Abstract
Introduction
Multiple sclerosis (MS) patients of African descent have increased risk for disease progression and may be less responsive to disease-modifying therapy.
Methods
Patients in the CARE-MS studies received alemtuzumab 12 mg/day [initial alemtuzumab treatment (IAT); baseline: 5 days; 12 months later: 3 days] or subcutaneous interferon beta-1a (SC IFNB-1a) 3 ×/week. Core study outcomes were compared between treatment groups. In the extension study CAMMS03409, SC IFNB-1a-treated patients switched to alemtuzumab [delayed alemtuzumab treatment (DAT)]. Data from IAT and DAT arms were pooled to assess outcomes through 6 years post alemtuzumab initiation; IAT patients had an additional 2 years of follow-up in TOPAZ.
Results
Of 1200 CARE-MS patients, 43 (4%) were of African descent (35 IAT; 8 DAT) and received alemtuzumab in the 2-year core and/or 6-year extension; 29 (67%) remained on study at the time of analysis (24 IAT patients completed year 8 post alemtuzumab; 5 DAT patients completed year 6 post alemtuzumab). In year 2, annualized relapse rate (ARR; 0.09 versus 0.42), percentage of patients with improved Expanded Disability Status Scale (EDSS; 18% versus 11%), 6-month confirmed disability improvement (CDI; 28% versus 13%), no evidence of disease activity (55% versus 13%), and cumulative brain volume loss (BVL; − 0.55% versus − 1.32%) favored alemtuzumab versus SC IFNB-1a. Alemtuzumab remained efficacious at year 6 (pooled IAT/DAT) and at year 8 (IAT only) post alemtuzumab (ARR: 0.15 and 0.30; improved EDSS: 17% and 25%; CDI: 47% and 55%; BVL: − 1.14% and − 0.70%, respectively). No safety signals were unique to this population.
Conclusions
Alemtuzumab was efficacious in a small cohort of relapsing-remitting MS patients of African descent over 8 years. Safety was consistent with the overall CARE-MS population, although the small sample size may have prevented the detection of known low-frequency adverse events.
ClinicalTrials.gov Registration Numbers
CARE-MS I, II, extension, TOPAZ: NCT00530348, NCT00548405, NCT00930553, NCT02255656.
Funding
Sanofi (Cambridge, MA, USA) and Bayer HealthCare Pharmaceuticals (Leverkusen, Germany).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Even though patients of African descent with multiple sclerosis (MS) have an increased risk for disease progression compared with their white counterparts, they remain an understudied population. |
Evidence suggests that patients of African descent may be less responsive to some disease-modifying therapies. |
To help address this gap in the literature and inform therapeutic decisions, we evaluated the efficacy and safety of alemtuzumab in MS patients of African descent over 8 years in the pooled CARE-MS trials and their extension studies. |
What was learned from the study? |
Alemtuzumab efficacy in a small cohort of MS patients of African descent was comparable with the overall (largely white) study population over 8 years, and included improvements in their clinical and radiologic outcomes. |
No safety signals unique to this population were observed; however, the small sample size may have limited the detection of known rare adverse events. |
Introduction
African ancestry is a predictive factor for disease progression in multiple sclerosis (MS) [1]. Patients of African descent may display rapid disability progression and a higher magnetic resonance imaging (MRI) disease burden compared with white patients [2,3,4,5,6]. Furthermore, while evidence suggests that patients of African descent are less responsive to some disease-modifying therapies (DMTs) [7, 8], this remains an understudied population.
Alemtuzumab (LEMTRADA®; Sanofi, Cambridge, MA) is a humanized monoclonal antibody effective for treatment of relapsing–remitting MS (RRMS). In two phase 3 clinical trials (CARE-MS I [NCT00530348] and II [NCT00548405]), alemtuzumab improved clinical and radiologic outcomes compared with subcutaneous interferon beta-1a (SC IFNB-1a; Rebif®; EMD Serono Inc., Rockland, MA) in RRMS patients who were either treatment naive (CARE-MS I) or had an inadequate response to prior therapy (CARE-MS II) [9, 10]. Clinical and MRI improvements were maintained over a total of 8 years in two consecutive open-label extension studies (CAMMS03409 [NCT00930553] [11,12,13] and TOPAZ [NCT02255656] [14, 15]). Alemtuzumab is dosed as two annual courses, with additional courses as needed in the European Union (up to two) and the United States (no restriction) [16, 17].
Studies investigating DMTs in MS patients of African descent are limited, in part because these patients are underrepresented in clinical trial populations [18, 19]. The objective of this analysis is to examine the efficacy and safety of alemtuzumab in RRMS patients of African descent over 8 years in the pooled CARE-MS I and II clinical trials and their extension studies.
