenson VL, Thompson AJ, et al. Cognitive function in primary progressive and transitional progressive multiple sclerosis: a controlled study with MRI correlates. Brain. 1999;122(Pt 7):1341–8.
Charil A, Zijdenbos AP, Taylor J, et al. Statistical mapping analysis of lesion location and neurological disability in multiple sclerosis: application to 452 patient data sets. Neuroimage. 2003;19:532–44.
Chiaravalloti N, Hillary F, Ricker J, et al. Cerebral activation patterns during working memory performance in multiple sclerosis using FMRI. J Clin Exp Neuropsychol. 2005;27:33–54.
Ciampi E, Uribe-San-Martin R, Vasquez M, et al. Relationship between social cognition and traditional cognitive impairment in progressive multiple sclerosis and possible implicated neuroanatomical regions. Mult Scler Relat Disord. 2018;20:122–8.
Comi G, Filippi M, Martinelli V, et al. Brain magnetic-resonance-imaging correlates of cognitive impairment in multiple-sclerosis. J Neurol Sci. 1993;115:S66–73.
Conway DS, Planchon SM, Oh SH, et al. Measures of thalamic integrity are associated with cognitive functioning in fingolimod-treated multiple sclerosis patients. Mult Scler Relat Disord. 2021;47: 102635.
Correale J, Peirano I, Romano L. Benign multiple sclerosis: a new definition of this entity is needed. Mult Scler J. 2012;18:210–8.
Cox D, Pelletier D, Genain C, et al. The unique impact of changes in normal appearing brain tissue on cognitive dysfunction in secondary progressive multiple sclerosis patients. Mult Scler. 2004;10:626–9.
Cruz-Gomez AJ, Ventura-Campos N, Belenguer A, Avila C, Forn C. The link between resting-state functional connectivity and cognition in MS patients. Mult Scler. 2014;20:338–48.
Czekoova K, Shaw DJ, Saxunova K, et al. Impaired self-other distinction and subcortical gray-matter alterations characterize socio-cognitive disturbances in multiple sclerosis. Front Neurol. 2019;10:525.
d’Ambrosio A, de la Cruz MH, Valsasina P, et al. Structural connectivity-defined thalamic subregions have different functional connectivity abnormalities in multiple sclerosis patients: implications for clinical correlations. Hum Brain Mapp. 2017;38:6005–18.
Daams M, Steenwijk MD, Schoonheim MM, et al. Multi-parametric structural magnetic resonance imaging in relation to cognitive dysfunction in long-standing multiple sclerosis. Mult Scler. 2016;22:608–19.
de Rodez Benavent SA, Nygaard GO, Harbo HF, et al. Fatigue and cognition: pupillary responses to problem-solving in early multiple sclerosis patients. Brain Behav. 2017;7: e00717.
Deloire MS, Ruet A, Hamel D, Bonnet M, Dousset V, Brochet B. MRI predictors of cognitive outcome in early multiple sclerosis. Neurology. 2011;76:1161–7.
Deloire MS, Salort E, Bonnet M, et al. Cognitive impairment as marker of diffuse brain abnormalities in early relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76:519–26.
Dineen RA, Vilisaar J, Hlinka J, et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain. 2009;132:239–49.
Faiss JH, Dahne D, Baum K, et al. Reduced magnetisation transfer ratio in cognitively impaired patients at the very early stage of multiple sclerosis: a prospective, multicenter, cross-sectional study. BMJ Open. 2014;4: e004409.
Faivre A, Rico A, Zaaraoui W, et al. Assessing brain connectivity at rest is clinically relevant in early multiple sclerosis. Mult Scler. 2012;18:1251–8.
Filippi M, Preziosa P, Copetti M, et al. Gray matter damage predicts the accumulation of disability 13 years later in MS. Neurology. 2013;81:1759–67.
Filippi M, Tortorella C, Rovaris M, et al. Changes in the normal appearing brain tissue and cognitive impairment in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;68:157–61.
Foong J, Rozewicz L, Davie CA, Thompson AJ, Miller DH, Ron MA. Correlates of executive function in multiple sclerosis: the use of magnetic resonance spectroscopy as an index of focal pathology. J Neuropsychiatry Clin Neurosci. 1999;11:45–50.
Foong J, Rozewicz L, Quaghebeur G, et al. Executive function in multiple sclerosis. The role of frontal lobe pathology. Brain. 1997;120(Pt 1):15–26.
Forn C, Rocca MA, Valsasina P, et al. Functional magnetic resonance imaging correlates of cognitive performance in patients with a clinically isolated syndrome suggestive of multiple sclerosis at presentation: an activation and connectivity study. Mult Scler. 2012;18:153–63.
Franklin GM, Heaton RK, Nelson LM, Filley CM, Seibert C. Correlation of neuropsychological and MRI findings in chronic/progressive multiple sclerosis. Neurology. 1988;38:1826–9.
Gabilondo I, Rilo O, Ojeda N, et al. The influence of posterior visual pathway damage on visual information processing speed in multiple sclerosis. Mult Scler. 2017;23:1276–88.
Giorgio A, De Stefano N. Cognition in multiple sclerosis: relevance of lesions, brain atrophy and proton MR spectroscopy. Neurol Sci. 2010;31(Suppl 2):S245–8.
Hanken K, Manousi A, Klein J, Kastrup A, Eling P, Hildebrandt H. On the relation between self-reported cognitive fatigue and the posterior hypothalamic-brainstem network. Eur J Neurol. 2016;23:101–9.
Hardmeier M, Schoonheim MM, Geurts JJ, et al. Cognitive dysfunction in early multiple sclerosis: altered centrality derived from resting-state functional connectivity using magneto-encephalography. PLoS ONE. 2012;7: e42087.
Hawellek DJ, Hipp JF, Lewis CM, Corbetta M, Engel AK. Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc Natl Acad Sci U S A. 2011;108:19066–71.
Hildebrandt H, Hahn HK, Kraus JA, Schulte-Herbruggen A, Schwarze B, Schwendemann G. Memory performance in multiple sclerosis patients correlates with central brain atrophy. Mult Scler. 2006;12:428–36.
Hillary FG, Chiaravalloti ND, Ricker JH, et al. An investigation of working memory rehearsal in multiple sclerosis using fMRI. J Clin Exp Neuropsychol. 2003;25:965–78.
Hohol MJ, Guttmann CR, Orav J, et al. Serial neuropsychological assessment and magnetic resonance imaging analysis in multiple sclerosis. Arch Neurol. 1997;54:1018–25.
Houtchens MK, Benedict RH, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology. 2007;69:1213–23.
