Abstract
Introduction
Acute traumatic intraparenchymal hematoma (tICH) expansion is a major cause of clinical deterioration after brain contusion. Here, an accurate prediction tool for acute tICH expansion is proposed.
Methods
A multicenter hospital-based study for multivariable prediction model was conducted among patients (889 patients in a development dataset and 264 individuals in an external validation dataset) with initial and follow-up computed tomography (CT) imaging for tICH volume evaluation. Semi-automated software was employed to assess tICH expansion. Two multivariate predictive models for acute tICH expansion were developed and externally validated.
Results
A total of 198 (22.27%) individuals had remarkable acute tICH expansion. The novel Traumatic Parenchymatous Hematoma Expansion Aid (TPHEA) model retained several variables, including age, coagulopathy, baseline tICH volume, time to baseline CT time, subdural hemorrhage, a novel imaging marker of multihematoma fuzzy sign, and an inflammatory index of monocyte-to-lymphocyte ratio. Compared with multihematoma fuzzy sign, monocyte-to-lymphocyte ratio, and the basic model, the TPHEA model exhibited optimal discrimination, calibration, and clinical net benefits for patients with acute tICH expansion. A TPHEA nomogram was subsequently introduced from this model to facilitate clinical application. In an external dataset, this device showed good predicting performance for acute tICH expansion.
Conclusions
The main predictive factors in the TPHEA nomogram are the monocyte-to-lymphocyte ratio, baseline tICH volume, and multihematoma fuzzy sign. This user-friendly tool can estimate acute tICH expansion and optimize personalized treatments for individuals with brain contusion.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Why carry out this study? |
Acute traumatic intraparenchymal hematoma (tICH) expansion after brain contusion is an important and common secondary injury leading to subsequent clinical deterioration. |
Although some clinical studies have attempted to predict hematoma expansion using various methods, a clinically accurate and robust predictive tool for acute traumatic intraparenchymal hematoma expansion is still lacking. |
What was learned from the study? |
The novel Traumatic Parenchymatous Hematoma Expansion Aid (TPHEA) nomogram exhibited optimal discrimination and calibration for acute traumatic intraparenchymal hematoma expansion. In an external validation dataset, this tool showed a robust performance across an extensive spectrum of individuals with brain contusion. |
The nomogram provides a clinically accurate and user-friendly prediction tool for tICH expansion in individuals with brain contusion. The TPHEA nomogram will optimize the personalized management and treatment of individuals with brain contusion. |
Introduction
Traumatic brain injury (TBI) is an important cause of global mortality and disability in the youth population and has shown no substantial decrease over the last 30 years [1]. One important reason is its heterogeneity that includes a range of pathological features from axonal to hemorrhagic injuries [2]. Hemorrhagic parenchyma contusion is a serious type of TBI that constitutes 20–30% of all TBI cases [3]. In particular, acute traumatic intraparenchymal hematoma (tICH) expansion after brain contusion is an important and common secondary injury that leads to subsequent clinical deterioration [1, 4,5,6,7]. Approximately 16–75% of brain contusions exhibit significant expansion on follow-up imaging [8,9,10,11]. In the majority of patients, acute tICH expansion occurs within the first 24 h and rarely progresses after 3–4 days [1, 5]. Identifying patients who are at high risk of acute tICH expansion is crucial because of their need for surgical intervention and/or intubation, ventilation, and neuromonitoring. Therefore, a timely prediction of tICH expansion will aid in the classification and individualized management of patients with brain contusion.
Some clinical studies have attempted to predict hematoma expansion using various imaging variables. Baseline tICH volume, subdural hemorrhage, subarachnoid hemorrhage, and multiple hematomas are potential predictors for tICH expansion [10, 12, 13]. Certain clinical variables, such as age and coagulation disorders, may also be associated with acute tICH expansion [11, 14, 15]. However, none of these individual variables can provide an accurate prediction for tICH expansion.
Several small retrospective studies have attempted to develop comprehensive models for tICH expansion based on clinical and imaging variables [11, 15, 16]. However, some crucial predictors were not considered, including baseline tICH volume, a novel imaging marker of multihematoma fuzzy sign, and an inflammatory marker of monocyte-to-lymphocyte ratio (MLR) [17, 18]. In addition, the predictive value of these models must be verified in a large multicenter sample size. These deficiencies may limit their clinical applicability. Hence, an accurate and robust predictive model for tICH expansion is still urgently needed.
In this multicenter hospital-based study, a novel comprehensive Traumatic Parenchymatous Hematoma Expansion Aid (TPHEA) model that combines several crucial and novel clinical and imaging variables on admission was developed. On the basis of this model, a TPHEA nomogram was introduced and externally validated to provide an easy-to-use and robust tool for predicting acute tICH expansion.
Methods
Study Design and Patients
This multicenter hospital-based study for multivariable prediction model was carried out from May 2012 to June 2019. Between May 2012 and June 2018, consecutive patients with primary brain contusion at three grade A teaching hospitals in eastern Guangdong province, China, the First and Second Affiliated Hospital of Shantou University Medical College, and the Affiliated Jieyang Hospital of Sun Yat-sen University, were retrospectively included in the development dataset. Between March 2014 and June 2019, patients with primary brain contusion at fourth grade A teaching hospital, the Affiliated East Hospital of Xiamen University in Fujian province, China, were retrospectively included in an external validation dataset.