Methods
Study Design, Patients, and Procedures
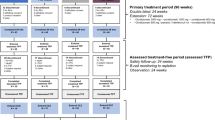
The CARE-MS I and II studies were randomized, rater-blinded, active controlled, head-to-head trials published previously [9, 10]. Patients with active RRMS were randomized to either intravenous (IV) alemtuzumab 12 mg/day on 5 consecutive days at baseline and on 3 consecutive days 12 months later or to SC IFNB-1a 44 µg 3 ×/week. In the extension study [11,12,13], patients randomized to alemtuzumab (initial alemtuzumab treatment; IAT) in the core studies could receive additional courses of alemtuzumab (12 mg/day on 3 consecutive days ≥ 12 months after the previous course) as needed for disease activity (≥ 1 protocol-defined relapse or ≥ 2 new/enlarging T2 hyperintense and/or gadolinium [Gd]-enhancing brain or spinal cord lesions on MRI) at the investigator’s discretion. Treatment with other approved DMTs was also permitted. Patients who received SC IFNB-1a in the core studies discontinued that therapy at the start of the extension study and were invited to initiate alemtuzumab (delayed alemtuzumab treatment; DAT) according to the same 5-day/3-day dosing schedule, with additional courses or other DMT as needed. Follow-up is ongoing in the TOPAZ study, wherein patients completing the extension study could receive additional alemtuzumab ≥ 12 months after the previous course at the investigator’s discretion (no defined disease criteria) or receive another DMT at any time [14, 15].
Patients self-identified their race from a predefined list of races including “American Indian or Alaska Native,” “Asian,” “Black,” “Native Hawaiian or Other Pacific Islander,” “White,” or “Other.”
Assessments
Details regarding efficacy end points have been described previously [9,10,11,12]. Confirmed relapses met the protocol-defined criteria of increase in the Expanded Disability Status Scale (EDSS) score and duration of ≥ 48 h in the absence of fever. EDSS evaluations were done quarterly. Improved, worsened, or stable EDSS scores were defined as ≥ 1.0-point decrease, ≥ 1.0-point increase, or ≤ 0.5-point change in either direction, respectively, since alemtuzumab initiation. Confirmed disability worsening (CDW) was defined as ≥ 1.0-point EDSS score increase (≥ 1.5 if baseline EDSS score = 0) confirmed over 6 months. Confirmed disability improvement (CDI) was defined as a 1.0-point decrease from baseline confirmed over 6 months and was assessed only in patients with baseline EDSS scores ≥ 2.0. No evidence of disease activity (NEDA) was defined as absence of both clinical disease activity (relapse and 6-month CDW) and MRI disease activity (new Gd-enhancing T1 lesions on current MRI or new/enlarging T2 hyperintense lesions since last MRI). Assessment of brain volume loss (BVL) was based on median percent change in brain parenchymal fraction (BPF) from baseline. Patients had MRI scans annually, scored by blinded imaging specialists at NeuroRx Research (Montréal, Canada; for lesion-based analyses) and the Cleveland Clinic MS MRI Analysis Center (Cleveland, OH, USA; for BPF analyses).
Patients were evaluated for safety throughout the studies and were monitored for autoimmune adverse events (AEs) by monthly questionnaires, complete blood count (CBC), serum creatinine, urinalysis, and quarterly thyroid function testing for 48 months after the last course of alemtuzumab. Safety is reported by year post-alemtuzumab initiation; AEs, serious AEs, and medical events of interest (MEOIs) were recorded. Serious AEs were defined as any AE resulting in death, life-threatening experience, prolonged hospitalization, significant disability, congenital anomaly, or any AE considered serious by the medical investigator. Infusion-associated reactions (IARs) were defined as any AE with onset during or ≤ 24 h after the end of infusion. MEOIs included autoimmune cytopenias, CBC values below threshold levels, anti-glomerular basement membrane disease, pregnancy, thyroid disorders, and cervical dysplasia.
Statistical Analysis
Analyses were based on all available pooled CARE-MS I and CARE-MS II data without imputation through the end of year 8. Efficacy end points from the 2-year core studies are reported separately by treatment arm for patients of African descent who were treated with either alemtuzumab or SC IFNB-1a. To maximize available longer-term data, efficacy and safety data from both treatment arms were pooled by year post-alemtuzumab initiation. Pooling was in effect through year 6 post alemtuzumab; only IAT patients had follow-up data for years 7 and 8 post alemtuzumab. Efficacy outcomes are also presented without pooled treatment arms (Supplementary Figs. 1, 2, and 3).