Hulst HE, Schoonheim MM, Roosendaal SD, et al. Functional adaptive changes within the hippocampal memory system of patients with multiple sclerosis. Hum Brain Mapp. 2012;33:2268–80.
Hulst HE, Steenwijk MD, Versteeg A, et al. Cognitive impairment in MS: impact of white matter integrity, gray matter volume, and lesions. Neurology. 2013;80:1025–32.
Hyncicova E, Kalina A, Vyhnalek M, et al. Health-related quality of life, neuropsychiatric symptoms and structural brain changes in clinically isolated syndrome. PLoS ONE. 2018;13: e0200254.
Iancheva D, Trenova A, Mantarova S, Terziyski K. Functional magnetic resonance imaging correlations between fatigue and cognitive performance in patients with relapsing remitting multiple sclerosis. Front Psychiatry. 2019;10:754.
Iancheva D, Trenova AG, Terziyski K, Kandilarova S, Mantarova S. Translational validity of PASAT and the effect of fatigue and mood in patients with relapsing remitting MS: a functional MRI study. J Eval Clin Pract. 2018;24:832–8.
Ifantopoulou P, Artemiadis AK, Bakirtzis C, et al. Cognitive and brain reserve in multiple sclerosis—a cross-sectional study. Mult Scler Relat Disord. 2019;35:128–34.
Joly H, Capet N, Mondot L, et al. Thalamic atrophy correlates with dysfunctional impulsivity in multiple sclerosis. Mult Scler Relat Disord. 2020;44: 102374.
Kern KC, Ekstrom AD, Suthana NA, et al. Fornix damage limits verbal memory functional compensation in multiple sclerosis. Neuroimage. 2012;59:2932–40.
Keser Z, Kamali A, Younes K, Schulz PE, Nelson FM, Hasan KM. Yakovlev’s basolateral limbic circuit in multiple sclerosis related cognitive impairment. J Neuroimaging. 2018;28:596–600.
Khalil M, Enzinger C, Langkammer C, et al. Cognitive impairment in relation to MRI metrics in patients with clinically isolated syndrome. Mult Scler. 2011;17:173–80.
Kiy G, Lehmann P, Hahn HK, Eling P, Kastrup A, Hildebrandt H. Decreased hippocampal volume, indirectly measured, is associated with depressive symptoms and consolidation deficits in multiple sclerosis. Mult Scler. 2011;17:1088–97.
Kletenik I, Alvarez E, Honce JM, Valdez B, Vollmer TL, Medina LD. Subjective cognitive concern in multiple sclerosis is associated with reduced thalamic and cortical gray matter volumes. Mult Scler J Exp Transl Clin. 2019;5:2055217319827618.
Kutzelnigg A, Lassmann H. Cortical demyelination in multiple sclerosis: a substrate for cognitive deficits? J Neurol Sci. 2006;245:123–6.
Lazeron RH, Langdon DW, Filippi M, et al. Neuropsychological impairment in multiple sclerosis patients: the role of (juxta)cortical lesion on FLAIR. Mult Scler. 2000;6:280–5.
Lin SJ, Vavasour I, Kosaka B, et al. Education, and the balance between dynamic and stationary functional connectivity jointly support executive functions in relapsing-remitting multiple sclerosis. Hum Brain Mapp. 2018;39:5039–49.
Louapre C, Perlbarg V, Garcia-Lorenzo D, et al. Brain networks disconnection in early multiple sclerosis cognitive deficits: an anatomofunctional study. Hum Brain Mapp. 2014;35:4706–17.
Lund H, Jonsson A, Andresen J, Rostrup E, Paulson OB, Sorensen PS. Cognitive deficits in multiple sclerosis: correlations with T2 changes in normal appearing brain tissue. Acta Neurol Scand. 2012;125:338–44.
Macaron G, Baldassari LE, Nakamura K, et al. Cognitive processing speed in multiple sclerosis clinical practice: association with patient-reported outcomes, employment and magnetic resonance imaging metrics. Eur J Neurol. 2020;27:1238–49.
Mainero C, Caramia F, Pozzilli C, et al. fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. Neuroimage. 2004;21:858–67.
Mathiesen HK, Jonsson A, Tscherning T, et al. Correlation of global N-acetyl aspartate with cognitive impairment in multiple sclerosis. Arch Neurol. 2006;63:533–6.
Mehndiratta A, Treaba CA, Barletta V, et al. Characterization of thalamic lesions and their correlates in multiple sclerosis by ultra-high-field MRI. Mult Scler. 2021;27:674–83.
Mesaros S, Rocca MA, Kacar K, et al. Diffusion tensor MRI tractography and cognitive impairment in multiple sclerosis. Neurology. 2012;78:969–75.
Mesaros S, Rocca MA, Riccitelli G, et al. Corpus callosum damage and cognitive dysfunction in benign MS. Hum Brain Mapp. 2009;30:2656–66.
Mike A, Glanz BI, Hildenbrand P, et al. Identification and clinical impact of multiple sclerosis cortical lesions as assessed by routine 3T MR imaging. AJNR Am J Neuroradiol. 2011;32:515–21.
Modica CM, Zivadinov R, Dwyer MG, Bergsland N, Weeks AR, Benedict RH. Iron and volume in the deep gray matter: association with cognitive impairment in multiple sclerosis. AJNR Am J Neuroradiol. 2015;36:57–62.
Morgen K, Sammer G, Courtney SM, et al. Evidence for a direct association between cortical atrophy and cognitive impairment in relapsing-remitting MS. Neuroimage. 2006;30:891–8.
Mowry EM, Beheshtian A, Waubant E, et al. Quality of life in multiple sclerosis is associated with lesion burden and brain volume measures. Neurology. 2009;72:1760–5.
Muhlert N, Atzori M, De Vita E, et al. Memory in multiple sclerosis is linked to glutamate concentration in grey matter regions. J Neurol Neurosurg Psychiatry. 2014;85:833–9.
Muller M, Esser R, Kotter K, Voss J, Muller A, Stellmes P. Third ventricular enlargement in early stages of multiple sclerosis is a predictor of motor and neuropsychological deficits: a cross-sectional study. BMJ Open. 2013;3:e003582.
Nelson F, Datta S, Garcia N, et al. Intracortical lesions by 3T magnetic resonance imaging and correlation with cognitive impairment in multiple sclerosis. Mult Scler. 2011;17:1122–9.
Nygaard GO, Walhovd KB, Sowa P, et al. Cortical thickness and surface area relate to specific symptoms in early relapsing-remitting multiple sclerosis. Mult Scler. 2015;21:402–14.
Okuda DT, Srinivasan R, Oksenberg JR, et al. Genotype-phenotype correlations in multiple sclerosis: HLA genes influence disease severity inferred by 1HMR spectroscopy and MRI measures. Brain. 2009;132(Pt 1):250–9.