This study was approved by the ethics committees of the First Affiliated Hospital of Shantou University Medical College, the Second Affiliated Hospital of Shantou University Medical College, the Affiliated Jieyang Hospital of Sun Yat-sen University, and the Affiliated East Hospital of Xiamen University. This approval covered the necessary ethics approval for all the study sites. The ethics approval reference number (No.: 2020-042) applies to all the above ethics committees. Data from the retrospective datasets are anonymous; hence, the requirement for informed consent was waived. This work was performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Inclusion and Exclusion Criteria
Consecutive patients with mild, moderate, or severe brain contusion were included in the analysis. Inclusion criteria were: (1) baseline computed tomography (CT) showing intraparenchymal bleeding and (2) record of a baseline CT scan and a follow-up CT after primary brain contusion. Exclusion criteria were: (1) less than 18 years old; (2) a documentation of the baseline CT scan over 6 h or a follow-up CT undertaken within 48 h following brain injury; (3) underwent surgery prior to the follow-up CT scan; (4) initial blood evaluation test over 24 h of occurrence of the injury; (5) history of head trauma, stroke, or brain tumor; and (6) history of anticoagulant use prior to cerebral contusion (Fig. 1).
Flowchart illustrating the selection of patients based on the inclusion and exclusion criteria. a Patients in the development dataset were selected from the First Affiliated Hospital of Shantou University Medical College, the Second Affiliated Hospital of Shantou University Medical College, and the Affiliated Jieyang Hospital of Sun Yat-sen University between May 2012 and June 2018. b Patients in the external validation dataset were selected from the Affiliated East Hospital of Xiamen University between March 2014 and June 2019. CT non-contrast computed tomography
Clinical Data
Demographic and clinical variables including sex, age, severity of injury mechanism, level on Glasgow Coma Scale score, mean arterial pressure, hypertension, diabetes, and coagulation function were acquired from the electronic medical record system of the hospitals. Venous puncture to collect venous blood samples was performed at admission into the hospital. Routine blood testing was conducted to determine white blood cell count (reference range of 3.5–9.5 × 109 cells/L), monocyte count (reference range, 0.1–0.6 × 109 cells/L), neutrophil count (normal range, 1.8–6.4 × 109 cells/L), and lymphocyte count (normal range, 1.1–3.2 × 109 cells/L). MLR was computed as the ratio of the absolute monocyte count to the lymphocytes count.
Routine coagulative function examinations were also performed at admission. A patient was considered having coagulopathy upon satisfying any one of the following indicators: international normalized ratio > 1.2, activated partial thromboplastin time ≥ 36 s, or platelet count < 120 × 109 platelets/L at admission [19].
Imaging Data
Imaging variables including time to baseline CT, time from baseline CT to follow-up CT, intraventricular hemorrhage, subarachnoid hemorrhage, subdural hemorrhage, extradural hemorrhage, location of contusion, multihematoma fuzzy sign, baseline tICH volume, and follow-up tICH volume were acquired from the electronic medical record system of the hospitals.
Two readers (D.Z. and S.C.) blinded to the other clinical data read the CT images with 5-mm slice thickness at admission (baseline) and follow-up. Hematoma volumes at baseline and follow-up were computed from the CT images through Semi-automated Computer Aided Volumetric Analysis (General Electric, USA) [20, 21]. First, the area of interest was manually selected, and software was then employed to separate this region from the environment on the basis of a fixed cut-off in Hounsfield units (HU). Visual inspection was conducted on the isolated regions, and manual adjustment was subsequently performed to visualize the hemorrhage in all three projections [20, 21]. The same two investigators participated in the manual selection and adjustment. An automatic summary of the neighboring voxels was provided to measure the volume of the hematoma by using a cut-off value of differentiating hematomas from the neighboring brain tissue. A fixed window set at 110 and 50 HU was employed to assess the CT images. Overall volume was computed when numerous intraparenchymal hematomas occurred in the contusion site.
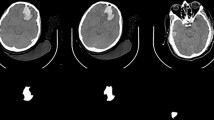
The latest noncontrast computed tomography (NCCT) marker for acute tICH expansion, namely, multihematoma fuzzy sign [9], was also introduced and is defined as: (1) three or more hematomas occurring next to each other in the contusion site was a manifestation of multiple hyperdense hematomas, with the maximum separation distance between hematomas before being regarded as unrelated equivalent to the diameter of the largest hematoma; (2) the fuzzy signal of the relative hypodense for the area flanked by hyperdense hematomas could be considered as hyperdense hematomas burring; and (3) the hypodense fuzzy area and the hyperdense hematomas are different by ≥ 20 HU. Multiple adjacent hematomas in the contusion regions must meet all three criteria mentioned above to be defined as a multihematoma fuzzy sign. Notably, multiple hyperdense hematomas separated by relatively hypodense gray/white matter must not be considered a multihematoma fuzzy sign.