Efficacy outcomes over 8 years. Results are shown for alemtuzumab- and SC IFNB-1a-treated patients in the 2-year core studies (left panels) and pooled patients from years 1–8 after initiation of alemtuzumab (right panels). Year 7 and year 8 outcomes represent IAT patients only. a Yearly ARR. b Percentage of patients with improved, stable, and worsened EDSS scores from core study baseline to the specified time point. c Kaplan-Meier estimates of the percentages of patients free of 6-month CDW. d Kaplan-Meier estimates of the percentages of patients with 6-month CDI. ARR annualized relapse rate, CDI confirmed disability improvement, CDW confirmed disability worsening, EDSS Expanded Disability Status Scale, IAT initial alemtuzumab treatment, SC IFNB-1a subcutaneous interferon beta-1a, Y year. aCategories may not sum appropriately because of rounding
Annual NEDA and freedom from MRI lesions over 8 years. Results are shown for alemtuzumab- and SC IFNB-1a-treated patients in the 2-year core studies (left panels) and pooled patients in years 2, 6, and 8 after initiation of alemtuzumab (right panels). Year 8 outcomes represent IAT patients only. a Percentage of patients achieving annual NEDA. b Percentage of patients free of new Gd-enhancing T1 lesions. c Percentage of patients free of new/enlarging T2 hyperintense lesions. d Percentage of patients free of new T1 hypointense lesions. CI confidence interval, Gd gadolinium, IAT initial alemtuzumab treatment, MRI magnetic resonance imaging, NEDA no evidence of disease activity, SC IFNB-1a subcutaneous interferon beta-1a, Y year. aAmong patients of African descent in the CARE-MS trials, 62% who received alemtuzumab and 36% who received SC IFNB-1a were free of Gd-enhancing T1 lesions at core study baseline
Annualized relapse rate (ARR) was determined using negative binomial regression with robust variance estimation. Percentages of patients free of 6-month CDW or achieving 6-month CDI were determined using Kaplan-Meier (KM) estimates. KM estimates of alemtuzumab-treated patients achieving CDI in the core study were based on patients who enrolled in the extension study, and KM estimates of SC IFNB-1a-treated patients achieving CDI in the core study were based on patients who enrolled in the core study. Freedom from MRI lesions was summarized descriptively with percentages, and confidence intervals (CIs) were obtained using the normal approximation to the binomial distribution. At visits where the sample size was small, exact CIs were computed. Safety data are reported as incidences (percentage of patients with ≥ 1 event). Exposure-adjusted incidence rates were reported per 100 patient-years (100 × [number of patients with the specific event divided by total follow-up time in years among patients at risk of initial occurrence of the event during the specified time interval]).
Compliance with Ethics Guidelines
The CARE-MS I and II studies, CARE-MS extension, and the TOPAZ study were registered with ClinicalTrials.gov (NCT00530348, NCT00548405, NCT00930553, and NCT02255656, respectively). The studies were conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The study protocol, informed consent forms, and other study-related documents were reviewed and approved by the local independent ethics committees and institutional review boards. Written informed consent was obtained from all patients.
Results
Patient Disposition, Baseline Characteristics, and Additional Treatment
Of the 1200 patients in the alemtuzumab (N = 811) and SC IFNB-1a (N = 389) treatment arms from the core CARE-MS studies, 46 were of African descent and had core study treatment with either alemtuzumab (n = 35 [CARE-MS I: n = 11; CARE-MS II: n = 24]) or SC IFNB-1a (n = 11 [CARE-MS I: n = 3; CARE-MS II: n = 8]); the total number of patients exposed to alemtuzumab in either the core and/or the extension was 43. Of the 40 (87%) patients who entered the extension, 27 IAT patients and 5 DAT patients completed year 6 post alemtuzumab; 24 IAT patients completed year 8 post alemtuzumab (Fig. 1). Over 8 years, 11 IAT patients discontinued for various reasons, including withdrawal of consent (n = 2), lost to follow-up (n = 2), other reasons (n = 2), not entering the CARE-MS extension (n = 2), and not entering TOPAZ (n = 3). Through the end of the DAT assessment period, six patients discontinued because of withdrawal of consent (n = 3), physician decision (n = 1), pregnancy (n = 1), and not entering the CARE-MS extension (n = 1).
Baseline characteristics in alemtuzumab-treated patients of African descent were similar to those of the overall study population, except the African descent population had a higher proportion of female patients and higher mean T2 hyperintense lesion volumes (Table 1).
Of the 40 patients who entered the extension, 14 (35%) (IAT: n = 11; DAT: n = 3) received no additional alemtuzumab courses and no other DMT in the extension and TOPAZ. Eighteen patients (45%) received no additional courses of alemtuzumab, and 12 (30%), 7 (18%), and 3 (8%) patients received 1, 2, and 3 additional courses, respectively. Reasons for additional courses were relapse activity (57.1%), MRI activity (22.9%), both relapse and MRI activity (14.3%), both relapse and EDSS progression (2.9%), and no reason provided (2.9%).