Pan JW, Krupp LB, Elkins LE, Coyle PK. Cognitive dysfunction lateralizes with NAA in multiple sclerosis. Appl Neuropsychol. 2001;8:155–60.
Papadopoulou A, Muller-Lenke N, Naegelin Y, et al. Contribution of cortical and white matter lesions to cognitive impairment in multiple sclerosis. Mult Scler. 2013;19:1290–6.
Parry AM, Scott RB, Palace J, Smith S, Matthews PM. Potentially adaptive functional changes in cognitive processing for patients with multiple sclerosis and their acute modulation by rivastigmine. Brain. 2003;126(Pt 12):2750–60.
Penner IK, Rausch M, Kappos L, Opwis K, Radu EW. Analysis of impairment related functional architecture in MS patients during performance of different attention tasks. J Neurol. 2003;250:461–72.
Penny S, Khaleeli Z, Cipolotti L, Thompson A, Ron M. Early imaging predicts later cognitive impairment in primary progressive multiple sclerosis. Neurology. 2010;74:545–52.
Pinter D, Khalil M, Pichler A, et al. Predictive value of different conventional and non-conventional MRI-parameters for specific domains of cognitive function in multiple sclerosis. Neuroimage Clin. 2015;7:715–20.
Piras MR, Magnano I, Canu ED, et al. Longitudinal study of cognitive dysfunction in multiple sclerosis: neuropsychological, neuroradiological, and neurophysiological findings. J Neurol Neurosurg Psychiatry. 2003;74:878–85.
Pravata E, Rocca MA, Valsasina P, et al. Gray matter trophism, cognitive impairment, and depression in patients with multiple sclerosis. Mult Scler. 2017;23:1864–74.
Rabins PV, Brooks BR, O’Donnell P, et al. Structural brain correlates of emotional disorder in multiple sclerosis. Brain. 1986;109(Pt 4):585–97.
Rachbauer D, Kronbichler M, Ropele S, Enzinger C, Fazekas F. Differences in cerebral activation patterns in idiopathic inflammatory demyelination using the paced visual serial addition task: an fMRI study. J Neurol Sci. 2006;244:11–6.
Randolph JJ, Wishart HA, Saykin AJ, et al. FLAIR lesion volume in multiple sclerosis: relation to processing speed and verbal memory. J Int Neuropsychol Soc. 2005;11:205–9.
Ranjeva JP, Audoin B, Au Duong MV, et al. Structural and functional surrogates of cognitive impairment at the very early stage of multiple sclerosis. J Neurol Sci. 2006;245:161–7.
Rao SM, Leo GJ, Haughton VM, St Aubin-Faubert P, Bernardin L. Correlation of magnetic resonance imaging with neuropsychological testing in multiple sclerosis. Neurology. 1989;39:161–6.
Reuter F, Zaaraoui W, Crespy L, et al. Cognitive impairment at the onset of multiple sclerosis: relationship to lesion location. Mult Scler. 2011;17:755–8.
Reuter F, Zaaraoui W, Crespy L, et al. Frequency of cognitive impairment dramatically increases during the first 5 years of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2011;82:1157–9.
Riccitelli G, Rocca MA, Pagani E, et al. Cognitive impairment in multiple sclerosis is associated to different patterns of gray matter atrophy according to clinical phenotype. Hum Brain Mapp. 2011;32:1535–43.
Rocca MA, Absinta M, Amato MP, et al. Posterior brain damage and cognitive impairment in pediatric multiple sclerosis. Neurology. 2014;82:1314–21.
Rocca MA, Valsasina P, Absinta M, et al. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology. 2010;74:1252–9.
Rocca MA, Valsasina P, Ceccarelli A, et al. Structural and functional MRI correlates of Stroop control in benign MS. Hum Brain Mapp. 2009;30:276–90.
Rocca MA, Valsasina P, Meani A, Falini A, Comi G, Filippi M. Impaired functional integration in multiple sclerosis: a graph theory study. Brain Struct Funct. 2016;221:115–31.
Rojas JI, Murphy G, Sanchez F, et al. Thalamus volume change and cognitive impairment in early relapsing-remitting multiple sclerosis patients. Neuroradiol J. 2018;31:350–5.
Roosendaal SD, Bendfeldt K, Vrenken H, et al. Grey matter volume in a large cohort of MS patients: relation to MRI parameters and disability. Mult Scler. 2011;17:1098–106.
Roosendaal SD, Geurts JJ, Vrenken H, et al. Regional DTI differences in multiple sclerosis patients. Neuroimage. 2009;44:1397–403.
Rossi F, Giorgio A, Battaglini M, et al. Relevance of brain lesion location to cognition in relapsing multiple sclerosis. PLoS ONE. 2012;7: e44826.
Rovaris M, Filippi M, Falautano M, et al. Relation between MR abnormalities and patterns of cognitive impairment in multiple sclerosis. Neurology. 1998;50:1601–8.
Rovaris M, Filippi M, Minicucci L, et al. Cortical/subcortical disease burden and cognitive impairment in patients with multiple sclerosis. AJNR Am J Neuroradiol. 2000;21:402–8.
Rovaris M, Iannucci G, Falautano M, et al. Cognitive dysfunction in patients with mildly disabling relapsing-remitting multiple sclerosis: an exploratory study with diffusion tensor MR imaging. J Neurol Sci. 2002;195:103–9.
Sanchez MP, Nieto A, Barroso J, Martin V, Hernandez MA. Brain atrophy as a marker of cognitive impairment in mildly disabling relapsing-remitting multiple sclerosis. Eur J Neurol. 2008;15:1091–9.
Sanfilipo MP, Benedict RH, Weinstock-Guttman B, Bakshi R. Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology. 2006;66:685–92.
Santa Maria MP, Benedict RH, Bakshi R, et al. Functional imaging during covert auditory attention in multiple sclerosis. J Neurol Sci. 2004;218:9–15.
Schoonheim MM, Hulst HE, Brandt RB, et al. Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology. 2015;84:776–83.
Schoonheim MM, Popescu V, Rueda Lopes FC, et al. Subcortical atrophy and cognition: sex effects in multiple sclerosis. Neurology. 2012;79:1754–61.
Schoonheim MM, Vigeveno RM, Rueda Lopes FC, et al. Sex-specific extent and severity of white matter damage in multiple sclerosis: implications for cognitive decline. Hum Brain Mapp. 2014;35:2348–58.
Sepulcre J, Masdeu JC, Pastor MA, et al. Brain pathways of verbal working memory: a lesion-function correlation study. Neuroimage. 2009;47:773–8.
