Abstract
New super-sensitive biomarker assay platforms for measuring Alzheimer’s disease (AD) core pathological markers in plasma have recently been developed and tested. Research findings from these technologies offer promising evidence for identifying the earliest stages of AD and correlating them with brain pathological progression. Here, we review findings using immunomagnetic reduction, one of these ultrasensitive technologies. The principles, technology and assays developed, along with selected published findings will be discussed. The major findings from this technology were significant increases of amyloid beta (Aβ) 42 and total tau (t-tau) levels in subjects clinically diagnosed with early AD when compared with cognitively normal control (NC) subjects. The composite marker of the product of Aβ42 and t-tau discriminated subjects with early AD from NC subjects with high accuracy. The potential of this technology for the purpose of early or preclinical disease stage detection has yet to be explored in subjects who have also been assessed with brain imaging and cerebrospinal fluid AD core biomarker measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Blood-based biomarkers are needed for identifying preclinical and/or high-risk subjects. |
Assays based on immunomagnetic reduction (IMR) and superconducting quantum interference device (SQUID) have consistently shown high sensitivity and specificity for discriminating between normal controls and subjects with early to mild Alzheimer’s diseases (AD). |
IMR-assayed Aβ42 in plasma increased in AD, opposite of CSF Aβ42 in AD; total tau (t-tau) levels in plasma and CSF are both increased in AD, comparing to levels in NC. Composite markers such as the product of Aβ42 and t-tau increased the sensitivity as discriminators. |
The potential to use IMR-assayed AD markers as surrogate for amyloid PET imaging to screen potential subjects for clinical trials is promising. |
Introduction
Blood-based Alzheimer’s disease (AD) biomarkers could have strategic roles in identifying suitable subjects before they are enrolled for neuroimaging and in clinical trials of disease-modifying therapeutics. Among the candidates as plasma biomarkers, the core AD markers amyloid beta (Aβ) and tau have been the most investigated. Recently, novel approaches and new technologies that increase the sensitivity and accuracy of the measurements of plasma Aβ42, total tau (t-tau), phosphorylated tau (p-tau), and neurofilament light (NFL) have emerged. There could be tremendous value in creating biomarker panels as screening tools for the assessment of degrees of AD pathology, much like hemoglobin A1C (HbA1C) for diabetes or prostate-specific antigen (PSA) for prostate cancer diagnosis.
There is a consensus that the current strategy for treating AD is not effective for slowing disease progression in subjects with clinically diagnosed AD [1]. A better strategy for modifying this disease would be to start treatments prior to the appearance of overt clinical symptoms; this requires methods to identify disease prior to cognitive impairment. As a result of recent failures in costly disease-modifying clinical trials, there is increased attention on lifestyle intervention or preventive approaches to preserve cognition with aging and prevent AD from developing [2,3,4]. One consistent issue raised with the failed clinical trials is that patients are treated too late in the disease process. Hence, the assessment of the effects of pharmacological or lifestyle approaches will benefit from the availability of routine, reliable, and easy-to-provide tests that measure the progression of disease pathological features as early as possible [5].
Using blood as the source of biomarkers has advantages of high repeatability, convenience, low cost, and minimal invasiveness. Thus, the development of blood-based molecular biomarkers to identify subjects with cognitive decline from those with normal cognition has become a research focus in recent years. If new technologies for blood biomarker measurement could provide accuracy comparable to cerebrospinal fluid (CSF) biomarkers in classifying cognitively normal control (NC), mild cognitive impairment (MCI), and subjects with early AD, the utility of blood-based biomarkers for clinical diagnosis or even preclinical stage detection of AD might become a reality.
New Ultrasensitive Biomarker Technologies
Innovative approaches and ultrasensitive technologies for blood-based biomarkers have been developed in last few years to address the limitations of the widely used enzyme-linked immunosorbent assay (ELISA) methodology. These technologies include immunomagnetic reduction (IMR), single molecule assay (SIMOA), immuno-infrared sensor technology, multimer detection system (MDS), immunoprecipitation/mass spectrometry (IP/MS), and liquid chromatography–mass spectrometry (LC–MS/MS) [6,7,8,9,10,11,12]. Except the LC–MS/MS analysis, all the technologies employ specific antibodies to target antigens, like ELISA, but incorporate new technologies to enhance sensitivity and accuracy of measurements. The MDS is a platform which uniquely targets Aβ oligomers. MS-based platforms have the advantages of obtaining both quantitative and qualitative information in plasma samples. This review will focus on IMR technology and results obtained using it, but will compare with findings using SIMOA, the main technology that has been widely used in AD biomarker research studies. We will first discuss some of the challenges in accurately and reliably measuring the AD pathological markers Aβ and tau in blood; then we review principles, assays, and findings with IMR methods. Lastly, we suggest what might be needed to bring this technology forwards as AD biomarkers in defined context of use.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Challenges in Measuring AD Pathological Components Aβ and Tau in Blood
As a result of their established association with amyloid plaques and neurofibrillary tangles, the AD brain pathological hallmarks, most biomarker studies in plasma and CSF have focused on measuring levels of Aβ (1–40) and Aβ (1–42), t-tau, and p-tau. Several studies have reported on measurements of plasma Aβ peptides in attempts to validate them as AD biomarkers, including identifying the threshold Aβ concentrations that correlate with disease stages. However, most of the previous studies based on conventional ELISA on plasma Aβ yielded conflicting results, and showed very marginal or no differences between patients with AD and NC subjects [13,14,15,16]. Problems have been identified with Aβ measurements in plasma in terms of biological and technical aspects. Firstly, the pool of plasma Aβ peptides are not stable, probably because of the presence of multiple sources of Aβ as well as ongoing production and degradation. Levels of Aβ peptides in biofluids fluctuated widely over time and varied among individuals [14]. Technically, the most commonly used analytical method ELISA involves specific antigen–antibody interactions, which can be easily affected by molecular hindrance due to high concentrations of proteins naturally present in plasma samples. In addition, levels of Aβ are also sensitive to methods of blood collection and processing and differences in the types of ELISA [17].