Efficacy Outcomes
Core Study with SC IFNB-1a Comparator
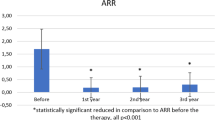
In patients of African descent, ARR in year 2 was significantly reduced with alemtuzumab compared with SC IFNB-1a treatment (0.09 versus 0.42, p = 0.037), and 91% of alemtuzumab-treated patients were relapse free (Fig. 2a). EDSS scores were stable or improved from baseline to year 2 in 74% of patients treated with alemtuzumab compared with 78% with SC IFNB-1a; improvement was seen in 18% of alemtuzumab-treated patients versus 11% of SC IFNB-1a-treated patients (Fig. 2b). Over 2 years, 78% (95% CI 60–89%) of alemtuzumab-treated patients and 80% (95% CI 41–95%) of SC IFNB-1a-treated patients remained free of 6-month CDW (Fig. 2c), and 28% (95% CI 13–54%) versus 13% (95% CI 2–61%), respectively, achieved 6-month CDI (Fig. 2d). In year 2, 55% of alemtuzumab-treated patients achieved NEDA compared with 13% of SC IFNB-1a-treated patients (Fig. 3a). More alemtuzumab-treated patients than SC IFNB-1a-treated patients of African descent were free of new Gd-enhancing T1 lesions (89% versus 75%; Fig. 3b), new/enlarging T2 hyperintense lesions (61% versus 13%; Fig. 3c), and new T1 hypointense lesions (88% versus 75%; Fig. 3d) in year 2. Alemtuzumab slowed BVL by 58% versus SC IFNB-1a over 2 years; median percent BVL in alemtuzumab-treated patients from core study baseline through year 2 was −0.55% (Fig. 4).
Change in BPF over time in pooled patients. Results are shown for alemtuzumab- and SC IFNB-1a-treated patients in the 2-year core studies (left panel) and pooled patients from years 1–8 after initiation of alemtuzumab (right panel). Year 7 and year 8 outcomes represent IAT patients only. BPF brain parenchymal fraction, IAT initial alemtuzumab treatment, SC IFNB-1a subcutaneous interferon beta-1a, Y year
Six-year Outcomes (Pooled Initial and Delayed Alemtuzumab-treated Patients)
Relapse rates remained low (range 0.10–0.23 per year), and 83–91% of patients per year were relapse free through year 6 post alemtuzumab (Fig. 2a). Cumulative ARR from years 0–6 was 0.17. Median EDSS score change from baseline to year 6 was + 0.25; scores were stable in 47% of patients and improved in 17% of patients at year 6 with respect to core study baseline (Fig. 2b). Over 6 years post alemtuzumab, 72% (95% CI 54–83%) of patients were free of 6-month CDW (Fig. 2c), and 47% (95% CI 29–70%) of patients achieved 6-month CDI (Fig. 2d). The majority (55%) of patients of African descent achieved annual NEDA in year 6, with 83% and 61% free of clinical disease activity and MRI disease activity, respectively (Fig. 3a). In year 6, high proportions of patients were free of new Gd-enhancing T1 lesions (77%; Fig. 3b), new/enlarging T2 hyperintense lesions (61%; Fig. 3c), and new T1 hypointense lesions (81%; Fig. 3d). Median percent BVL from baseline to year 6 was − 1.14% (Fig. 4), and annual BVL was not more than − 0.28% per year in years 3–6.
Outcomes at Year 8 (Initial Alemtuzumab-Treated Patients)
IAT patients of African descent showed a low ARR (0.30) at year 8 with 79% free of relapse (Supplementary Fig. 1a). Cumulative ARR from years 0–8 in the IAT arm was 0.20. EDSS scores were improved (25%) or stable (35%) at year 8 compared with baseline, 55% (95% CI 34–71%) of patients were free of 6-month CDW over 8 years, and 57% (95% CI 36–80%) achieved CDI (Supplementary Fig. 1b–d). In year 8, 40% of patients achieved NEDA, with 75% and 53% free of clinical disease activity and MRI disease activity, respectively (Supplementary Fig. 2). All patients of African descent were free of new Gd-enhancing T1 lesions in year 8, 53% were free of new/enlarging T2 hyperintense lesions, and 80% were free of new T1 hypointense lesions. Median percent BVL from baseline to year 8 was − 0.70% (Supplementary Fig. 3), and annual BVL was + 0.26% in each of years 7 and 8.
Safety
The low number of patients of African descent treated with SC IFNB-1a may confound incidence comparisons between the two treatment arms during the core study. However, the incidences of overall serious AEs over 2 years were similar between groups (alemtuzumab, 22.9% versus SC IFNB-1a, 25.0%), and incidences of infections were not higher in alemtuzumab- versus SC IFNB-1a-treated patients (alemtuzumab: 77.1% versus SC IFNB-1a: 87.5%). No serious infections occurred in the SC IFNB-1a group compared with serious infections in two patients in the alemtuzumab group.
In pooled IAT and DAT patients, the incidence of infections was highest in year 1 (74%) and lowest in year 8 (32%; Table 2). In all, five patients (11.6%) had serious infections over 8 years post alemtuzumab, including one patient with uterine infection and cesarean section wound infection in year 1 post alemtuzumab (recovered), one patient with grade 3 varicella zoster virus meningitis in year 1 (recovered), one patient with grade 3 Legionella sepsis in year 1 and severe Legionella pneumonia sepsis in year 7 (recovered), one patient with grade 4 sepsis during hospitalization for ischemic colitis and acute tubular necrosis in year 4 (recovered), and one with grade 3 pneumonia and grade 2 sepsis in year 7 (recovered) [20].