Shinoda K, Matsushita T, Nakamura Y, et al. Contribution of cortical lesions to cognitive impairment in Japanese patients with multiple sclerosis. Sci Rep. 2020;10:1–8.
Sicotte NL, Kern KC, Giesser BS, et al. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131:1134–41.
Smith AM, Walker LA, Freedman MS, DeMeulemeester C, Hogan MJ, Cameron I. fMRI investigation of disinhibition in cognitively impaired patients with multiple sclerosis. J Neurol Sci. 2009;281:58–63.
Sperling RA, Guttmann CR, Hohol MJ, et al. Regional magnetic resonance imaging lesion burden and cognitive function in multiple sclerosis: a longitudinal study. Arch Neurol. 2001;58:115–21.
Staffen W, Mair A, Zauner H, et al. Cognitive function and fMRI in patients with multiple sclerosis: evidence for compensatory cortical activation during an attention task. Brain. 2002;125(Pt 6):1275–82.
Staffen W, Zauner H, Mair A, et al. Magnetic resonance spectroscopy of memory and frontal brain region in early multiple sclerosis. J Neuropsychiatry Clin Neurosci. 2005;17:357–63.
Summers M, Fisniku L, Anderson V, Miller D, Cipolotti L, Ron M. Cognitive impairment in relapsing-remitting multiple sclerosis can be predicted by imaging performed several years earlier. Mult Scler. 2008;14:197–204.
Summers M, Swanton J, Fernando K, et al. Cognitive impairment in multiple sclerosis can be predicted by imaging early in the disease. J Neurol Neurosurg Psychiatry. 2008;79:955–8.
Sumowski JF, Wylie GR, Deluca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain. 2010;133:362–74.
Sundgren M, Wahlin A, Maurex L, Brismar T. Event related potential and response time give evidence for a physiological reserve in cognitive functioning in relapsing-remitting multiple sclerosis. J Neurol Sci. 2015;356:107–12.
Sweet LH, Rao SM, Primeau M, Durgerian S, Cohen RA. Functional magnetic resonance imaging response to increased verbal working memory demands among patients with multiple sclerosis. Hum Brain Mapp. 2006;27:28–36.
Sweet LH, Rao SM, Primeau M, Mayer AR, Cohen RA. Functional magnetic resonance imaging of working memory among multiple sclerosis patients. J Neuroimaging. 2004;14:150–7.
Swirsky-Sacchetti T, Mitchell DR, Seward J, et al. Neuropsychological and structural brain lesions in multiple sclerosis: a regional analysis. Neurology. 1992;42:1291–5.
Tiemann L, Penner IK, Haupts M, Schlegel U, Calabrese P. Cognitive decline in multiple sclerosis: impact of topographic lesion distribution on differential cognitive deficit patterns. Mult Scler. 2009;15:1164–74.
Till C, Deotto A, Tipu V, et al. White matter integrity and math performance in pediatric multiple sclerosis: a diffusion tensor imaging study. NeuroReport. 2011;22:1005–9.
Till C, Ghassemi R, Aubert-Broche B, et al. MRI correlates of cognitive impairment in childhood-onset multiple sclerosis. Neuropsychology. 2011;25:319–32.
Tovar-Moll F, Evangelou IE, Chiu AW, et al. Thalamic involvement and its impact on clinical disability in patients with multiple sclerosis: a diffusion tensor imaging study at 3T. AJNR Am J Neuroradiol. 2009;30:1380–6.
Tur C, Penny S, Khaleeli Z, et al. Grey matter damage and overall cognitive impairment in primary progressive multiple sclerosis. Mult Scler. 2011;17:1324–32.
Van Schependom J, Gielen J, Laton J, et al. The effect of morphological and microstructural integrity of the corpus callosum on cognition, fatigue and depression in mildly disabled MS patients. Magn Reson Imaging. 2017;40:109–14.
Wilting J, Rolfsnes HO, Zimmermann H, et al. Structural correlates for fatigue in early relapsing remitting multiple sclerosis. Eur Radiol. 2016;26:515–23.
Yaldizli O, Penner IK, Frontzek K, et al. The relationship between total and regional corpus callosum atrophy, cognitive impairment and fatigue in multiple sclerosis patients. Mult Scler. 2014;20:356–64.
Yildiz M, Tettenborn B, Radue EW, Bendfeldt K, Borgwardt S. Association of cognitive impairment and lesion volumes in multiple sclerosis—a MRI study. Clin Neurol Neurosurg. 2014;127:54–8.
Zaaraoui W, Reuter F, Rico A, et al. Occurrence of neuronal dysfunction during the first 5 years of multiple sclerosis is associated with cognitive deterioration. J Neurol. 2011;258:811–9.
Zhou F, Zhuang Y, Gong H, et al. Altered inter-subregion connectivity of the default mode network in relapsing remitting multiple sclerosis: a functional and structural connectivity study. PLoS ONE. 2014;9: e101198.
Andreasen AK, Iversen P, Marstrand L, Siersma V, Siebner HR, Sellebjerg F. Structural and cognitive correlates of fatigue in progressive multiple sclerosis. Neurol Res. 2019;41:168–76.
Andreasen AK, Jakobsen J, Soerensen L, et al. Regional brain atrophy in primary fatigued patients with multiple sclerosis. Neuroimage. 2010;50:608–15.
Biberacher V, Schmidt P, Selter RC, et al. Fatigue in multiple sclerosis: associations with clinical MRI and CSF parameters. Mult Scler. 2018;24:1115–25.
Calabrese M, Rinaldi F, Grossi P, et al. Basal ganglia and frontal/parietal cortical atrophy is associated with fatigue in relapsing-remitting multiple sclerosis. Mult Scler. 2010;16:1220–8.
Chalah MA, Kauv P, Creange A, Hodel J, Lefaucheur JP, Ayache SS. Neurophysiological, radiological and neuropsychological evaluation of fatigue in multiple sclerosis. Mult Scler Relat Disord. 2019;28:145–52.
Colombo B, Martinelli Boneschi F, Rossi P, et al. MRI and motor evoked potential findings in nondisabled multiple sclerosis patients with and without symptoms of fatigue. J Neurol. 2000;247:506–9.
Cruz Gomez AJ, Ventura Campos N, Belenguer A, Avila C, Forn C. Regional brain atrophy and functional connectivity changes related to fatigue in multiple sclerosis. PLoS ONE. 2013;8:e77914.
Damasceno A, Damasceno BP, Cendes F. Atrophy of reward-related striatal structures in fatigued MS patients is independent of physical disability. Mult Scler. 2016;22:822–9.
DeLuca J, Genova HM, Hillary FG, Wylie G. Neural correlates of cognitive fatigue in multiple sclerosis using functional MRI. J Neurol Sci. 2008;270:28–39.
