Abstract
Introduction
Fingolimod was the first oral therapy approved for the treatment of relapsing-remitting multiple sclerosis (RRMS). Due to its action on cardiac sphingosine 1-phosphate receptors, fingolimod is leading to a transient decrease in heart rate (HR) and the occurrence of rare and asymptomatic self-limited atrioventricular (AV) blocks. This German non-interventional clinical study aimed to assess the cardiac safety profile in RRMS patients during at least 6 h after the initial treatment or restart after interruption of fingolimod in real-world settings.
Methods
The GoCARD study (German National Health Authorities, BfArM, CFTY720DDE18, NIS334) was a prospective, multi-center non-interventional study which was conducted in neurological and other medical practices or hospitals, qualified to routinely assess electrocardiogram (ECG) findings. Data were collected through interviews, clinical evaluations (notably ECGs), and laboratory tests. Medical history, vital signs, and a 12-lead ECG were assessed before fingolimod administration. After the first dose, a 6 h ECG was performed and vital signs (blood pressure and HR) were measured hourly. The occurrence of bradycardia (HR ≤45 beats per minute [BPM]), AV blocks (2nd degree Mobitz type I or higher), and corrected QT interval (QTc) intervals was also documented.
Results
More than 95% of physicians adhered to the cardiac monitoring recommendations. The observation of 217 patients in 42 study centers showed that while 35.9% of the patients had any cardiac risk profile, none of them experienced a bradycardia during the 6 h post-dose observation. Overall, only 1.8% of all patients displayed bradycardia (HR ≤45 BPM) during 6 h after treatment initiation. Moreover, in this cohort, none of the patients showed a new or persistent onset AV block (2nd degree Mobitz type I or higher) or QTc ≥500 ms.
Conclusion
Altogether, these data confirm that the first-dose observation after fingolimod initiation is usually uneventful (even in patients with pre-existing cardiovascular risk factors of this cohort) and that the rarely observed events remained asymptomatic and self-limited.
Funding
Novartis Pharma GmbH, Nürnberg, Germany.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a demyelinating disease of the central nervous system leading to inflammation and destruction of myelin and axons [1]. A worldwide average incidence of 2.5 per 100,000 people with a variance of 1.1–4.0 and a prevalence of 30.0 per 100,000 people with a variance of 5.0–80.0 is estimated (Word Health Organization, 2008). The relapsing-remitting (RRMS) course of the disease is typically characterized by recurrent acute episodes with neurologic symptoms, followed by complete or partial recovery.
Fingolimod (FTY720, brand name Gilenya® Novartis Pharma AG, Basel, Switzerland) has been approved for the treatment of RRMS. The safety profile of fingolimod has been proven by treatment of over 30,000 MS patients in clinical studies. Altogether, worldwide, more than 148,000 patients were treated with the substance, according to an experience of 316,000 patient years (data obtained from Q1 Novartis Pharmaceuticals Interim Financial Report, April 2016).
Due to the expression of sphingosine 1-phosphate receptors on cardiomyocytes, it was known that the first dose of fingolimod may lead to a transient, usually asymptomatic bradycardia and may possibly trigger rare and self-limited atrioventricular-blocks (AV blocks) [2–4]. Therefore, the European Medicines Agency recommended a 6 h continuous cardiac monitoring after the first administration of fingolimod.
Therefore, the aims of this non-interventional study (NIS) were the following: (1) to assess the cardiac risk profile in RRMS patients who intended to be treated with fingolimod, (2) to assess the cardiac safety profile in RRMS patients during at least 6 h after the initial treatment or restart after interruption, and (3) to assess the adherence of treating physicians regarding the new cardiovascular monitoring.
Methods
Subjects and Study Design
This prospective, multi-center NIS (registered by the German National Health Authorities, [BfArM], CFTY720DDE18, NIS334) was conducted in neurological and other medical practices or hospitals in Germany, qualified to routinely assess electrocardiogram (ECG) findings and to conduct first treatment with fingolimod, including the appropriate cardiovascular monitoring. The patient was observed for 6 h or longer, depending on whether or not cardiac abnormalities arose. Only data of patients under therapy with fingolimod under the routine treatment conditions were documented on the day of the visit and evaluated. If any adverse events (AEs) were reported after the 6 h observation period, a documentation of these events was obligatory. The prescription of fingolimod was solely based on the treatment decision of the physician. 217 patients with a mean age of 39.8 ± 9.8 years were enrolled at 42 German study centers.
Ethics Statement
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. The protocol was approved by an independent Ethics Committee. All patients gave written informed consent before any study-related procedures were performed.