Acute tICH Expansion Definition
Compared with that in previous studies [20, 21], a more stringent definition of tICH expansion was developed to further improve the correlation between acute tICH expansion and clinical deterioration. Acute tICH expansion was defined as no less than a 33% increase and no less than a 5-mL increase in absolute hematoma volume from the baseline CT. Patients must meet both of these criteria to be recognized as having an acute tICH expansion. Examples of acute tICH expansion in two patients with brain contusion are shown in Fig. 2.
Statistical Analyses
Continuous variables were presented as means (standard deviations) or medians (interquartile ranges). Categorical variables were shown as counts (percentages). Distribution of the data was tested using quantile–quantile plots and the Shapiro–Wilk test for normal or non-normal distribution. Except for age and mean arterial pressure, the remaining continuous variables were nonnormally distributed. Comparison of characteristics was conducted between individuals with and without tICH expansion. The chi-squared test was used for categorical data, the two-tailed Student’s t test was employed for normally distributed variables, and nonparametric data were examined by the Mann–Whitney U test. Intraclass correlation efficient (ICC) was applied to estimate the consistency of the machine and the investigators for tICH volume.
Missing Data
Data on the following variables were missing from the datasets: severity of injury mechanism (with 22.86% missing data), coagulation function parameters (with 18.86% missing data), MLR (with 12.27% missing data), hypertension (with 3.74% missing data), diabetes (with 2.67% missing data), and Glasgow Coma Scale (GCS) level (with 0.62% missing data). For all the variables with missing data, less than 25% of data points were unavailable. Missing values were imputed through multiple imputation by chained equations to preserve the sample size while limiting the selection bias [22].
Admission Predictor Selection
Logistic regression analysis was adopted to train a multivariate estimation model for tICH expansion. In univariate regression analyses, variables with P < 0.20 were incorporated in the multivariate analysis [23]. In multivariate regression, stepwise selection with the Akaike information criterion was adopted to select the independent risk factors of acute tICH expansion. Some important risk factors previously reported such as coagulopathy were also included in multivariate regression. Collinearity was assessed via the variance inflation factor (VIF), and features with VIF values > 10 were excluded [24]. Variables for inclusion were carefully chosen to ensure the parsimony of the final model. In the final multivariate logistic regression, baseline tICH volume (< 5, 5–10, or > 10 mL), and time to baseline CT (< 3 vs. ≥ 3 h) were included as categorical variables.
Construction of Predictive Models
After multivariate logistic regression, the following two predictive models for acute tICH expansion were established: a basic model with predictors of age, baseline tICH volume, time from the occurrence of brain trauma to the time of the initial CT (time to baseline CT), subdural hemorrhage, and coagulopathy and the TPHEA) model constituting all the predictors in the basic model together with multihematoma fuzzy sign and MLR. Their performance was assessed by the receiver operator characteristic (ROC) curve, calibration curve, and clinical decision curve (DCA) in the development dataset [25, 26]. The area under the ROC curve (AUC) was used to examine the model discrimination, which is equivalent to c statistics [27]. Calibration was evaluated with a graphical calibration plot of actual versus predicted probabilities with 1000 bootstrap samples to decrease the overfit bias [28]. DCA was also employed to evaluate the clinical utility of the models [26]. This tool gives insights into the scope of estimated risks for which the model possesses a high net benefit, rather than simply either categorizing all patients as having acute tICH expansion or no patient as having acute tICH expansion [29]. DCA can also be adopted to compare the net benefit of models.
Construction of TPHEA Nomogram
A TPHEA nomogram was subsequently constructed from the TPHEA model. Here, each regression coefficient in multivariate logistic regression model is proportionally converted to a 0- to 100-point scale. The effect of the variable with the highest β coefficient (absolute value) is assigned 100 points. These points are added across independent variables to derive the total points, which are then converted to predicted probabilities [30]. ROC and calibration curves were used to evaluate the performance of the TPHEA nomogram in the external dataset.
All remarkable tests were two-tailed, with P < 0.05 signifying statistical significance. SPSS v.22 (IMB, Armonk, NY, USA) and R statistical software (v3.5.1; http://www.r-project.org) with associated packages were used for all the analyses [31,32,33]. Multiple imputations for missing data were performed through the “MICE” package. Nomogram and calibration curves were plotted with the ‘‘rms’’ package. ROC curves were plotted by using the ‘‘ROCR’’ package. The Delong test was carried out to assess differences in the AUCs of various models. The Hosmer–Lemeshow test was used to examine nomogram calibration. The DCA curve was generated with the ‘‘rmda’’ package. This manuscript was prepared on the basis of the Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis [34].
Results
Patient Characteristics
A total of 889 patients with tICH expansion were included in the development dataset (Fig. 1; Table 1). Among them, 487 (55.22%) patients had a mild GCS level (13–15 score) at admission, and 779 (88.73%) brain contusions occurred in the frontal and temporal lobes. The ICC was 0.812, suggesting a good agreement between automatic volume calculation with and without fine-tuning. Among these individuals with brain contusion, 198 (22.27%) had significant acute tICH expansion, and 47 (5.29%) died in hospital. Compared with the patients without tICH expansion, those with tICH expansion had a higher rate of in-hospital mortality. In terms of clinical variables, individuals with acute tICH expansion had relatively high mean arterial pressure, MLR, and frequency of hypertension, coagulopathy, and diabetes, and a severe or moderate GCS level on admission. In terms of imaging variables, individuals with acute tICH expansion had a relatively large baseline tICH volume, short time from brain trauma to initial CT scan, and a high frequency of subarachnoid hemorrhage, subdural hemorrhage, and multihematoma fuzzy sign.