IARs were frequent (course 1: 88%; course 2: 73%; course 3: 82%; course 4: 60%; course 5: 33%). One serious IAR occurred as a grade 3 allergic reaction during course 3. The patient was treated with IV corticosteroids and recovered 13 days after diagnosis.
The incidence of thyroid AEs peaked in year 3 (12.5%) but subsequently declined (Table 2); the same trend was seen in the overall population [14, 15] (Coles et al. in preparation). Overall incidence of thyroid AEs in the African descent subgroup over 8 years was 27.9% compared with > 40% in the overall CARE-MS population [14, 15] (Coles et al. in preparation). The KM estimate for thyroid AEs over 8 years in the African descent subgroup was 31.8%. No serious thyroid AEs were reported. There were two cases of immune thrombocytopenia (ITP), with onset at 4 years and 6 years after initiating alemtuzumab and 15 months and 11 months after the last dose of alemtuzumab [21]. Both patients required second-line therapy with rituximab after first-line therapy with IV immunoglobulin and corticosteroids. After rituximab treatment, both patients were in remission from ITP for > 11 months at the time of last follow-up. There was one case of nephropathy in the African descent subgroup, which occurred in year 2 as grade 3 membranous glomerulonephritis [22] and was treated with angiotensin-converting enzyme inhibitors and diuretics. The patient was in spontaneous remission 45 months after diagnosis, until last follow-up almost 2 years later, with glomerular filtration rate within normal limits.
There was a single case of malignancy that occurred in year 1 post alemtuzumab as breast cancer. The patient underwent mastectomy and breast reconstruction and was in remission at 13 months after diagnosis with no evidence of malignancy.
Discussion
Patients of African descent have increased risk of developing MS compared with their white counterparts, along with greater morbidity [23,24,25]. MS disease in many of these patients is more aggressive [25], characterized by more rapid disability progression [1, 5, 6], a higher incidence of cerebellar dysfunction [26], and a greater risk for severe disability and ambulatory disability [3, 26]. Opticospinal MS, accelerated thinning of the retinal nerve fiber layer, and a greater loss of visual acuity are more frequent in patients of African descent [2, 27]. Increased MRI-detected tissue damage is evident in these patients compared with white patients, including higher T2 hyperintense and T1 hypointense lesion volumes. Additionally, brain atrophy rates in patients of African descent have been reported to be nearly double those of white patients (gray matter: − 0.9% versus − 0.5% per year, p = 0.02; white matter: − 0.7% versus − 0.3% per year, p = 0.04; whole brain: − 0.5% versus − 0.3%, p = 0.08) [6, 28,29,30,31]. Consistent with trends in MRI-measured damage, greater cognitive impairment has been documented in patients of African descent compared with white patients [25, 28, 32]. These disease features in patients of African descent are also evident on patient-reported disability measures [33].
In CARE-MS patients of African descent, increased disease burden at baseline versus the overall study population manifested mainly as increased T2 hyperintense lesion volume; greater disability was not evident in these patients. This may be explained by the low numbers of patients of African descent (i.e., 4% of the overall CARE-MS study population), prior treatment exposure in most of these patients, or the selection for patients in a relatively early stage of disease imposed by inclusion criteria of the study.
In addition to a more aggressive disease course, patients of African descent may also have reduced responses to DMT. Treatment with SC IFNB-1a showed reduced efficacy in patients of African descent (n = 36) compared with white patients on relapse and MRI lesion outcomes in the EVIDENCE study [7]. Similar efficacy outcomes in 76 Afro-Caribbean patients treated with IFNB (either intramuscular IFNB-1a, SC IFNB-1a, or IFNB-1b) were reported in an uncontrolled observational study [34], and a retrospective chart review comparing 66 patients of African descent with white patients showed greater EDSS worsening on DMT (mainly IFNB or glatiramer acetate) in the African descent group [8]. Newer DMTs may hold more promise for efficacy in these patients. A real-world retrospective study showed similar responses to treatment with dimethyl fumarate when comparing patients of African descent with white patients [35]. Natalizumab treatment showed improvements in relapse rates, Gd-enhancing lesions, and T2 hyperintense lesions in patients of African descent over 2 years compared with placebo [36]. However, no active comparator data or long-term data describing natalizumab use in the African descent population are available.