Both brain and peripheral tissues contribute to the pool of Aβ in the plasma. Soluble forms of Aβ species in the brain can enter the circulation through low-density lipoprotein receptor-related protein-mediated transport across the blood–brain barrier [18]. More recently, the astrocyte-mediated glymphatic transport system and meningeal lymphatic vessels have been suggested to provide important pathways for brain Aβ to enter the peripheral circulation [19,20,21]. Peripheral tissues also produce Aβ, as amyloid precursor protein (APP) mRNA has been detected in the heart, liver, kidney, pancreas, lymph nodes, spleen, skeletal muscle, aorta, lung, skin, intestines, testis, leukocytes, platelets as well as in the salivary, thyroid, and adrenal glands [22,23,24,25,26,27,28,29]. Among these tissues/cells, Aβ peptides have been biochemically or immunochemically identified in skeletal muscle, platelets, vascular walls, and liver [14, 26, 30, 31]. Platelets have been suggested to be the primary source of circulating Aβ. Upon activation, platelets secrete substantial amounts of Aβ peptides, especially Aβ40 [26]. The average level of Aβ in the serum (after platelet activation) was about 1.6-fold higher than in serum where platelets are not activated [26]. Together, these distinct reservoirs will contribute to plasma Aβ separately from what is derived from the brain.
Recent studies have found that the amount of Aβ in the brain correlates with wakefulness [32]. Sleep deprivation acutely increases levels of Aβ in the interstitial fluid (ISF). Furthermore, the concentration of Aβ in the brain fluctuates with the sleep–wake cycle [33]. It remains unclear whether such a diurnal fluctuation of Aβ concentrations occurs in the blood.
Once Aβ enters the plasma, it appears to have a short half-life. Using an approach that combined stable isotope labeling kinetics (SILK) with LC–MS/MS analysis, Ovod et al. found that Aβ isoforms have a mean half-life of approximately 3 h [8]. Interestingly, Aβ42 has a faster turnover rate than Aβ40.
The accurate measurement of free plasma Aβ levels can be confounded by its interactions with plasma/serum proteins. Aβ species are known to interact specifically or non-specifically with a large number of plasma proteins, including albumin, α2-macroglobulin, α1-antichymotrypsin, amyloid P component, complement proteins, transthyretin, apoferritin, hemoglobin, fibrinogen, apolipoproteins, and lipoproteins [31, 34,35,36,37]. In addition to binding to plasma proteins, Aβ is also known to interact with blood cells, including erythrocytes and macrophages [38]. Binding of Aβ to plasma proteins can mask the epitopes for specific antibodies used in different assays [36, 39, 40]. It has been reported that to accurately quantify this “sequestered” pool of Aβ, it is necessary to release Aβ from binding molecules [41].
Plasma levels of t-tau, p-tau, or a combination of both have also been under development as AD biomarkers. Validating plasma tau as an AD biomarker has encountered different challenges compared to Aβ. Significant changes in plasma tau between patients with AD and controls were evident in some studies [42,43,44,45]. However, the specificity of plasma tau alone as an AD biomarker appears relatively low, possibly because extracellular tau represents evidence of non-specific neuronal injury [46]. Increased plasma tau levels have been associated with several neurodegenerative diseases, not just AD [46,47,48,49]. Therefore, it has been generally accepted that plasma tau is not a reliable and specific AD biomarker [49, 50]. Similar observations can also be applied to other neural injury biomarkers present in plasma, such as NFL [51].
Innovative Technologies: Immunomagnetic Reduction (IMR)
Magnetic particles have many useful applications in biomedical field: biosensing, separation of cellular components and molecules, imaging, and delivery of therapeutics. The IMR biomarker detection system uses antibody-conjugated magnetic nanoparticles as bioprobes and a high TC, alternating current (ac) magneto-susceptometer, superconducting quantum interference device (SQUID), to detect the amounts of antigens bound to the bioprobes [52, 53]. Several assays are available from this technology for the quantification of pathological markers involved in neurodegenerative diseases (http://www.magqu.com). AD-focused IMR assays and their associated technology are described in the following sections (Fig. 1).
Assay Reagents
IMR assay reagents contain antibody-conjugated magnetic nanoparticles suspended in pH 7.4-phosphate-buffered saline (PBS). The magnetic nanoparticles are 50–60 nm in size and have magnetite (Fe3O4) in the core and hydrophilic dextran coating on the surface for antibody conjugation. The concentration of the magnetic particles is 8 mg Fe/ml. For the AD-focused markers Aβ40, Aβ42, and t-tau, the IMR assay reagents contain anti-β-amyloid (A3981, Sigma-Aldrich, St. Louis, MO, USA), anti-β-amyloid 37–42 (ab34376, Abcam, Cambridge, UK), and anti-tau (T9450, Sigma)-conjugated magnetic nanoparticles. The IMR assay reagents remain homogeneously suspended for 10 months at 2–8 °C storage [43, 52, 54].
Assay Preparation
Plasma samples are obtained from whole blood collected in K2EDTA tubes after centrifugation at 1500–2500 g for 15 min at room temperature. Aliquots of plasma samples need to be stored at − 80 °C and assayed within 1 year. During preparation of the assay, both samples and IMR reagents are brought to room temperature and mixed in differing sample-to-reagent ratios depending on the markers being measured: for example, 40 µl of plasma sample is mixed with 80 µl of IMR reagents for t-tau (MF-TAU-0060, MagQu), p-Tau (MF-PT1-0060), Aβ40 (MF-AB0-0060, MagQu); 60 µl of sample is mixed with 60 µl of reagent (MF-AB2-0060, MagQu) for Aβ42 assay. The total volume of assay reagent and sample mixture is 120 µl. Sample and IMR assay reagent are mixed by vortexing, followed by centrifugation to settle floating particulates and DNA. The tubes are immediately placed into individual holders inside the assay cells of the equipment (Model χacPro-S, MagQu) for immunoreaction.