Predictive Model Creation
Univariate logistic regression analysis revealed that several clinical variables including age, level of GCS score, mean arterial pressure, hypertension, coagulopathy, and MLR were linked to an elevated risk of tICH expansion (Table S1). In addition, multiple imaging variables, including subarachnoid hemorrhage, subdural hemorrhage, time from brain trauma to initial CT, initial tICH volume, and multihematoma fuzzy sign, were linked to an elevated risk of acute tICH expansion. The variables retained in the final multivariate logistic regression included age [odds ratio (OR)1.49; 95% confidence interval (95% CI) 0.86–2.58; 41–65 years vs. 18–40 years; OR 3.23; 95% CI 1.72–6.05; > 65 years vs. 18–40 years], baseline tICH volume (OR 3.37; 95% CI 1.87–6.08; 5–10 mL vs. < 5 mL; OR 9.04; 95% CI 4.85–16.87; > 10 mL vs. < 5 mL), time to baseline CT (OR 2.21; 95% CI 1.36–3.62; < 3 vs. ≥ 3 h), subdural hemorrhage (OR 2.72; 95% CI 1.60–4.66; yes vs. no), coagulopathy (OR 1.82; 95% CI 0.94–3.55; yes vs. no), multihematoma fuzzy sign (OR 12.41; 95% CI 7.74–19.87;; yes vs. no), and MLR (OR 2.10; 95% CI 1.38–3.19) (Table 2).
On the basis of multivariate logistic regression, a basic model and a TPHEA model for acute tICH expansion were introduced. The former consisted of initial tICH volume, time to initial CT, subdural hemorrhage, and coagulopathy, and the latter included all the risk factors in the basic model and two novel predictors of multihematoma fuzzy sign and MLR.
In discrimination, the AUC of the TPHEA model [AUC 0.90 (95% CI 0.88–0.93)] was larger than that of the basic model [AUC 0.80 (95% CI 0.77–0.84)], multihematoma fuzzy sign [AUC 0.78 (95% CI 0.74–0.81)], and MLR [AUC 0.75 (95% CI 0.70–0.77)] (Fig. 3a). In DCA, all the models performed certain clinical practicality of which the TPHEA model showed the largest net benefit between threshold probabilities 5% and 95% (Fig. 3b). Its calibration was better than that of the basic model in patients with brain contusion (Fig. 3c, d). Compared with the basic model or novel individual predictors, the TPHEA model had better performance in discrimination, calibration, and clinical utility for tICH expansion.
ROC curve, calibration curve, and clinical decision curve for basic model, TPHEA model, multihematoma fuzzy sign, and MLR in predicting acute tICH expansion. a ROC curves for basic model (black line), multihematoma fuzzy sign (blue line), MLR (green line) and TPHEA model (red line) in forecasting acute tICH expansion in the development dataset. b In the DCA curve, the net benefit curves of all predictive models are shown: yellow line exhibits the basic model, red line the TPHEA model, blue line the multihematoma fuzzy sign, green line the MLR, light gray line the net benefit when all the subjects are regarded as manifesting with acute tICH expansion, and black line the absolute net benefit when all the subjects are regarded as not manifesting with tICH expansion; red line (the TPHEA model) had the highest net benefit at any given threshold. Calibration curves of the basic model (c) and the TPHEA model (d) showing the observed versus predicted probabilities of acute tICH expansion across risk levels. The light gray line represents an ideal model, and the red line designates the observed frequencies of estimated probability. The light red area shows the 95% confidence interval of the red line
TPHEA Nomogram
A TPHEA nomogram derived from the TPHEA model was introduced to facilitate clinical application (Fig. 4). Here, all the predictors are assigned a point, and the total points are calculated by adding the points of every predictor. The nomogram has a scale from 10 to 90%. For any particular patient, the use of total points allows for the easy exploration of the corresponding probability of acute tICH expansion.
A TPHEA nomogram abstracted from the TPHEA model to forecast acute tICH expansion. For the computation of the probability of acute tICH expansion of a patient, each parameter’s points are given by corresponding score from the value axis, and the sum of the points is then plotted on the Total Points axis. The probability of acute tICH expansion of a patient is the corresponding value on the Acute tICH expansion axis
TPHEA Nomogram Validation
A total of 264 individuals with brain contusion were included in an external validation dataset from the Affiliated East Hospital of Xiamen University (Fig. 1; Table S2). Among them, 22.35% (59) had acute tICH expansion. Compared with those in the development dataset, the patients with acute tICH expansion usually have a higher mean arterial pressure, a higher MLR, a larger baseline tICH volume, a shorter time from brain trauma to baseline CT scan, and a higher frequency of hypertension, diabetes, coagulopathy, subarachnoid hemorrhage, subdural hemorrhage, and multihematoma fuzzy sign.