Given the lower efficacy of some DMTs in patients of African descent and a lack of data on newer drugs, our findings on alemtuzumab effectiveness over 8 years in these patients are relevant to practitioners and patients despite the small sample size. Alemtuzumab treatment in patients of African descent improved clinical and MRI outcomes over 2 years compared with SC IFNB-1a, and outcomes over 6 and 8 years in this subgroup were generally comparable with those observed in the overall CARE-MS study population [11, 12, 14, 15] (Coles et al. in preparation). Proportions of patients with 6-month CDI at year 8 are higher in the African descent subgroup than the overall CARE-MS populations (CARE-MS I: 41%; CARE-MS II: 47%; African descent: 57%) [14, 15]. This increase appears to be driven by CDI events rather than selective dropout of patients with poorer outcomes. Proportions of patients free of 6-month CDW were lower in the African descent subgroup than overall CARE-MS patients at year 8 (CARE-MS I: 71%; CARE-MS II: 64%; African descent: 55%) [14, 15] and were driven by CDW events. However, for both of these outcomes, the low numbers of patients "at risk" in the analysis in later years led to more variability in KM estimates, as reflected by their large CIs. Similarly, BVL data may have been affected by low patient numbers in the African descent subgroup, evidenced by the apparent uptick in brain volume at years 7 and 8. Alternatively, a higher degree of inflammation in patients of African descent may have contributed to an increase in BVL in later years.
Alemtuzumab has previously shown efficacy in other underrepresented patient populations. Treatment with alemtuzumab improved relapse rates, disability outcomes, and freedom from MRI lesions in a cohort of Korean patients with RRMS over 1.5 years [37].
Alemtuzumab depletes circulating T and B lymphocytes, leading to a distinct pattern of immune cell repopulation [38, 39]. A relative increase in immunoregulatory T cells and decrease in proinflammatory cytokines during and after repopulation may underlie prolonged clinical efficacy in the absence of regular dosing [40, 41]. Relative increases in immunoregulatory B and natural killer cells may also contribute to alemtuzumab’s effect [42,43,44].
The safety profile in alemtuzumab-treated patients of African descent was similar to that of the overall CARE-MS population [9,10,11,12, 14, 15] (Coles et al. in preparation), and no new safety signals were evident in this subgroup of patients. Incidences of infections or malignancies were not increased compared with the overall CARE-MS population. The frequency of thyroid AEs peaked in year 3 post alemtuzumab, as expected, and they occurred at annual incidences similar to those observed in the overall cohort. ITP and nephropathy autoimmune AEs occurred in few patients, consistent with the overall study population, and risk did not appear to increase with time. AEs associated with alemtuzumab treatment in clinical trials and post-marketing experience include IARs, increased frequency of infection and the potential for opportunistic infections, secondary autoimmunity (thyroid disorders, ITP, nephropathies, autoimmune cytopenias, autoimmune hepatitis, and other less common autoimmune events), acute acalculous cholecystitis, and cardiovascular and pulmonary events possibly related to infusion. Awareness of these rare but serious risks by clinicians is important for prompt recognition and optimal management of patients treated with alemtuzumab [16, 17].
The small sample size of patients is the primary limitation of our analyses. This factor hinders the interpretability of AE incidence data in this population, especially for known low-frequency AEs, and may have also impacted efficacy data, particularly in the later years. Additionally, a comparator arm was not available during the extension studies.
Conclusion
We conclude that alemtuzumab improves efficacy outcomes over 2 years in CARE-MS patients of African descent compared with SC IFNB-1a, and efficacy was maintained over 6 and 8 years in a smaller subset of these patients. There were no safety concerns with alemtuzumab unique to patients of African descent from the CARE-MS studies. Findings were promising; however, further confirmation in a larger study or a real-world setting is needed to characterize the efficacy and safety of alemtuzumab in this understudied patient population.
References
Ferreira Vasconcelos CC, Cruz Dos Santos GA, Thuler LC, Camargo SM, Papais Alvarenga RM. African ancestry is a predictor factor to secondary progression in clinical course of multiple sclerosis. ISRN Neurol. 2012; 2012:410629.
Cree BA, Khan O, Bourdette D, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology. 2004;63(11):2039–45.
Kaufman MD, Johnson SK, Moyer D, Bivens J, Norton HJ. Multiple sclerosis: severity and progression rate in African Americans compared with whites. Am J Phys Med Rehabil. 2003;82(8):582–90.
Kister I, Chamot E, Bacon JH, et al. Rapid disease course in African Americans with multiple sclerosis. Neurology. 2010;75(3):217–23.
Marrie RA, Cutter G, Tyry T, Vollmer T, Campagnolo D. Does multiple sclerosis-associated disability differ between races? Neurology. 2006;66(8):1235–40.
Weinstock-Guttman B, Ramanathan M, Hashmi K, et al. Increased tissue damage and lesion volumes in African Americans with multiple sclerosis. Neurology. 2010;74(7):538–44.
Cree BA, Al-Sabbagh A, Bennett R, Goodin D. Response to interferon beta-1a treatment in African American multiple sclerosis patients. Arch Neurol. 2005;62(11):1681–3.
Klineova S, Nicholas J, Walker A. Response to disease modifying therapies in African Americans with multiple sclerosis. Ethn Dis. 2012;22(2):221–5.
Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–28.
Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–39.
Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology. 2017;89(11):1117–26.
Havrdova E, Arnold DL, Cohen JA, et al. Alemtuzumab CARE-MS I 5-year follow-up: durable efficacy in the absence of continuous MS therapy. Neurology. 2017;89(11):1107–16.
Ziemssen T, Thomas K. Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: an update on the clinical trial evidence and data from the real world. Ther Adv Neurol Disord. 2017;10(10):343–59.
Comi G, Arnold DL, Boyko AN, et al. Alemtuzumab improves clinical and MRI disease activity outcomes, including slowing of brain volume loss, in RRMS patients over 8 years: CARE-MS I follow-up (TOPAZ study). Mult Scler. 2018;24:S702–3.
Singer BA, Alroughani R, Broadley S, et al. Alemtuzumab improves clinical and MRI disease activity outcomes including slowing of brain volume loss, in RRMS patients over 8 years: CARE-MS II follow-up (TOPAZ study). Mult Scler. 2018;24:S493–4.
LEMTRADA (alemtuzumab) [Prescribing Information]. Genzyme Corporation, USA.
LEMTRADA [Summary of Product Characteristics] April 2019. Diegem, Belgium: Sanofi Belgium.
Khan O, Williams MJ, Amezcua L, et al. Multiple sclerosis in US minority populations: clinical practice insights. Neurol Clin Pract. 2015;5(2):132–42.
Branson RD, Davis K Jr, Butler KL. African Americans’ participation in clinical research: importance, barriers, and solutions. Am J Surg. 2007;193(1):32–9.
Wray S, Havrdova E, Snydman DR, et al. Infection risk with alemtuzumab decreases over time: pooled analysis of 6-year data from the CAMMS223, CARE-MS I, and CARE-MS II studies and the CAMMS03409 extension study. Mult Scler. 2018:1352458518796675.
Cuker A, Bass AD, Nadj C, et al. Immune thrombocytopenia in alemtuzumab-treated MS patients: incidence, detection, and management. Mult Scler. 2019:1352458518816612.
Phelps R, Winston JA, Wynn D, et al. Incidence, management, and outcomes of autoimmune nephropathies following alemtuzumab treatment in patients with multiple sclerosis. Mult Scler. 2019:1352458519841829.
Amezcua L, Rivas E, Joseph S, Zhang J, Liu L. Multiple sclerosis mortality by race/ethnicity, age, sex, and time period in the United States, 1999–2015. Neuroepidemiology. 2018;50(1–2):35–40.
Langer-Gould A, Brara SM, Beaber BE, Zhang JL. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology. 2013;80(19):1734–9.
Rivas-Rodriguez E, Amezcua L. Ethnic considerations and multiple sclerosis disease variability in the United States. Neurol Clin. 2018;36(1):151–62.
Naismith RT, Trinkaus K, Cross AH. Phenotype and prognosis in African-Americans with multiple sclerosis: a retrospective chart review. Mult Scler. 2006;12(6):775–81.
Kimbrough DJ, Sotirchos ES, Wilson JA, et al. Retinal damage and vision loss in African American multiple sclerosis patients. Ann Neurol. 2015;77(2):228–36.
Al-Kawaz M, Monohan E, Morris E, et al. Differential impact of multiple sclerosis on cortical and deep gray matter structures in African Americans and Caucasian Americans. J Neuroimaging. 2017;27(3):333–8.
Caldito NG, Saidha S, Sotirchos ES, et al. Brain and retinal atrophy in African-Americans versus Caucasian-Americans with multiple sclerosis: a longitudinal study. Brain. 2018;141(11):3115–29.
Howard J, Battaglini M, Babb JS, et al. MRI correlates of disability in African-Americans with multiple sclerosis. PLoS One. 2012;7(8):e43061.
Petracca M, Zaaraoui W, Cocozza S, et al. An MRI evaluation of grey matter damage in African Americans with MS. Mult Scler Relat Disord. 2018;25:29–36.
Buchanan RJ, Martin RA, Wang S, Kim M. Racial analyses of longer-stay nursing home residents with multiple sclerosis. Ethn Dis. 2006;16(1):159–65.
Ventura RE, Antezana AO, Bacon T, Kister I. Hispanic Americans and African Americans with multiple sclerosis have more severe disease course than Caucasian Americans. Mult Scler. 2017;23(11):1554–7.
Jeannin S, Deschamps R, Chausson N, Cabre P. Response to interferon-beta treatment in Afro-Caribbeans with multiple sclerosis. Mult Scler Int. 2011;2011:950126.
Zhovtis Ryerson L, Green R, Confident G, et al. Efficacy and tolerability of dimethyl fumarate in White-, African- and Hispanic-Americans with multiple sclerosis. Ther Adv Neurol Disord. 2016;9(6):454–61.