Magnetic Signal Collection
Magnetic nanoparticles oscillate in a magnetic field generated by external ac. The feature of the magnetic signals corresponding to oscillation pace and magnitude in mixed frequency ac is termed mixed-frequency ac magnetic susceptibility, χac. In the current model of the IMR-SQUID equipment, each sample holder is surrounded by a set of excitation coils and a pickup coil. The excitation coils are driven by ac voltage generators, and magnetic signals emitted from assay mixtures are picked up by an axial gradiometer and transmitted in real time to a flux coupling coil connecting to the SQUID. The SQUID is immersed in an optimal amount of liquid nitrogen and shielded from potential noise-generating interfering environmental magnetic sources.
During sample assay, magnetic nanoparticles become larger as a result of antigen binding with antibody and eventually immune complexes form, which result in reduction of oscillation and changes in magnetic signals. Thus, by comparing magnetic signals before immune reaction (χac,0) and after immune reaction (χac), the percentage of IMR% can be calculated with the equation [(χac,0 − χac)/χac] × 100%. The real-time magneto-susceptibility during the assay is recorded for 4–5 h [10]. During the initial 100 min, high-pace magnetic signals χac are picked up, representing a stage when immune complexes are not yet formed. In the following 100 min or so, χac descends gradually during formation of immune complexes. When high-affinity binding forms stable immune complexes, χac signals also become stable. A 4- to 5-h recording provides the basis for the IMR% calculation [52, 55, 56].
From IMR% to Concentrations
The concentrations of markers being analyzed can be calculated from IMR% by reference to a standard curve specific for the marker being analyzed. An example of the equation for marker concentration (∅marker) and IMR% is shown below (A, B, γ are fitting parameters):
A standard curve for each marker is usually established first after the IMR-SQUID analyzer is assembled and set up in a laboratory. Once established, each sample assay run only required inclusion of standard proteins at the highest and lowest concentrations, along with an IMR reagent control. Currently, the IMR-SQUID analyzer is a 36-magnetic channel software-guided system (model χac-Pro-S). The equipment can analyze 3–4 different samples in duplicate if three markers, Aβ40, Aβ42, and t-tau, are to be simultaneously assayed. At completion of an assay, IMR% and corresponding concentrations are calculated automatically by the machine software. The lower detection limits of various IMR assays are in the low pico- to femto-gram per milliliter range according to MagQu company’s information (Table 1). These values indicate that IMR-SQUID technology is an ultrasensitive immunoassay platform for the quantification of low-abundance proteins.
Long-Term Sample Storage
The effect of long-term sample storage on IMR assays was evaluated recently in plasma samples collected from 53 NCs and 46 subjects with amnestic mild cognitive impairment (aMCI) or AD [57]. In this study, plasma samples stored at − 80 °C for at least 3 months after sample collection were compared to measurements of aliquots from the same case after storage for 1.1–5.4 years. The results showed that Aβ40 and Aβ42 levels were not affected by storage for up to 5.4 years at − 80 °C, but t-tau levels were reduced by 20% after storage for 2 years at − 80 °C, and by 33% after storage for 4–5 years. The study also assessed whether storage-related decreases in t-tau measures affected the ability of the composite marker of Aβ42 × t-tau to discriminate between AD and NC but found little effect. It is unclear why t-tau levels are more prone to decline during storage. It remains to be determined whether plasma t-tau is more susceptible to aggregation, degradation, or association with other molecules during or after low temperature storage; these factors could result in loss of epitopes or weaker binding with antibody. A comprehensive study that assessed the effects of various pre-analytical procedures on plasma AD markers showed that most factors affecting Aβ also affected t-tau, but decrease in t-tau was particularly noticeable when tubes with half-full samples were examined [17]. There were 12–14% reductions in plasma t-tau levels in half-filled aliquot tubes, which would suggest that sample drying (freezer burn) or oxidation was a factor in t-tau stability.
Current Findings in AD Biomarkers Using IMR Assays
Most of the developed IMR assays for AD pathological markers have focused on Aβ40, Aβ42, and t-tau and their measurements in clinically diagnosed patients. The IMR assay for p-tau is new with only one publication [54] and will not be featured in this review. Most published findings have assessed the potential of Aβ40, Aβ42, and t-tau in discriminating clinically diagnosed groups; some have also assessed the relationship between levels of IMR plasma markers and amyloid PET and MRI imaging. A summary of major findings in research using IMR technology is outlined in Table 2 [52, 55, 58,59,60,61,62]. The table is organized from most recent publications to earliest publications. In addition to highlights of the major finding, the number of subjects studied and type of IMR assays used are also indicated.
Levels of IMR Plasma AD Markers
Compiled in Table 3 are the ranges of means for each marker reported in the IMR studies. The ranges of the mean Aβ42 levels were 15.3–16.1 for NC and 16.8–34.2 pg/ml AD, while the ranges of mean t-tau levels were 13.5–20.5, 29.7–33.5, and 34.5–47.1 pg/ml for NC, clinically diagnosed MCI, and AD, respectively. Aβ40 concentrations detected have higher values than t-tau and Aβ42; the ranges of the means were 59.2–65.8, 41.4–48.0, and 36.9–53.2 pg/ml for NC, MCI, and AD, respectively.
As IMR employs a very different technology from frequently used ELISA, the reported range of AD marker levels can be expected to be different from those reported using ELISA. The best example is illustrated in a systematic review and meta-analysis of plasma t-tau [63]. The plasma t-tau values by ELISA were between 200 and 900 pg/ml. However, the digital ELISA technology, SIMOA (Quanterix, MA, USA), which is gaining popularity for plasma tau measurements, detected t-tau levels in low picogram per milliliiter values. The discrepancy in detected values in published studies is most likely related to the antibodies used and assay features of the platforms; however, sample processing, demographics and cognitive stages of the study subjects, and experimental design will also be factors. The comparison of biological markers between different platforms has to be approached with caution because of these many factors involved [64].