In the validation dataset, the TPHEA nomogram maintained optimal discrimination [AUC 0.87 (95% CI 0.81–0.92)] compared with the basic model [AUC 0.79 (95% CI 0.67–0.82)], multihematoma fuzzy sign [AUC 0.74 (95% CI 0.71–0.84)], and MLR [AUC 0.70 (95% CI 0.62–0.77)] (Fig. 5a). The calibration curve also indicated that the TPHEA nomogram exhibited good calibration for acute tICH expansion (Fig. 5b).
Discrimination and calibration of TPHEA nomogram for acute tICH expansion in the external dataset. a ROC curves for basic model, multihematoma fuzzy sign, MLR and TPHEA nomogram in predicting acute tICH expansion. b Calibration curve showing the observed versus predicted probabilities of acute tICH expansion across risk levels. The light gray line represents an ideal model, and the red line designates the observed frequencies of estimated probability. The light red area shows the 95% confidence interval of the red line
TPHEA Nomogram Performance in Subgroups
In the validation dataset, the TPHEA nomogram performance was generally consistent across different subgroups including sex, age, severity of trauma, baseline tICH volume, and coagulation function. Its discrimination was considerably better than that of the basic model across all the subgroups (Table 3). Moreover, the TPHEA nomogram exhibited a balanced performance in sensitivity and specificity.
Discussion
In this multicenter retrospective study, a novel TPHEA model was developed for acute tICH expansion among individuals with hemorrhagic brain contusion. Compared with the basic model, multihematoma fuzzy sign, and MLR, the TPHEA model had optimal discrimination, calibration, and clinical utility. On the basis of this model, a TPHEA nomogram was derived and externally validated. The main predictors of this tool are multihematoma fuzzy sign, baseline tICH volume, and inflammatory index of MLR. The TPHEA nomogram maintained an excellent and robust discrimination for tICH expansion across a wide spectrum of patients with brain contusion.
In pathophysiology, several predictive NCCT variables, including the early presentation of hematoma, large baseline tICH volume, and the presence of subdural hemorrhage and multihematoma fuzzy sign, all contribute to acute tICH expansion after brain contusion. Theoretical tICH expansion models indicated that the mechanical injury tears and shears brain parenchyma and blood vessels, resulting in initial hematoma [35, 36], and further shears rupture the surrounding microvessels, resulting in progressive bleeding [37]. A large initial tICH volume severely shears microvessels and exacerbates persistent bleeding [38]. Early presentation of hemorrhage on a CT scan may signify the early stage of acute tICH expansion, thus providing an opportunity to block persistent bleeding. The presence of subdural hemorrhage may reflect rupture to the bridging veins and the presence of venous sinuses that induce brain venous congestion and increase the risk of hematoma expansion [3]. The new NCCT marker of multihematoma fuzzy sign usually indicates the concurrence of blood clot with fresh liquid blood in contusion regions [17]. The existence of fresh liquid blood could explain the predictive ability of multihematoma fuzzy sign for acute tICH growth.
In the clinical aspect, coagulation disorder and neuroinflammation are important risk factors involved in tICH expansion. The pathophysiological mechanisms of coagulopathy after brain trauma involve platelet dysfunction, fibrinogen modifications, endogenous anticoagulation, inflammation hyperfibrinolysis, and endothelial activation, all of which may induce continued bleeding [19]. Laroche et al. reported that coagulopathy is associated with prolonged bleeding and parenchymatous hemorrhagic progression [39]. In the present study, no significant association was found between coagulopathy and tICH expansion. To date, the role of coagulation dysfunction in the progression of traumatic parenchymal hematoma remains controversial. Consistent with the current findings, several studies have reported that many patients with coagulopathy did not develop hemorrhagic expansion [40, 41]. Another explanation for this difference is that the current data analysis was underpowered to detect a significant association between coagulopathy and tICH growth. Nevertheless, coagulation dysfunction as a widely reported risk factor for tICH expansion was finally included in the TPHEA model/nomogram.
Neuroinflammation is also an indispensable manifestation of acute brain contusion linked to tICH expansion and can affect its course [42]. The invasion of monocytes from the periphery contributes to unfavorable outcomes in patients with brain contusion [43, 44]. Consistent with our previous study [18], the present work found a positive association between MLR and acute tICH expansion. The inflammatory marker of MLR reflects the activation of the innate immune system numerator and the suppression of the adaptive immune system (denominator). A high MLR may indicate a high level of intracerebral monocyte infiltration in patients with brain contusion. Given its easy accessibility, this indicator may provide new insights into neuroinflammation for promoting acute tICH expansion [18].
Consistent with its performance in the development dataset, the TPHEA nomogram maintained optimal discrimination and good calibration in an external validation dataset. Moreover, its predictive value for acute tICH expansion was relatively robust in different patient subgroups. High-risk patients could benefit from using safe cost-effective treatments consisting of hematoma/contusion evacuation, recombinant activated factor VII administration [45], and intensive surveillance. Meanwhile, low-risk patients may avoid unnecessary repeat CT scans and unnecessary intensive monitoring. In the acute phase of brain contusion, the TPHEA nomogram has the potential to optimize the personalized management and treatment of individuals.