Derache N, Grassiot B, Mezenge F, et al. Fatigue is associated with metabolic and density alterations of cortical and deep gray matter in relapsing-remitting-multiple sclerosis patients at the earlier stage of the disease: a PET/MR study. Mult Scler Relat Disord. 2013;2:362–9.
Dobryakova E, Hulst HE, Spirou A, et al. Fronto-striatal network activation leads to less fatigue in multiple sclerosis. Mult Scler. 2018;24:1174–82.
Engstrom M, Flensner G, Landtblom AM, Ek AC, Karlsson T. Thalamo-striato-cortical determinants to fatigue in multiple sclerosis. Brain Behav. 2013;3:715–28.
Fernandez-de-Las-Penas C, Ortega-Santiago R, Ortiz-Gutierrez R, Caminero AB, Salom-Moreno J, Arendt-Nielsen L. Widespread pressure pain hypersensitivity in patients with multiple sclerosis with and without pain as sign of central sensitization. Clin J Pain. 2015;31:66–72.
Filippi M, Rocca MA, Colombo B, et al. Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage. 2002;15:559–67.
Finke C, Schlichting J, Papazoglou S, et al. Altered basal ganglia functional connectivity in multiple sclerosis patients with fatigue. Mult Scler. 2015;21:925–34.
Genova HM, Rajagopalan V, Deluca J, et al. Examination of cognitive fatigue in multiple sclerosis using functional magnetic resonance imaging and diffusion tensor imaging. PLoS ONE. 2013;8: e78811.
Gobbi C, Rocca MA, Riccitelli G, et al. Influence of the topography of brain damage on depression and fatigue in patients with multiple sclerosis. Mult Scler. 2014;20:192–201.
Gschwind M, Hardmeier M, Van De Ville D, et al. Fluctuations of spontaneous EEG topographies predict disease state in relapsing-remitting multiple sclerosis. Neuroimage Clin. 2016;12:466–77.
Hanken K, Eling P, Klein J, Klaene E, Hildebrandt H. Different cortical underpinnings for fatigue and depression in MS? Mult Scler Relat Disord. 2016;6:81–6.
Hanken K, Francis Y, Kastrup A, Eling P, Klein J, Hildebrandt H. On the role of the amygdala for experiencing fatigue in patients with multiple sclerosis. Mult Scler Relat Disord. 2018;20:67–72.
Jaeger S, Paul F, Scheel M, et al. Multiple sclerosis-related fatigue: altered resting-state functional connectivity of the ventral striatum and dorsolateral prefrontal cortex. Mult Scler J. 2019;25:554–64.
Kantorova E, Polacek H, Bittsansky M, et al. Hypothalamic damage in multiple sclerosis correlates with disease activity, disability, depression, and fatigue. Neurol Res. 2017;39:323–30.
Lazzarotto A, Margoni M, Franciotta S, et al. Selective cerebellar atrophy associates with depression and fatigue in the early phases of relapse-onset multiple sclerosis. Cerebellum. 2020;19:192–200.
Leocani L, Colombo B, Magnani G, et al. Fatigue in multiple sclerosis is associated with abnormal cortical activation to voluntary movement–EEG evidence. Neuroimage. 2001;13:1186–92.
Marchesi O, Vizzino C, Meani A, et al. Fatigue in multiple sclerosis patients with different clinical phenotypes: a clinical and magnetic resonance imaging study. Eur J Neurol. 2020;27:2549–60.
Marrie RA, Fisher E, Miller DM, Lee JC, Rudick RA. Association of fatigue and brain atrophy in multiple sclerosis. J Neurol Sci. 2005;228:161–6.
Niepel G, Tench ChR, Morgan PS, Evangelou N, Auer DP, Constantinescu CS. Deep gray matter and fatigue in MS: a T1 relaxation time study. J Neurol. 2006;253:896–902.
Palotai M, Cavallari M, Koubiyr I, et al. Microstructural fronto-striatal and temporo-insular alterations are associated with fatigue in patients with multiple sclerosis independent of white matter lesion load and depression. Mult Scler. 2020;26:1708–18.
Palotai M, Nazeri A, Cavallari M, et al. History of fatigue in multiple sclerosis is associated with grey matter atrophy. Sci Rep. 2019;9:14781.
Pardini M, Bonzano L, Mancardi GL, Roccatagliata L. Frontal networks play a role in fatigue perception in multiple sclerosis. Behav Neurosci. 2010;124:329–36.
Pellicano C, Gallo A, Li X, et al. Relationship of cortical atrophy to fatigue in patients with multiple sclerosis. Arch Neurol. 2010;67:447–53.
Pravata E, Zecca C, Sestieri C, et al. Hyperconnectivity of the dorsolateral prefrontal cortex following mental effort in multiple sclerosis patients with cognitive fatigue. Mult Scler. 2016;22:1665–75.
Riccitelli G, Rocca MA, Forn C, Colombo B, Comi G, Filippi M. Voxelwise assessment of the regional distribution of damage in the brains of patients with multiple sclerosis and fatigue. AJNR Am J Neuroradiol. 2011;32:874–9.
Rocca MA, Absinta M, Valsasina P, et al. Abnormal cervical cord function contributes to fatigue in multiple sclerosis. Mult Scler. 2012;18:1552–9.
Rocca MA, Parisi L, Pagani E, et al. Regional but not global brain damage contributes to fatigue in multiple sclerosis. Radiology. 2014;273:511–20.
Roelcke U, Kappos L, Lechner-Scott J, et al. Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology. 1997;48:1566–71.
Russo M, Calamuneri A, Cacciola A, et al. Neural correlates of fatigue in multiple sclerosis: a combined neurophysiological and neuroimaging approach (R1). Arch Ital Biol. 2017;155:142–51.
Sepulcre J, Masdeu JC, Goni J, et al. Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult Scler. 2009;15:337–44.
Specogna I, Casagrande F, Lorusso A, et al. Functional MRI during the execution of a motor task in patients with multiple sclerosis and fatigue. Radiol Med. 2012;117:1398–407.
Stefancin P, Govindarajan ST, Krupp L, Charvet L, Duong TQ. Resting-state functional connectivity networks associated with fatigue in multiple sclerosis with early age onset. Mult Scler Relat Disord. 2019;31:101–5.
Tartaglia MC, Narayanan S, Arnold DL. Mental fatigue alters the pattern and increases the volume of cerebral activation required for a motor task in multiple sclerosis patients with fatigue. Eur J Neurol. 2008;15:413–9.
Tartaglia MC, Narayanan S, Francis SJ, et al. The relationship between diffuse axonal damage and fatigue in multiple sclerosis. Arch Neurol. 2004;61:201–7.