How plasma marker levels assayed by IMR compared to CSF levels assayed by ELISA was investigated in a recent study consisting of 43 NC and 63 European Caucasian subjects with AD [65]. The ratios of plasma to CSF Aβ42 were 1.6% in NC and 4.1% in patients with AD. In this study, the increase in Aβ42 levels in plasma samples from subjects with AD was highly significant (P < 0.001), consistent with other IMR findings. A moderately negative correlation between plasma and CSF Aβ42 levels (r = − 0.352) in AD and a weakly positive correlation in NC (r = 0.186) were detected. In this study, Teunissen et al. suggested two potential explanations for the inverse correlation in AD [65]. Firstly, it could be due to a disease-associated compensatory mechanism that increases central to peripheral clearance of Aβ42 when brain amyloid accumulates. Secondly, the differences could be caused by the abundance of carrier proteins to transport Aβ.
To elucidate the dynamic mechanisms affecting CSF and plasma Aβ levels, more research is needed. As the assay platform is a technical factor affecting the outcomes of assays, more valid comparisons between CSF and plasma markers can be made if samples are analyzed with the same assay. In a study using INNO-BIA ELISA for both plasma and CSF samples (Fujirebio Europe NV, formerly Innogenetics, Belgium), the mean plasma Aβ42 concentrations were 36.9 ± 11.7 pg/ml from 426 AD samples and 38.2 ± 11.9 pg/ml from 373 subjects with aMCI [66]. The ratios of plasma to CSF Aβ42 levels were 5.1% and 4.4%, respectively, for AD and aMCI groups. The study did not detect any correlation between CSF and plasma samples for Aβ42 levels.
Although the ELISA-based platform is adequate for measuring AD core markers in CSF samples as CSF contains lower concentrations of potentially interfering proteins, there is an advantage if the same markers in CSF and plasma samples from the same patient could be measured by the same assay platform. This could provide a better assessment of the interrelationship of these markers between central and peripheral systems.
There have been no published IMR-assayed values for CSF AD markers, but to perform initial assessment of IMR for CSF samples, we carried out a pilot study using CSF samples from nine elderly subjects enrolled at the Banner Sun Health Research Institute (BSHRI, Sun City, AZ, USA). Although undiluted CSF samples were within the dynamic range of IMR assays, a 20-fold dilution was needed to achieve optimal IMR sensitivity. The CSF Aβ42 and t-tau concentrations detected were approximately 3- to 4-fold greater than reported for CSF Aβ42 levels assayed by multiplexed ELISA platforms, such as the multiplex xMAP Luminex platform (Luminex Corp., Austin, TX, USA) and the INNO-BIA AlzBio3 kit (Innogenetics, Ghent, Belgium) (our unpublished data). Further studies to directly compare IMR-assayed CSF and IMR-assayed plasma results from the same subjects could determine if they correlate. The differences in the detected levels between platforms might not be surprising. IMR is an ultrasensitive platform and has best sensitivity in the low picogram per milliliter range. To determine protein concentrations which are abundant, it requires samples to be diluted to ensure the signals are within optimal range of linearity. When the detected results are corrected by the dilution factor, the numbers could represent extrapolated results in some cases.
Plasma AD Marker Levels by Clinical Diagnosis Groups
IMR assays have detected significant or almost significant increases in plasma Aβ42 in clinically diagnosed AD cases. Aβ42 levels in plasma from subjects with AD measured by IMR assays were 1.1- to 2.2-fold greater than the levels in NC plasma. Similarly, IMR t-tau levels were significantly increased in subjects with MCI and early AD when compared with NC. The t-tau levels in plasma from subjects with AD were 1.7- to 4-fold greater than the levels detected in the NC group [61, 62]. However, in subjects with AD, IMR-measured Aβ40 levels changed in the opposite direction to those of Aβ42 and t-tau with Aβ40 levels in subjects with AD being 61–80% of levels in NC subjects [59, 60, 62]. In fact, not all IMR-assayed Aβ40 levels in plasma showed consistent findings. Chiu et al. detected a broad range of plasma Aβ40 levels in subjects with very mild AD or mild-to-severe AD and the results were unable to be used for disease group classification [58]. Another way of analyzing the biomarker utility of these measures was to calculate the ratio of Aβ42/Aβ40. Significantly higher ratios were obtained in subjects with AD and MCI when compared with NC values [58, 62]. Although Aβ40 IMR assay seems to be robust, the exact types of Aβ peptides being measured in plasma samples require further characterization as the antibody used in the assay was not targeting C-terminal epitopes [67].
For comparison, we present results from studies using the ultrasensitive SIMOA technology. Using samples obtained from the community-based Framingham Heart Study (FHS), and the multi-cohort Memeto study, Pase et al. used SIMOA to assess plasma t-tau for predicting AD in a total of 1852 participants [45]. They found that FHS subjects with plasma t-tau levels greater than the median had a 62% greater risk of dementia and a 76% greater risk of AD dementia. The findings were replicated in the Memeto cohort in the same study. The median baseline levels of plasma t-tau were around 4 pg/ml. Another large study with a total of 1284 participants from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and the BioFinder study to determine whether plasma t-tau could be useful for AD biomarkers also used the SIMOA method to measure plasma t-tau [49]. This study also detected plasma t-tau in a narrow range of low picogram per milliliter values. Notably, there was no significant correlation between CSF and plasma t-tau levels from this study, even though plasma t-tau and CSF t-tau were all significantly increased in the disease groups.