Cree BA, Stuart WH, Tornatore CS, et al. Efficacy of natalizumab therapy in patients of African descent with relapsing multiple sclerosis: analysis of AFFIRM and SENTINEL data. Arch Neurol. 2011;68(4):464–8.
Kim H, Lee EJ, Kim SK, Kim KK, Lim YM. Efficacy and safety of alemtuzumab in Korean multiple sclerosis patients. Mult Scler Relat Disord. 2019;30:247–51.
Cox AL, Thompson SA, Jones JL, et al. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur J Immunol. 2005;35(11):3332–42.
Hu Y, Turner MJ, Shields J, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology. 2009;128(2):260–70.
Zhang X, Tao Y, Chopra M, et al. Differential reconstitution of T cell subsets following immunodepleting treatment with alemtuzumab (anti-CD52 monoclonal antibody) in patients with relapsing-remitting multiple sclerosis. J Immunol. 2013;191(12):5867–74.
De Mercanti S, Rolla S, Cucci A, et al. Alemtuzumab long-term immunologic effect: Treg suppressor function increases up to 24 months. Neurol Neuroimmunol Neuroinflamm. 2016;3(1):e194.
Kim Y, Kim G, Shin HJ, et al. Restoration of regulatory B cell deficiency following alemtuzumab therapy in patients with relapsing multiple sclerosis. J Neuroinflamm. 2018;15(1):300.
Lund BT, Traboulsee A, Javed A, et al. Leukocyte repopulation following alemtuzumab treatment in relapsing-remitting MS contains multiple regulatory immune cell types. Mult Scler. 2017;24:S506–7.
Gross CC, Ahmetspahic D, Ruck T, et al. Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3(6):e289.
Acknowledgements
The authors and Sanofi would like to thank the patients for their participation in the trials, as well as the CARE-MS I and II Steering Committees, along with the CAMMS03409 and TOPAZ investigators. CARE-MS I, CARE-MS II, the CAMMS03409 extension study, and TOPAZ were supported by Sanofi and Bayer HealthCare Pharmaceuticals.
Funding
The study was supported by Sanofi (Cambridge, MA, USA) and Bayer HealthCare Pharmaceuticals (Leverkusen, Germany). All authors had full access to study data and take responsibility for the integrity of the data and accuracy of the data analysis, and have given their approval for this version to be published. The journal’s Rapid Service Fee was provided by Sanofi.
Medical Writing, Editorial and Other Assistance
Critical review of the manuscript was provided by Darren P. Baker, PhD, Ericka M. Bueno, PhD, and Colin Mitchell, PhD, of Sanofi. Additional statistical support was provided by Cytel, Inc. (Cambridge, MA, USA). Editorial and writing assistance was provided by Valerie P. Zediak, PhD, and Ritama Gupta, PhD, of Eloquent Scientific Solutions, Philadelphia, PA, USA, and was funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Annette F. Okai reports receiving speaking and consulting fees from Biogen, Genentech, Novartis, Sanofi, Teva Neuroscience, and TG Therapeutics and research support from Alexion, Novartis, Sanofi, and Sun Pharma. Lilyana Amezcua reports receiving consulting fees from Celgene and Genzyme and research support from Biogen and Novartis. Regina R. Berkovich reports receiving consulting fees and fees for serving on an advisory board from Acorda, Avanir, Bayer, Biogen, Novartis, Questcor, Sanofi, and Teva. Angel R. Chinea reports receiving speaking/consulting fees from Allergan, Biogen, Genentech, Novartis, Sanofi, and Teva Neuroscience and research support from Novartis. Keith R. Edwards reports receiving speaking and consulting fees from Biogen and Genzyme and research support from Biogen, Eli Lilly, Genentech, Novartis, and Sanofi. Brian Steingo reports receiving speaking and consulting fees and/or grant/research support from Acorda, Biogen, EMD Serono, Mallinckrodt, Novartis, Sanofi, and Teva. Aljoeson Walker reports receiving consulting fees and fees for serving on an advisory board from Bayer, Biogen, EMD Serono, Genentech, and Sanofi. Alan K. Jacobs reports receiving personal compensation as an employee of Sanofi. Nadia Daizadeh reports receiving personal compensation as an employee of Sanofi. Mitzi J. Williams reports receiving consulting/speaking fees from Biogen, EMD Serono, Genentech, Novartis, Sanofi, and Teva Neuroscience.
Compliance with Ethics Guidelines
The studies were conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The study protocol, informed consent forms, and other study-related documents were reviewed and approved by the local independent ethics committees and institutional review boards. Written informed consent was obtained from all patients.
Data Availability
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.9918761.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Okai, A.F., Amezcua, L., Berkovich, R.R. et al. Efficacy and Safety of Alemtuzumab in Patients of African Descent with Relapsing-Remitting Multiple Sclerosis: 8-Year Follow-up of CARE-MS I and II (TOPAZ Study). Neurol Ther 8, 367–381 (2019). https://doi.org/10.1007/s40120-019-00159-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-019-00159-2