Several comprehensive models for acute tICH expansion have been developed. First, Yuan et al. reported a risk score based on a retrospective cohort with 468 cases. Its discrimination was good (c statistic = 0.86), and its main predictors included age, brain contusion, midline shift ≥ 5 mm, platelet count, prothrombin time, d-dimer, and glucose [15]. In a retrospective study with 286 moderate or severe patients, Allison et al. developed a similar hemorrhagic progression of contusion score [11] that had an AUC curve of 0.77 and was incorporated with several predictors including age, subarachnoid hemorrhage, subdural hemorrhage, convexity skull fracture, and time to initial CT. Zhang et al. created a combined radiomics model in a small dataset of 88 patients with brain contusion [16]. The main predictors included international normalized ratio, blood platelet count, fibrinogen, and one comprehensive radiomics feature with five imaging variables. Among these models, the radiomics model exhibited the best discrimination for acute tICH expansion with an AUC curve of 0.90.
Similar predictors, such as age, subdural hemorrhage, time to baseline CT, and a combined coagulation function feature with international normalized ratio, activated partial thromboplastin time, and platelet count, were also included in the TPHEA model. In univariate regression analysis, other variables, such as GCS level, mean arterial pressure, hypertension, location of contusion, and subarachnoid hemorrhage, were found to be associated with tICH expansion. However, multivariate regression did not confirm this association and thus failed to improve the discrimination of TPHEA model/nomogram for acute tICH expansion. Therefore, these variables were not included in the final THPEA model/nomogram. The TPHEA nomogram was also incorporated with other important predictors, such as baseline tICH volume, the novel NCCT marker of multihematoma fuzzy sign, and the novel inflammatory index of MLR. As a result, its discrimination of tICH expansion was significantly improved, with an AUC curve of 0.90, which is the same as that for the radiomics model. Moreover, the TPHEA nomogram based on easily accessible clinical variables and noncontrast CT sign could be easily generalized in other trauma centers, as opposed to the radiomics model that needs complex algorithms for the extraction of imaging features.
In terms of datasets, all three reported models were derived from a single center small dataset. In particular, HCP score was developed from a dataset of patients with only moderate and severe TBI. The bias may lead to its inadaptability for patients with mild brain contusion. Meanwhile, the TPHEA nomogram originated from a multicenter larger dataset and exhibited a high and stable performance for predicting acute tICH expansion in different subgroups in an external dataset. Compared with previous models, this tool provides a more robust predicting strategy for acute tICH expansion.
Although tICH expansion has been associated with poor clinical prognosis, the relationship between acute tICH expansion and clinical outcome is controversial. First, the presence of acute tICH expansion/progression contributes to subsequent clinical deterioration and requirement for surgical intervention [8]. Chang et al. reported that patients with contusion growth > 5 mL are seven times more likely to require surgery than those whose contusions grew by < 5 mL [46]. Although univariate associations between contusion progression and short and long-term mortality have been reported [6, 14, 47], these associations have not been observed in multivariate analyses. One reason might be the inconsistent definition of acute tICH expansion. Previous combined models define tICH expansion/progression as ≥ 15%, or ≥ 25%, or ≥ 30%, or ≥ 5 mL increase to the initial CT scan [11, 15, 16]. In this study, acute tICH expansion was defined as ≥ 33% increase in size and ≥ 5 mL increase in absolute hematoma volume from the initial CT. Patients must meet both of these criteria mentioned above to be recognized as having an acute tICH expansion. Compared with that in previous studies, this high threshold of acute tICH expansion may further enhance the correlation between acute tICH expansion/progression and deterioration or poor prognosis in patients with brain contusion.
In summary, the strengths of this work include its multicenter design, large sample size, and external validation of the TPHEA nomogram for acute tICH expansion following the primary brain contusion. Multiple crucial risk factors for acute tICH expansion were incorporated into the TPHEA nomogram and significantly improved its accuracy and robustness in predicting tICH expansion. Given that it is based on several clinical and NCCT imaging variables that are easily assessed at admission, the TPHEA nomogram can serve as a practical and handy tool to support neurosurgeons in their pre-judgement for acute tICH expansion in patients with brain contusion.
Some limitations of this work should be considered during the interpretation of findings. First, a crucial limitation is the lack of a subset of patients without follow-up CT or with an urgent need for hematoma evacuation prior to follow-up CT. A large baseline tICH volume likely leads to an urgent need for hematoma evacuation or early death. Therefore, the inclusion and exclusion criteria could have potentially led to selection bias in excluding patients with a large initial hematoma volume. Subgroup analysis suggested that the discrimination of the TPHEA nomogram for acute tICH expansion was comparable between patients with small or large initial tICH volumes. Second, other variables such as fibrinogen, D-dimer, and glucose were non-routine items or lost at admission in the four hospitals. Further comparison on the predictive value of these scores/nomograms for acute tICH expansion is not possible. Finally, the TPHEA nomogram is not a substitute for a surgeon’s clinical evaluation of an individual patient and should be used with caution during clinical decision-making.
Conclusions
The main predictors of the novel TPHEA nomogram are MLR, baseline tICH volume, and multihematoma fuzzy sign. Integrating multiple hematoma fuzzy sign and MLR significantly improved the predictive value of the TPHEA nomogram for acute tICH expansion. This study provides a user-friendly tool for acute traumatic intraparenchymal hematoma expansion to optimize the individualized management and treatment of patients with brain contusion.
References
Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J Neurotrauma. 2012;29:19–31.