SIMOA was also used to determine the utility of plasma AD markers to screen subjects with subjective memory decline [68]. The presence of abnormal amyloid in the brain was determined by CSF AD core marker levels or 11C-Pittsburgh compound (11C-PiB) PET imaging. They found that plasma t-tau levels did not correlate with CSF amyloid status, and single Aβ markers did not perform as well as the ratio of plasma Aβ species, Aβ42/Aβ40. It is important to point out that this study also showed low levels of plasma Aβ42 and t-tau in PiB amyloid normal and abnormal groups. The plasma Aβ42 level was 1.3% of CSF Aβ42 level in the amyloid abnormal group and 0.9% in the amyloid normal group, whereas plasma t-tau level was 1.2% of CSF t-tau level in the amyloid normal group and 0.6% in the amyloid abnormal groups. These findings demonstrated the SIMOA platform’s ultrasensitivity for measuring both plasma Aβ and t-tau.
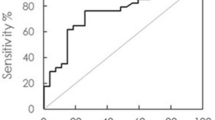
Findings from Receiver Operating Characteristic (ROC) Curve Analysis
The potential clinical utility of IMR-assayed AD markers to identify AD or MCI has been the focus in several studies. In one study consisting of 68 NC, 24 subjects with MCI due to AD, and 31 subjects with early AD, plasma Aβ42 and t-tau showed high sensitivity and specificity (greater than 90%) in discriminating between NC and subjects clinically diagnosed with AD [55]. However, these markers lacked the ability to discriminate MCI from early-stage AD as the ROC sensitivity and specificity were lower than 80% [55, 58]. There was a slight improvement of sensitivity and specificity observed by multiplying the concentrations of Aβ42 and t-tau markers (the product), but this measure was still not useful for distinguishing between MCI and early AD [55].
Application of the Aβ42/Aβ40 ratio has been investigated in IMR studies. As IMR-assayed Aβ42 increased and Aβ40 decreased in subjects with MCI and AD when compared with NC, the increase in the ratios become significant between all pairs of group comparisons [62]. This ratio used for classifying AD and NC outperformed single markers in one earlier IMR study [52]. In a later study, Aβ42/Aβ40 plasma ratio had significant association with the PiB standardized uptake value ratio (SUVR; R2 = 0.326–0.449, P < 0.001).
The first IMR study outside Taiwan was a study of 16 NC and 16 subjects with AD carried out in a Caucasian population at the BSHRI (Sun City, AZ, USA). These subjects had an older mean age (NC 81.9 ± 6.0 years; AD 82.5 ± 5.6 years) and lower Mini Mental State Examination (MMSE) score (NC 29.3 ± 1.2; AD 16.1 ± 4.0) than the Chinese-ethic subjects studied by IMR in Taiwan [61]. The sensitivity for distinguishing AD and cognitively normal was 56% for Aβ42 and 75% for t-tau; the specificity was 81% for Aβ42 and 88% for t-tau. Using the product of Aβ42 and t-tau increased sensitivity to 88%, but with no improvement in specificity. In the same study, ROC analysis was also performed in the combined BSHRI and National Taiwan University Hospital (NTUH) cohorts. Aβ42 and t-tau markers had 89% sensitivity and specificity of at least 90% or greater. However, using their product with a cutoff value of greater than 383 (pg/ml)2 to identify AD, the sensitivity was improved to 96%, but not the specificity [61]. In an earlier study conducted by Chiu et al., a higher cutoff value of greater than 455 (pg/ml)2 for the product of two markers achieved 96% sensitivity and 97% specificity for distinguishing between NC and AD, and 80% sensitivity and 82% specificity between MCI and AD groups [58].
IMR AD Markers and Imaging Markers
IMR-assayed plasma AD markers have been assessed for their relationship with brain atrophy, measured by MRI, and amyloid deposition, measured by 11C-PiB SUVR [59, 60, 62]. Chiu et al. reported that plasma t-tau levels could be predicted by a regression model that includes total hippocampus volume (predicting 0.394 of the variance in the plasma tau level) and gray matter density of the superior frontal gyrus (predicting 0.054 of the variance in the plasma tau level) [59]. Plasma t-tau levels did not predict PiB+ SUVR in bilateral frontal, parietal, temporal lobes, and the precuneus [62]. Higher plasma t-tau levels in SIMOA were associated with smaller hippocampi, poorer cognitive function, as well as pathological evidence of microinfarct and neurofibrillary tangles [45]. IMR plasma t-tau levels had a strong correlation with MMSE in a PiB+ group (r = − 0.624, P < 0.001), but not in a PiB− group (r = − 0.295, P = 0.072) [60].
Brain amyloid measured by 11C-PiB PET imaging significantly correlated with the ratios of IMR plasma Aβ42/Aβ40 (R2 = 0.326–0.449, P < 0.01) [62]. IMR-assayed plasma markers (Aβ42 and t-tau) have also been studied for their ability to distinguish between PiB-positive (PiB+) and PiB-negative (PiB−) subjects [60]. Using the cutoff values for Aβ42 and t-tau of 21.92 and 37.54 pg/ml made it possible to discriminate between PiB− and PiB+ subjects with 100% sensitivity and 100% specificity. To identify the PiB+ group, lower Aβ42 and t-tau cutoff values (16.81 and 25.57 pg/ml) were needed to achieve 86.7% and 93.3% sensitivity and 82% and 100% specificity (respectively for Aβ42 and t-tau). By comparison, using a cortical SUVR 1.2167 cutoff value for PiB imaging achieved 93% sensitivity and 100% specificity could be for identifying the PiB+ group. Thus, the IMR-assayed plasma AD markers had high concordance with PiB positivity, at least as good as CSF core AD markers [69]. The possibility of using IMR-assayed AD markers as surrogates for amyloid PET imaging to screen potential subjects for clinical trials shows promise.