Tedeschi G, Dinacci D, Lavorgna L, et al. Correlation between fatigue and brain atrophy and lesion load in multiple sclerosis patients independent of disability. J Neurol Sci. 2007;263:15–9.
Tellez N, Alonso J, Rio J, et al. The basal ganglia: a substrate for fatigue in multiple sclerosis. Neuroradiology. 2008;50:17–23.
Weier K, Penner IK, Magon S, et al. Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis. PLoS ONE. 2014;9: e86916.
White AT, Lee JN, Light AR, Light KC. Brain activation in multiple sclerosis: a BOLD fMRI study of the effects of fatiguing hand exercise. Mult Scler. 2009;15:580–6.
Yaldizli O, Glassl S, Sturm D, et al. Fatigue and progression of corpus callosum atrophy in multiple sclerosis. J Neurol. 2011;258:2199–205.
Zellini F, Niepel G, Tench CR, Constantinescu CS. Hypothalamic involvement assessed by T1 relaxation time in patients with relapsing-remitting multiple sclerosis. Mult Scler. 2009;15:1442–9.
Bakshi R, Czarnecki D, Shaikh ZA, et al. Brain MRI lesions and atrophy are related to depression in multiple sclerosis. NeuroReport. 2000;11:1153–8.
Colasanti A, Guo Q, Giannetti P, et al. Hippocampal neuroinflammation, functional connectivity, and depressive symptoms in multiple sclerosis. Biol Psychiatry. 2016;80:62–72.
Fassbender K, Schmidt R, Mossner R, et al. Mood disorders and dysfunction of the hypothalamic-pituitary-adrenal axis in multiple sclerosis: association with cerebral inflammation. Arch Neurol. 1998;55:66–72.
Feinstein A, O’Connor P, Akbar N, Moradzadeh L, Scott CJ, Lobaugh NJ. Diffusion tensor imaging abnormalities in depressed multiple sclerosis patients. Mult Scler. 2010;16:189–96.
Feinstein A, Roy P, Lobaugh N, Feinstein K, O’Connor P, Black S. Structural brain abnormalities in multiple sclerosis patients with major depression. Neurology. 2004;62:586–90.
Gold SM, Kern KC, O’Connor MF, et al. Smaller cornu ammonis 2–3/dentate gyrus volumes and elevated cortisol in multiple sclerosis patients with depressive symptoms. Biol Psychiatry. 2010;68:553–9.
Gold SM, O’Connor MF, Gill R, et al. Detection of altered hippocampal morphology in multiple sclerosis-associated depression using automated surface mesh modeling. Hum Brain Mapp. 2014;35:30–7.
Nigro S, Passamonti L, Riccelli R, et al. Structural “connectomic” alterations in the limbic system of multiple sclerosis patients with major depression. Mult Scler. 2015;21:1003–12.
Nunnari D, De Cola MC, D’Aleo G, et al. Impact of depression, fatigue, and global measure of cortical volume on cognitive impairment in multiple sclerosis. Biomed Res Int. 2015;2015: 519785.
Passamonti L, Cerasa A, Liguori M, et al. Neurobiological mechanisms underlying emotional processing in relapsing-remitting multiple sclerosis. Brain. 2009;132(Pt 12):3380–91.
Pujol J, Bello J, Deus J, Cardoner N, Marti-Vilalta JL, Capdevila A. Beck depression inventory factors related to demyelinating lesions of the left arcuate fasciculus region. Psychiatry Res. 2000;99:151–9.
Pujol J, Bello J, Deus J, Marti-Vilalta JL, Capdevila A. Lesions in the left arcuate fasciculus region and depressive symptoms in multiple sclerosis. Neurology. 1997;49:1105–10.
Riccelli R, Passamonti L, Cerasa A, et al. Individual differences in depression are associated with abnormal function of the limbic system in multiple sclerosis patients. Mult Scler. 2016;22:1094–105.
Rocca MA, Pravata E, Valsasina P, et al. Hippocampal-DMN disconnectivity in MS is related to WM lesions and depression. Hum Brain Mapp. 2015;36:5051–63.
Sabatini U, Pozzilli C, Pantano P, et al. Involvement of the limbic system in multiple sclerosis patients with depressive disorders. Biol Psychiatry. 1996;39:970–5.
Shen Y, Bai L, Gao Y, et al. Depressive symptoms in multiple sclerosis from an in vivo study with TBSS. Biomed Res Int. 2014;2014: 148465.
Stuke H, Hanken K, Hirsch J, et al. Cross-sectional and longitudinal relationships between depressive symptoms and brain atrophy in MS patients. Front Hum Neurosci. 2016;10:622.
Yaldizli O, Penner IK, Yonekawa T, et al. The association between olfactory bulb volume, cognitive dysfunction, physical disability and depression in multiple sclerosis. Eur J Neurol. 2016;23:510–9.
Zorzon M, Zivadinov R, Nasuelli D, et al. Depressive symptoms and MRI changes in multiple sclerosis. Eur J Neurol. 2002;9:491–6.
Cruccu G, Biasiotta A, Di Rezze S, et al. Trigeminal neuralgia and pain related to multiple sclerosis. Pain. 2009;143:186–91.
Grau-Lopez L, Sierra S, Martinez-Caceres E, Ramo-Tello C. Analysis of the pain in multiple sclerosis patients. Neurologia. 2011;26:208–13.
Love S, Gradidge T, Coakham HB. Trigeminal neuralgia due to multiple sclerosis: ultrastructural findings in trigeminal rhizotomy specimens. Neuropathol Appl Neurobiol. 2001;27:238–44.
Scherder RJ, Kant N, Wolf ET, Pijnenburg BCM, Scherder EJA. Sensory function and chronic pain in multiple sclerosis. Pain Res Manag. 2018;2018:1924174.
Seixas D, Palace J, Tracey I. Chronic pain disrupts the reward circuitry in multiple sclerosis. Eur J Neurosci. 2016;44:1928–34.
Heesen C, Koehler G, Gross R, Tessmer W, Schulz KH, Gold SM. Altered cytokine responses to cognitive stress in multiple sclerosis patients with fatigue. Mult Scler. 2005;11:51–7.
Heesen C, Nawrath L, Reich C, Bauer N, Schulz KH, Gold SM. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry. 2006;77:34–9.
Heesen C, Schulz KH, Fiehler J, et al. Correlates of cognitive dysfunction in multiple sclerosis. Brain Behav Immun. 2010;24:1148–55.
Brenner P, Granqvist M, Konigsson J, Al Nimer F, Piehl F, Jokinen J. Depression and fatigue in multiple sclerosis: relation to exposure to violence and cerebrospinal fluid immunomarkers. Psychoneuroendocrinology. 2018;89:53–8.