Adatia K, Newcombe VFJ, Menon DK. Contusion progression following traumatic brain injury: a review of clinical and radiological predictors, and influence on outcome. Neurocrit Care. 2021;34:312–24.
Regner A, Meirelles LDS, Ikuta N, Cecchini A, Simon D. Prognostic utility of circulating nucleic acids in acute brain injuries. Expert Rev Mol Diagn. 2018;18(11):925–38.
Sharma M, Mittal R, Sharma A, Gandhi A. Posttraumatic contusion: clinical and radiologic factors for progression in early postinjury period. Indian J Neurotrauma. 2016;13(1):1–6.
Yadav Y, Basoor A, Jain G, Nelson A. Expanding traumatic intracerebral contusion/hematoma. Neurol India. 2006;54(4):377.
White CL, Griffith S, Caron J-L. Early progression of traumatic cerebral contusions: characterization and risk factors. J Trauma Inj Infect Crit Care. 2009;67(3):508–15.
Narayan RK, Maas AIR, Servadei F, Skolnick BE, Tillinger MN, Marshall LF. Progression of traumatic intracerebral hemorrhage: a prospective observational study. J Neurotrauma. 2008;25(6):629–39.
Alahmadi H, Vachhrajani S, Cusimano MD. The natural history of brain contusion: an analysis of radiological and clinical progression. J Neurosurg. 2010;112(5):1139–45.
Rehman L, Afzal A, Aziz HF, Akbar S, Abbas A, Rizvi R. Radiological parameters to predict hemorrhagic progression of traumatic contusional brain injury. J Neurosci Rural Pract. 2019;10(02):212–7.
Carnevale JA, Segar DJ, Powers AY, Shah M, Doberstein C, Drapcho B, et al. Blossoming contusions: identifying factors contributing to the expansion of traumatic intracerebral hemorrhage. J Neurosurg. 2018;129(5):1305–16.
Allison RZ, Nakagawa K, Hayashi M, Donovan DJ, Koenig MA. Derivation of a predictive score for hemorrhagic progression of cerebral contusions in moderate and severe traumatic brain injury. Neurocrit Care. 2017;26(1):80–6.
Nasi D, di Somma L, Gladi M, Moriconi E, Scerrati M, Iacoangeli M, et al. New or blossoming hemorrhagic contusions after decompressive craniectomy in traumatic brain injury: analysis of risk factors. Front Neurol. 2019;9:1186.
Cepeda S, Gómez PA, Castaño-Leon AM, Munarriz PM, Paredes I, Lagares A. Contrecoup traumatic intracerebral hemorrhage: a geometric study of the impact site and association with hemorrhagic progression. J Neurotrauma. 2016;33(11):1034–46.
Juratli TA, Zang B, Litz RJ, Sitoci KH, Aschenbrenner U, Gottschlich B, et al. Early hemorrhagic progression of traumatic brain contusions: frequency, correlation with coagulation disorders, and patient outcome: a prospective study. J Neurotrauma. 2014;31(17):1521–7.
Yuan F, Ding J, Chen H, Guo Y, Wang G, Gao WW, et al. Predicting progressive hemorrhagic injury after traumatic brain injury: derivation and validation of a risk score based on admission characteristics. J Neurotrauma. 2012;29(12):2137–42.
Zhang L, Zhuang Q, Wu G, Yu J, Shi Z, Yuan Q, et al. Combined radiomics model for prediction of hematoma progression and clinical outcome of cerebral contusions in traumatic brain injury. Neurocrit Care. 2021. https://doi.org/10.1007/s12028-021-01320-2.
Sheng J, Yang J, Cai S, Zhuang D, Li T, Chen X, et al. Development and external validation of a novel multihematoma fuzzy sign on computed tomography for predicting traumatic intraparenchymal hematoma expansion. Sci Rep. 2021;11(1):1–9.
Sheng J, Li T, Zhuang D, Cai S, Yang J, Ding F, et al. The monocyte-to-lymphocyte ratio at hospital admission is a novel predictor for acute traumatic intraparenchymal hemorrhage expansion after cerebral contusion. Mediat Inflamm. 2020;2020:1–12.
Maegele M, Schöchl H, Menovsky T, Maréchal H, Marklund N, Buki A, et al. Coagulopathy and haemorrhagic progression in traumatic brain injury: advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017;16(8):630–47.
Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. 2009;40(9):2994–3000.
Rosa M, da Rocha M, Saade N, Veiga E, Romero M. Contusion contrast extravasation depicted on multidetector computed tomography angiography predicts growth and mortality in traumatic brain contusion. J Neurotrauma. 2016;33(11):1015–22.
Van BS, Groothuis OK. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011. https://doi.org/10.18637/jss.v045.i03.
Greenberg JK, Yan Y, Carpenter CR, Lumba-Brown A, Keller MS, Pineda JA, et al. Development and internal validation of a clinical risk score for treating children with mild head trauma and intracranial injury. JAMA Pediatr. 2017;171(4):342–9.
Wang H, Sun Y, Ge Y, Wu PY, Lin J, Zhao J, et al. A clinical-radiomics nomogram for functional outcome predictions in ischemic stroke. Neurol Ther. 2021;10:819–32.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36.