Clinical Potentials of IMR-Assayed Plasma AD Markers
Validated blood biomarkers for detecting preclinical stages of AD, identifying subjects at risk of AD, and pre-screening subjects in clinical trials prior to expensive amyloid or tau imaging would have significant use in research and clinical practice. To achieve this will require routine, reliable assays that can sensitively and accurately detect the earliest biochemical changes that distinguish ongoing pathological abnormality from normal aging in the brain. This still poses great challenges at both technical and knowledge levels. The former relies on innovation in the technology, and the latter is dependent on further understanding of the early-stage events that precipitate AD pathology. IMR-SQUID technology has demonstrated consistent ultrasensitivity to detect Aβ42 and t-tau levels in plasma from clinically diagnosed groups. On the basis of the published findings, it would be beneficial to incorporate IMR-based blood tests in initial and follow-up diagnostic procedures in clinics. However, it remains to be determined whether IMR-assayed plasma markers could be used as prediction markers. Further vigorous research in well-designed multicenter longitudinal studies is needed to determine the potential of using IMR assays for plasma biomarkers to identify at-risk, preclinical subjects, and subjects with MCI in those who also have undergone PiB imaging and CSF core AD marker testing.
Blood-based biomarkers could be the quickest and most straightforward way of detecting the pathological and biochemical changes associated with clinical symptoms as in most community-based clinical practice in the USA, CSF samples are not usually collected. On the basis of combined use of CSF and imaging biomarker findings, Jack et al. proposed that alterations in Aβ levels in CSF could reflect the earliest pathological events, which precede the amyloid deposition observed by PET imaging [70]. Aβ in CSF of subjects with no cognitive impairment results from normal metabolism of brain-derived APP. As mentioned previously, this material is rapidly degraded. Now, it is believed that Aβ levels in CSF start to decrease as this material is incorporated into brain or cerebrovascular plaques. The Aβ levels present in plasma is affected by more complicated factors. Nevertheless, if the more complex sequence of changes of biomarkers in plasma compared to CSF and brain imaging could be established, it would help determine feasibility of plasma changes as a substitute for CSF markers. Additionally, it could also help assess how the periphery might be involved in the pathological changes in CSF and brain. As plasma AD biomarkers have potential utility in monitoring the effects of drugs in clinical trials, whether these markers could be used to assess the effects of current acetylcholineesterase inhibitor treatment in patients with MCI and AD will also be a relevant research area to be investigated.
Conclusions
The IMR-SQUID technology has demonstrated consistent findings in its ability to distinguish cognitively normal subjects from those with very early AD and MCI, as well as to predict brain atrophy, amyloid positivity determined by 11C-PiB PET, and cognitive decline in subjects with MCI. This situates IMR assays among those promising blood-based biomarker platforms for diagnosis of AD at early stages. The research findings also provide strong rationales for further validation of IMR technology in large-cohort longitudinal study to confirm the accuracy for predicting conversion to AD in subjects with MCI and identifying Aβ amyloid PET positive subjects. The National Institute on Aging and Alzheimer’s Association (NIA-AA) diagnostic criteria for AD had recently proposed preclinical diagnosis to include imaging and CSF amyloid/tau/neurodegeneration (A/T/N) biomarkers [71,72,73]. New technologies available have significantly improved the accuracy of detecting AD plasma markers. This opens the opportunity of exploring the potentials of A/T/N biomarkers in plasma. As IMR technology has assays for detecting Aβ, t-tau, p-tau and NFL, future study could determine whether an IMR-based plasma panel of A/T/N biomarkers could have the sensitivity to characterize the stages of AD pathological development as CSF biomarkers have shown.
References
Aisen PS, Andrieu S, Sampaio C, et al. Report of the task force on designing clinical trials in early (predementia) AD. Neurology. 2011;76:280–6.
Lista S, Dubois B, Hampel H. Paths to Alzheimer’s disease prevention: from modifiable risk factors to biomarker enrichment strategies. J Nutr Health Aging. 2015;19:154–63.
Galvin JE. Prevention of Alzheimer’s disease: lessons learned and applied. J Am Geriatr Soc. 2017;65:2128–33.
Aridi YS, Walker JL, Wright ORL. The association between the Mediterranean dietary pattern and cognitive health: a systematic review. Nutrients. 2017;9:nu9070674.
Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92.
Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature. 2018;554:249–54.
Nabers A, Ollesch J, Schartner J, et al. An infrared sensor analysing label-free the secondary structure of the Abeta peptide in presence of complex fluids. J Biophotonics. 2016;9:224–34.
Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13:841–9.
Song L, Lachno DR, Hanlon D, et al. A digital enzyme-linked immunosorbent assay for ultrasensitive measurement of amyloid-beta 1-42 peptide in human plasma with utility for studies of Alzheimer’s disease therapeutics. Alzheimers Res Ther. 2016;8:58–0225.
Yang CC, Yang SY, Chieh JJ, et al. Biofunctionalized magnetic nanoparticles for specifically detecting biomarkers of Alzheimer’s disease in vitro. ACS Chem Neurosci. 2011;2:500–5.
Mawuenyega KG, Kasten T, Sigurdson W, Bateman RJ. Amyloid-beta isoform metabolism quantitation by stable isotope labeled kinetics. Anal Biochem. 2013;440:56–62.
Wang MJ, Yi S, Han J, et al. Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimers Res Ther. 2017. https://doi.org/10.1186/s13195-017-0324-0.
Song F, Poljak A, Valenzuela M, Mayeux R, Smythe GA, Sachdev PS. Meta-analysis of plasma amyloid-beta levels in Alzheimer’s disease. J Alzheimers Dis. 2011;26:365–75.
Roher AE, Esh CL, Kokjohn TA, et al. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimers Dement. 2009;5:18–29.
Hansson O, Zetterberg H, Vanmechelen E, et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging. 2010;31:357–67.
Frankfort SV, Tulner LR, van Campen JP, Verbeek MM, Jansen RW, Beijnen JH. Amyloid beta protein and tau in cerebrospinal fluid and plasma as biomarkers for dementia: a review of recent literature. Curr Clin Pharmacol. 2008;3:123–31.