Flachenecker P, Bihler I, Weber F, Gottschalk M, Toyka KV, Rieckmann P. Cytokine mRNA expression in patients with multiple sclerosis and fatigue. Mult Scler. 2004;10:165–9.
Gold SM, Kruger S, Ziegler KJ, et al. Endocrine and immune substrates of depressive symptoms and fatigue in multiple sclerosis patients with comorbid major depression. J Neurol Neurosurg Psychiatry. 2011;82:814–8.
Malekzadeh A, Van de Geer-Peeters W, De Groot V, Teunissen CE, Beckerman H, Group T-AS. Fatigue in patients with multiple sclerosis: is it related to pro- and anti-inflammatory cytokines? Dis Markers. 2015;2015:758314.
Pokryszko-Dragan A, Frydecka I, Kosmaczewska A, et al. Stimulated peripheral production of interferon-gamma is related to fatigue and depression in multiple sclerosis. Clin Neurol Neurosurg. 2012;114:1153–8.
Foley FW, Traugott U, LaRocca NG, et al. A prospective study of depression and immune dysregulation in multiple sclerosis. Arch Neurol. 1992;49:238–44.
Kahl KG, Kruse N, Faller H, Weiss H, Rieckmann P. Expression of tumor necrosis factor-alpha and interferon-gamma mRNA in blood cells correlates with depression scores during an acute attack in patients with multiple sclerosis. Psychoneuroendocrinology. 2002;27:671–81.
Kallaur AP, Lopes J, Oliveira SR, et al. Immune-inflammatory and oxidative and nitrosative stress biomarkers of depression symptoms in subjects with multiple sclerosis: increased peripheral inflammation but less acute neuroinflammation. Mol Neurobiol. 2016;53:5191–202.
Gottschalk M, Kumpfel T, Flachenecker P, et al. Fatigue and regulation of the hypothalamo-pituitary-adrenal axis in multiple sclerosis. Arch Neurol. 2005;62:277–80.
Powell DJ, Moss-Morris R, Liossi C, Schlotz W. Circadian cortisol and fatigue severity in relapsing-remitting multiple sclerosis. Psychoneuroendocrinology. 2015;56:120–31.
Tellez N, Comabella M, Julia E, et al. Fatigue in progressive multiple sclerosis is associated with low levels of dehydroepiandrosterone. Mult Scler. 2006;12:487–94.
Kern S, Schultheiss T, Schneider H, Schrempf W, Reichmann H, Ziemssen T. Circadian cortisol, depressive symptoms and neurological impairment in early multiple sclerosis. Psychoneuroendocrinology. 2011;36:1505–12.
Niepel G, Bibani RH, Vilisaar J, et al. Association of a deficit of arousal with fatigue in multiple sclerosis: effect of modafinil. Neuropharmacology. 2013;64:380–8.
Pardini M, Capello E, Krueger F, Mancardi G, Uccelli A. Reward responsiveness and fatigue in multiple sclerosis. Mult Scler. 2013;19:233–40.
Aeinehband S, Brenner P, Stahl S, et al. Cerebrospinal fluid kynurenines in multiple sclerosis; relation to disease course and neurocognitive symptoms. Brain Behav Immun. 2016;51:47–55.
Heitmann H, Andlauer TFM, Korn T, et al. Fatigue, depression, and pain in multiple sclerosis: how neuroinflammation translates into dysfunctional reward processing and anhedonic symptoms. Mult Scler. 2020. https://doi.org/10.1177/1352458520972279:1352458520972279 (2020/11/13 [Epub ahead of print]).
Dunn AJ. Cytokine activation of the HPA axis. Ann N Y Acad Sci. 2000;917:608–17.
Andravizou A, Dardiotis E, Artemiadis A, et al. Brain atrophy in multiple sclerosis: mechanisms, clinical relevance and treatment options. Autoimmun Highlights. 2019;10:7.
Yeshurun Y, Nguyen M, Hasson U. The default mode network: where the idiosyncratic self meets the shared social world. Nat Rev Neurosci. 2021;22:181–92.
Leavitt V, Paxton J, Sumowski J. Default network connectivity is linked to memory status in multiple sclerosis. J Int Neuropsychol Soc. 2014;20:937–44.
Filippi M, Rocca MA. Cortical reorganisation in patients with MS. J Neurol Neurosurg Psychiatry. 2004;75:1087–9.
Aslan D, Hernandez M, Frechette M, Gephart A, Soloveychik I, Sosnoff J. The neural underpinnings of motor learning in people with neurodegenerative diseases: A scoping review. Neurosci Biobehav Rev. 2021;131:882–98.
Ksiazek-Winiarek D, Szpakowski P, Glabinski A. Neural plasticity in multiple sclerosis: the functional and molecular background. Neural Plast. 2015;2015:307175.
Bonavita S, Gallo A, Sacco R, et al. Distributed changes in default-mode resting-state connectivity in multiple sclerosis. Mult Scler. 2011;17:411–22.
Leocani L, Colombo B, Comi G. Physiopathology of fatigue in multiple sclerosis. Neurol Sci. 2008;29:241–3.
Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–43.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56.
Liu Y, Ho R, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139:230–9.
Valkanova V, Ebmeier K, Allan C. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150:736–44.
Nickerson M, Cofield SS, Tyry T, Salter AR, Cutter GR, Marrie RA. Impact of multiple sclerosis relapse: the NARCOMS participant perspective. Mult Scler Relat Disord. 2015;4:234–40.
Galea I, Ward-Abel N, Heesen C. Relapse in multiple sclerosis. BMJ. 2015;350:h1765.
Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:54–63.
Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry. 2000;61(6):7–11.
Dobryakova E, Genova HM, DeLuca J, Wylie GR. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front Neurol. 2015;6:52.
Watzlawik JO, Wootla B, Rodriguez M. Tryptophan catabolites and their impact on multiple sclerosis progression. Curr Pharm Des. 2016;22:1049–59.
Gardner EL. Addiction and brain reward and antireward pathways. Adv Psychosom Med. 2011;30:22–60.
Huecker MR, Smiley A, Saadabadi A. Bupropion. Treasure Island: StatPearls; 2021.
Carandini T, Mancini M, Bogdan I, et al. Disruption of brainstem monoaminergic fibre tracts in multiple sclerosis as a putative mechanism for cognitive fatigue: a fixel-based analysis. Neuroimage Clin. 2021;30: 102587.
Cercignani M, Dipasquale O, Bogdan I, et al. Cognitive fatigue in multiple sclerosis is associated with alterations in the functional connectivity of monoamine circuits. Brain Commun. 2021;3:fcab023.