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Mak. 2016;26(6):565–74.
Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for some traditional and novel measures. Epidemiology. 2010;21(1):128.
Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35(29):1925–31.
Lamain-de Ruiter M, Kwee CA, Naaktgeboren CA, de Groot I, Evers IM, Groenendaal F, et al. External validation of prognostic models to predict risk of gestational diabetes mellitus in one Dutch cohort: prospective multicenter cohort study. BMJ. 2016;354:i4338.
Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the milan criteria. JAMA Surg. 2016;151(4):356–63.
Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2010;45(3):1–68.
R Core Team. R Foundation for Statistical Computing. R: a language and environment for statistical computing. http://www.R-project.org/. Accessed 10 Oct 2016.
Sullivan LM, Massaro JM, D’Agostino RB Sr. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23(10):1631–60.
Moons KGM, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Annals Intern Med. 2015;162(1):W1–73.
Simard JM, Kilbourne M, Tsymbalyuk O, Tosun C, Caridi J, Ivanova S, et al. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J Neurotrauma. 2009;26(12):2257–67.
Simard JM, Geng Z, Woo SK, Ivanova S, Tosun C, Melnichenko L, et al. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2009;29:317–30.
Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30(3):536–50.
Brouwers HB, Biffi A, Ayres AM, Schwab K, Cortellini L, Romero JM, et al. Apolipoprotein E genotype predicts hematoma expansion in lobar intracerebral hemorrhage. Stroke. 2012;43(6):1490–5.
Laroche M, Kutcher ME, Huang MC, Cohen MJ, Manley GT. Coagulopathy after traumatic brain injury. Neurosurgery. 2012;70:1334–45.
Patel Y, Hoyt B, Nakaji P, Marshall L, Holbrook T, Coimbra R, et al. Traumatic brain injury: patterns of failure of nonoperative management. J Trauma. 2000;48:367–74.
Kaups L, Davis W, Parks N. Routinely repeated computed tomography after blunt head trauma: does it benefit patients? J Trauma. 2004;56:475–80.
Sulhan S, Lyon KA, Shapiro LA, Huang JH. Neuroinflammation and blood–brain barrier disruption following traumatic brain injury: pathophysiology and potential therapeutic targets. J Neurosci Res. 2020;98:19–28.
Makinde HM, Cuda CM, Just TB, Perlman HR, Schwulst SJ. Nonclassical monocytes mediate secondary injury, neurocognitive outcome, and neutrophil infiltration after traumatic brain injury. J Immunol. 2017;199(10):3583–91.
Chou A, Krukowski K, Morganti J, Riparip LK, Rosi S. Persistent infiltration and impaired response of peripherally derived monocytes after traumatic brain injury in the aged brain. Int J Mol Sci. 2018;19(6):1616.
Stein DM, Dutton RP, Kramer ME, Scalea TM. Reversal of coagulopathy in critically ill patients with traumatic brain injury: recombinant factor VIIa is more costeffective than plasma. J Trauma. 2009;66:63–72.
Chang EF, Meeker M, Holland MC. Acute traumatic intraparenchymal hemorrhage: risk factors for progression in the early post-injury period. Neurosurgery. 2006;58(4):647–56.
Qureshi AI, Malik AA, Adil MM, Defillo A, Sherr GT, Suri MFK. Hematoma enlargement among patients with traumatic brain injury: analysis of a prospective multicenter clinical trial. J Vasc Interv Neurol. 2015;8(3):42–9.
Acknowledgements
The authors would like to thank the patients, their families, and all investigators involved in this study.
Funding
This study was supported by China Postdoctoral Science Foundation (2018M633091); the Natural Science Foundation of Guangdong Province (2019A1515010649); Medical Scientific Research Foundation of Guangdong Province, China (A2017168). Jiangtao Sheng will be funding the journal’s Rapid Service Fee for this study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ contributions
Study concept and design: JS, WC and KL; analysis and interpretation of data: JS, WC, DZ, SC and JY; drafting of the manuscript: JS and WC; data collection: DZ, TL, JY, SC, XC, XL, FT, MH and LL; critical revision of the manuscript for important intellectual content: JS, WC and KL.
Disclosures
Jiangtao Sheng, Weiqiang Chen, Dongzhou Zhuang, Tian Li, Jinhua Yang, Shirong Cai, Xiaoxuan Chen, Xueer Liu, Fei Tian, Mindong Huang, Lianjie Li and Kangsheng Li declare no conflict of interest.
Compliance with Ethics Guidelines
This study had approval from the ethics committees of the First Affiliated Hospital of Shantou University Medical College, the Affiliated Jieyang Hospital of Sun Yat-sen University, the Second Affiliated Hospital of Shantou University Medical College, and the Affiliated East Hospital of Xiamen University. This approval covered the necessary ethics approval for all study sites. The ethics approval reference number (No.: 2020-042) applies to all above ethics committees. All data are anonymous, and the requirement for informed consent was therefore waived. This work was performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Data Availability
All data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sheng, J., Chen, W., Zhuang, D. et al. A Clinical Predictive Nomogram for Traumatic Brain Parenchyma Hematoma Progression. Neurol Ther 11, 185–203 (2022). https://doi.org/10.1007/s40120-021-00306-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-021-00306-8