Rozga M, Bittner T, Batrla R, Karl J. Preanalytical sample handling recommendations for Alzheimer’s disease plasma biomarkers. Alzheimers Dement (Amst). 2019;11:291–300. https://doi.org/10.1016/j.dadm.2019.02.002.
Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid beta-peptide elimination from the brain. J Neurochem. 2010;115:1077–89.
Peng W, Achariyar TM, Li B, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215–25. https://doi.org/10.1016/j.nbd.2016.05.015.
Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Investig. 2017;127:3210–9.
Da MS, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560:185–91.
Selkoe DJ, Podlisny MB, Joachim CL, et al. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci USA. 1988;85:7341–5.
Schubert W, Prior R, Weidemann A, et al. Localization of Alzheimer beta A4 amyloid precursor protein at central and peripheral synaptic sites. Brain Res. 1991;563:184–94.
Sandbrink R, Masters CL, Beyreuther K. Beta A4-amyloid protein precursor mRNA isoforms without exon 15 are ubiquitously expressed in rat tissues including brain, but not in neurons. J Biol Chem. 1994;269:1510–7.
Nordstedt C, Naslund J, Thyberg J, Messamore E, Gandy SE, Terenius L. Human neutrophil phagocytic granules contain a truncated soluble form of the Alzheimer beta/A4 amyloid precursor protein (APP). J Biol Chem. 1994;269:9805–10.
Li QX, Whyte S, Tanner JE, Evin G, Beyreuther K, Masters CL. Secretion of Alzheimer’s disease Abeta amyloid peptide by activated human platelets. Lab Investig. 1998;78:461–9.
Joachim CL, Mori H, Selkoe DJ. Amyloid beta-protein deposition in tissues other than brain in Alzheimer’s disease. Nature. 1989;341:226–30.
Golde TE, Estus S, Usiak M, Younkin LH, Younkin SG. Expression of beta amyloid protein precursor mRNAs: recognition of a novel alternatively spliced form and quantitation in Alzheimer’s disease using PCR. Neuron. 1990;4:253–67.
Catteruccia N, Willingale-Theune J, Bunke D, et al. Ultrastructural localization of the putative precursors of the A4 amyloid protein associated with Alzheimer’s disease. Am J Pathol. 1990;137:19–26.
Van Nostrand WE, Melchor JP. Disruption of pathologic amyloid beta-protein fibril assembly on the surface of cultured human cerebrovascular smooth muscle cells. Amyloid. 2001;8(Suppl 1):20–7.
Kuo YM, Kokjohn TA, Watson MD, et al. Elevated abeta42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AbetaPP metabolism. Am J Pathol. 2000;156:797–805.
Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7.
Roh JH, Huang Y, Bero AW, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4:150ra122.
Zamolodchikov D, Berk-Rauch HE, Oren DA, et al. Biochemical and structural analysis of the interaction between beta-amyloid and fibrinogen. Blood. 2016;128:1144–51.
Shih YH, Tsai KJ, Lee CW, et al. Apolipoprotein C-III is an amyloid-beta-binding protein and an early marker for Alzheimer’s disease. J Alzheimers Dis. 2014;41:855–65.
Kuo YM, Emmerling MR, Lampert HC, et al. High levels of circulating Abeta42 are sequestered by plasma proteins in Alzheimer’s disease. Biochem Biophys Res Commun. 1999;257:787–91.
Chuang JY, Lee CW, Shih YH, Yang T, Yu L, Kuo YM. Interactions between amyloid-beta and hemoglobin: implications for amyloid plaque formation in Alzheimer’s disease. PLoS One. 2012;7:e33120.
Crane A, Brubaker WD, Johansson JU, et al. Peripheral complement interactions with amyloid beta peptide in Alzheimer’s disease: 2. Relationship to amyloid beta immunotherapy. Alzheimers Dement. 2018;14:243–52.
Van Giau V, An SSA. Epitope mapping immunoassay analysis of the interaction between beta-amyloid and fibrinogen. Int J Mol Sci. 2019;20:ijms20030496.
Matsumoto A, Minami M, Matsumoto R. The beta-amyloid epitope masking activity in human brain is identified as albumin. Neuroreport. 1997;8:3297–301.
Kuo YM, Kokjohn TA, Kalback W, et al. Amyloid-beta peptides interact with plasma proteins and erythrocytes: implications for their quantitation in plasma. Biochem Biophys Res Commun. 2000;268:750–6.
Zetterberg H, Wilson D, Andreasson U, et al. Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther. 2013;5:9.
Yang SY, Chiu MJ, Chen TF, et al. Analytical performance of reagent for assaying tau protein in human plasma and feasibility study screening neurodegenerative diseases. Sci Rep. 2017;7:9304.
Tatebe H, Kasai T, Ohmichi T, et al. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol Neurodegener. 2017;12:63.
Pase MP, Beiser AS, Himali JJ, et al. Assessment of plasma total tau level as a predictive biomarker for dementia and related endophenotypes. JAMA Neurol. 2019;76(5):598–606.
Kovacs GG, Andreasson U, Liman V, et al. Plasma and cerebrospinal fluid tau and neurofilament concentrations in rapidly progressive neurological syndromes: a neuropathology-based cohort. Eur J Neurol. 2017;24:1326-1e77.
Startin CM, Ashton NJ, Hamburg S, et al. Plasma biomarkers for amyloid, tau, and cytokines in Down syndrome and sporadic Alzheimer’s disease. Alzheimers Res Ther. 2019;11:26.
Staffaroni AM, Kramer AO, Casey M, et al. Association of blood and cerebrospinal fluid tau level and other biomarkers with survival time in sporadic Creutzfeldt-Jakob disease. JAMA Neurol. 2019;76(8):969–77.
Mattsson N, Zetterberg H, Janelidze S, et al. Plasma tau in Alzheimer disease. Neurology. 2016;87:1827–35.