Liyanarachchi K, Ross R, Debono M. Human studies on hypothalamo-pituitary-adrenal (HPA) axis. Best Pract Res Clin Endocrinol Metab. 2017;31:459–73.
Gold SM, Mohr DC, Huitinga I, Flachenecker P, Sternberg EM, Heesen C. The role of stress-response systems for the pathogenesis and progression of MS. Trends Immunol. 2005;26:644–52.
Dean J, Keshavan M. The neurobiology of depression: an integrated view. Asian J Psychiatr. 2017;27:101–11.
Cleare AJ. The HPA axis and the genesis of chronic fatigue syndrome. Trends Endocrinol Metab. 2004;15:55–9.
Schoonheim MM, Geurts JJG, Wiebenga OT, et al. Changes in functional network centrality underlie cognitive dysfunction and physical disability in multiple sclerosis. Mult Scler. 2014;20:1058–65.
Tahedl M, Levine S, Greenlee M, Weissert R, Schwarzbach J. Functional connectivity in multiple sclerosis: recent findings and future directions. Front Neurol. 2018;9:828.
Hutchison R, Womeldsorf T, Gati J, Everling S, Menon R. Resting-state networks show dynamic functional connectivity in awake humans and anaesthetized macaques. Hum Brain Mapp. 2013;34:2154–77.
Amin M, Ontaneda D. Thalamic injury and cognition in multiple sclerosis. Front Neurol. 2020;11: 623914.
Carandini T, Cercignani M, Galimberti D, Scarpini E, Bozzali M. The distinct roles of monoamines in multiple sclerosis: a bridge between the immune and nervous systems? Brain Behav Immun. 2021;94:381–91.
Van Schependom J, Nagels G. Targeting cognitive impairment in multiple sclerosis-the road toward an imaging-based biomarker. Front Neurosci. 2017;11:380.
Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron. 2018;97:742–68.
Broicher SD, Filli L, Geisseler O, et al. Positive effects of fampridine on cognition, fatigue and depression in patients with multiple sclerosis over 2 years. J Neurol. 2018;265:1016–25.
Morrow S, Kaushik T, Zarevics P, et al. The effects of l-amphetamine sulfate on cognition in MS patients: results of a randomized controlled trial. J Neurol. 2009;256:1095–102.
Benedict R, Munschauer F, Zarevics P, et al. Effects of l-amphetamine sulfate on cognitive function in multiple sclerosis patients. J Neurol. 2008;255:848–52.
Pavsic K, Pelicon K, Ledinek AH, Sega S. Short-term impact of fampridine on motor and cognitive functions, mood and quality of life among multiple sclerosis patients. Clin Neurol Neurosurg. 2015;139:35–40.
Knippenberg S, Damoiseaux J, Bol Y, et al. Higher levels of reported sun exposure, and not vitamin D status, are associated with less depressive symptoms and fatigue in multiple sclerosis. Acta Neurol Scand. 2014;129:123–31.
Rodriguez M, Wootla B, Anderson G. Multiple sclerosis, gut microbiota and permeability: role of tryptophan catabolites, depression and the driving down of local melatonin. Curr Pharm Des. 2016;22:6134–41.
Miller AH, Haroon E, Felger JC. Therapeutic implications of brain-immune interactions: treatment in translation. Neuropsychopharmacology. 2017;42:334–59.
Wijenberg ML, Stapert SZ, Kohler S, Bol Y. Explaining fatigue in multiple sclerosis: cross-validation of a biopsychosocial model. J Behav Med. 2016;39:815–22.
Dalgas U, Langeskov-Christensen M, Stenager E, Riemenschneider M, Hvid LG. Exercise as medicine in multiple sclerosis-time for a paradigm shift: preventive, symptomatic, and disease-modifying aspects and perspectives. Curr Neurol Neurosci Rep. 2019;19:88.
Langeskov-Christensen M, Bisson EJ, Finlayson ML, Dalgas U. Potential pathophysiological pathways that can explain the positive effects of exercise on fatigue in multiple sclerosis: a scoping review. J Neurol Sci. 2017;373:307–20.
Anderson G, Maes M. A role for the regulation of the melatonergic pathways in Alzheimer’s disease and other neurodegenerative and psychiatric conditions. In: Ravishankar G, Ramakrishna A, editors. Serotonin and melatonin. Their functional role in plants, food, phytomedicine, and human health. CRC Press; 2016. p. 24.
Rommer PS, Eichstadt K, Ellenberger D, et al. Symptomatology and symptomatic treatment in multiple sclerosis: results from a nationwide MS registry. Mult Scler. 2019;25:1641–52.
Sandi D, Fricska-Nagy Z, Bencsik K, Vecsei L. Neurodegeneration in multiple sclerosis: symptoms of silent progression, biomarkers and neuroprotective therapy-kynurenines are important players. Molecules. 2021;26:3423.
Acknowledgements
Funding
This review, as well as the journal’s Rapid Service Fee, was funded by Novartis Pharma AG, Basel, Switzerland.
Medical Writing Assistance
Medical writing support was provided by David McMinn, PhD, and Emer Power, PhD, of Novartis CONEXTS, Dublin, Ireland, and was funded by Novartis Pharma AG, Basel, Switzerland.
Author Contributions
Tanuja Chitnis, Jo Vandercappellen, Miriam King, and Giampaolo Brichetto all meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article. All named authors helped to conceive the article, contributed to the reviewing and revision of the manuscript, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
Certain aspects of this review have been presented previously as part of broader presentations at the 37th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, 13–15 October 2021 (online), and at the 29th annual meeting of the European Charcot Foundation, November 14–18, 2021, Baveno, Italy.
Disclosures
Tanuja Chitnis has received compensation for consulting from Biogen, Novartis Pharmaceuticals, Roche Genentech, and Sanofi Genzyme. She has received research support from the National Institutes of Health, National MS Society, US Department of Defense, Sumaira Foundation, Brainstorm Cell Therapeutics, EMD Serono, I-Mab Biopharma, Mallinckrodt ARD, Novartis Pharmaceuticals, Octave Bioscience, Roche Genentech, and Tiziana Life Sciences. Miriam King and Jo Vandercappellen are employees of Novartis Pharma AG. In the last 3 years, Giampaolo Brichetto has been member on advisory board of Novartis and Roche.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
There are no data, per se, associated with this manuscript. Information extracted from the articles identified from the search can be requested from the authors.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chitnis, T., Vandercappellen, J., King, M. et al. Symptom Interconnectivity in Multiple Sclerosis: A Narrative Review of Potential Underlying Biological Disease Processes. Neurol Ther 11, 1043–1070 (2022). https://doi.org/10.1007/s40120-022-00368-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00368-2