Mielke MM, Hagen CE, Wennberg AMV, et al. Association of plasma total tau level with cognitive decline and risk of mild cognitive impairment or dementia in the Mayo Clinic study on aging. JAMA Neurol. 2017;74:1073–80.
Olsson B, Portelius E, Cullen NC, et al. Association of cerebrospinal fluid neurofilament light protein levels with cognition in patients with dementia, motor neuron disease, and movement disorders. JAMA Neurol. 2019;76(3):318–25.
Chiu MJ, Yang SY, Chen TF, et al. New assay for old markers-plasma beta amyloid of mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer Res. 2012;9:1142–8.
Huang KW, Yang SY, Hong YW, et al. Feasibility studies for assaying alpha-fetoprotein using antibody-activated magnetic nanoparticles. Int J Nanomed. 2012;7:1991–6. https://doi.org/10.2147/ijn.s28245.
Yang CC, Chiu MJ, Chen TF, Chang HL, Liu BH, Yang SY. Assay of plasma phosphorylated tau protein (threonine 181) and total tau protein in early-stage Alzheimer’s disease. J Alzheimers Dis. 2018;61:1323–32.
Yang SY, Chiu MJ, Chen TF, Horng HE. Detection of plasma biomarkers using immunomagnetic reduction: a promising method for the early diagnosis of Alzheimer’s disease. Neurol Ther. 2017;6:37–56.
Chieh JJ, Wei WC, Liao SH, et al. Eight-channel AC magnetosusceptometer of magnetic nanoparticles for high-throughput and ultra-high-sensitivity immunoassay. Sensors (Basel). 2018;18:s18041043.
Chiu MJ, Lue LF, Sabbagh MN, Chen TF, Chen HH, Yang SY. Long-term storage effects on stability of Abeta1-40, Abeta1-42, and total tau proteins in human plasma samples measured with immunomagnetic reduction assays. Dement Geriatr Cogn Dis Extra. 2019;9:77–86.
Chiu MJ, Yang SY, Horng HE, et al. Combined plasma biomarkers for diagnosing mild cognition impairment and Alzheimer’s disease. ACS Chem Neurosci. 2013;4:1530–6.
Chiu MJ, Chen YF, Chen TF, et al. Plasma tau as a window to the brain-negative associations with brain volume and memory function in mild cognitive impairment and early Alzheimer’s disease. Hum Brain Mapp. 2014;35:3132–42.
Fan LY, Tzen KY, Chen YF, et al. The relation between brain amyloid deposition, cortical atrophy, and plasma biomarkers in amnesic mild cognitive impairment and Alzheimer’s disease. Front Aging Neurosci. 2018;10:175. https://doi.org/10.3389/fnagi.2018.00175.
Lue LF, Sabbagh MN, Chiu MJ, et al. Plasma levels of Abeta42 and tau identified probable Alzheimer’s dementia: findings in two cohorts. Front Aging Neurosci. 2017;9:226. https://doi.org/10.3389/fnagi.2017.00226.
Tzen KY, Yang SY, Chen TF, et al. Plasma Abeta but not tau is related to brain PiB retention in early Alzheimer’s disease. ACS Chem Neurosci. 2014;5:830–6.
Shanthi KB, Krishnan S, Rani P. A systematic review and meta-analysis of plasma amyloid 1–42 and tau as biomarkers for Alzheimer’s disease. SAGE Open Med. 2015;3:2050312115598250. https://doi.org/10.1177/2050312115598250.
O’Bryant SE, Lista S, Rissman RA, et al. Comparing biological markers of Alzheimer’s disease across blood fraction and platforms: comparing apples to oranges. Alzheimers Dement (Amst). 2015;3:27–34. https://doi.org/10.1016/j.dadm.2015.12.003.
Teunissen CE, Chiu MJ, Yang CC, et al. Plasma amyloid-beta (Abeta42) correlates with cerebrospinal fluid Abeta42 in Alzheimer’s disease. J Alzheimers Dis. 2018;62:1857–63.
Hanon O, Vidal JS, Lehmann S, et al. Plasma amyloid levels within the Alzheimer’s process and correlations with central biomarkers. Alzheimers Dement. 2018;14:858–68.
Lue LF, Guerra A, Walker DG. Amyloid beta and tau as Alzheimer’s disease blood biomarkers: promise from new technologies. Neurol Ther. 2017;6:25–36.
Verberk IMW, Slot RE, Verfaillie SCJ, et al. Plasma amyloid as pre-screener for the earliest Alzheimer’s pathological changes. Ann Neurol. 2018;84(5):648–58.
Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer’s disease concord with amyloid-beta PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14(11):1470–81.
Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16.
Jack CR Jr, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–47.
Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62.
Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323.
Acknowledgements
Funding
The authors are supported by State of Arizona funding ADHS Grant no. ADHS14-052688 (Lue), and National Institute on Aging, 5P20GM109025 and Keep Memory Alive Foundation (Sabbagh). No funding or sponsorship was received for this study or publication of this article.
Medical Writing and/or Editorial Assistance
We thank Douglas G. Walker, Ph.D. for English editing of this manuscript (http://www.expertsciencewriting.com).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Lue: the author has served as a technical consultant for MagQu, Ltd. (Oct 2018–Feb 2019). She has no proprietary interest in the investigational products or of the company. Kuo: no conflict of interest. Sabbagh: the author served as an investigator in the biomarker development plan. He has no proprietary interest in the investigational products or of the company.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.9959051.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License ( http://creativecommons.org/licenses/by-nc/4.0/ ), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lue, LF., Kuo, YM. & Sabbagh, M. Advance in Plasma AD Core Biomarker Development: Current Findings from Immunomagnetic Reduction-Based SQUID Technology. Neurol Ther 8 (Suppl 2), 95–111 (2019). https://doi.org/10.1007/s40120-019-00167-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-019-00167-2