Data Collection
Data were collected through interviews, clinical evaluations (notably ECGs), and laboratory tests. Each study site was equipped with the ECG-device Custo Cardio® 100/110/130 (Customed GmbH, Ottobrun, Germany), a 12-lead-continuous-ECG-digital recorder. The ECG recordings were pseudonymized and immediately transmitted via the Internet to a central reading site (Cormed Services Ltd., Dinslaken, Germany) for evaluation. Medical history, laboratory values, MS history, Expanded Disability Status Scale [5], cardiologic parameters, body mass index, vital signs, and a pre-dose 12-lead ECG were assessed before fingolimod administration. After the first dose, a 6 h continuous ECG was performed and vital signs (blood pressure and heart rate [HR]) were measured and documented hourly. The occurrence of bradycardia (HR ≤45 BPM), AV blocks (2nd degree Mobitz type I or higher), and corrected QT interval (QTc) interval was also documented.
Statistical Analysis
Outcomes of this study were the incidence with 95% confidence intervals of the aforementioned cardiac parameters, occurring during or at the end of the 6 h observation period after therapy start with fingolimod. The analysis was performed using the SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA). The number of participating centers and enrolled patients (42 centers and 217 patients) was lower than expected (2000 patients), resulting in a relatively small sample size. Therefore, only descriptive statistics are presented in this paper.
Results
From February 2012 to April 2015, 217 patients were enrolled at 42 study centers (5.2 ± 5.5 patients/center). The mean age of the 58 males (26.7%) and 159 females (73.3%) included was 39.8 ± 9.8 years (see Table 1). Data on MS history are also summarized in Table 1. For the evaluation of the cardiac risk profile, the history of cardiovascular risk factors in patients and their families was assessed. A family history of cardiovascular risk factors was reported in 43.8% of the patients, mostly hypertension (66 patients, 30.4%) and diabetes mellitus (35 patients, 16.1%). A cardiovascular risk factor was directly observed in 78 patients (35.9%), mostly tobacco use (40 patients, 18.4%), overweight (27 patients, 12.4%), and hypertension (24 patients, 11.1%). Ten patients (4.6%) had a history of cardiac arrhythmias. Twenty nine (13.4%) of the 217 patients enrolled took concomitantly cardiovascular medication, mainly antihypertensive (25 patients, 11.5%), beta blockers (two patients, 0.9%), calcium-channel blockers (one patient, 0.5%), or lipid-lowering drugs (eight patients, 3.7%).
ECG examinations were mainly performed by cardiologists (61.7% and 62.7%), followed by neurologists (16.2% and 16.6%), general practitioners (13.1% and 10.6%), and specialists in internal medicine (8.1% and 8.3%). Overall, more than 95% of physicians adhered to the cardiac monitoring recommendations.
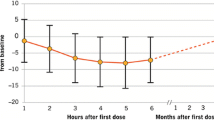
Course of blood pressure and HR course at baseline and during the 6 h observation period are summarized in Fig. 1a and b. The detailed HR course is also represented in Table 2. During the observation period, blood pressure remained relatively stable; only a slight decrease in the mean blood pressure (systolic and diastolic) was observed (Fig. 1a). The lowest mean systolic blood pressure (120.4 ± 13.9 mmHg) was observed, 2 h after treatment start and 5 h after treatment start, the lowest mean diastolic blood pressure (76.7 ± 10.0 mmHg). Patients receiving the first dose of fingolimod experienced, on average, a slight and transient decline in HR (Table 2; Fig. 1b). These patients had a maximum mean decline in HR of 9.2 beats per minute (BPM) from 74.9 ± 11.5 to 65.8 ± 9.9 BPM with the lowest values measured at 5 h after application.
Evolution of vital signs and cardiac events during and after the 6 h monitoring post-treatment initiation. a Systolic and diastolic blood pressure course at baseline and during the 6 h observation period (in mmHg, each value represents the mean ± SD, n = 215). b Heart rate course at baseline and during the 6 h observation period (in BPM, each value represents the mean ± SD, n = 215). BPM beats per minute, SD standard deviation
Overall, 33 patients (15.2%) were documented with cardiac events during or at the end of the observation period, summarized in Table 3 (including asymptomatic blood pressure increase or HR increase) [data on file, (Novartis Pharma GmbH, Nürnberg, Germany)]. None of the patients displayed a new or persistent onset AV block [2nd degree (Mobitz type I) or 3rd degree] or QTc ≥500 ms. Bradycardia, defined as a decrease in HR <45 BPM during or at the end of the observation period, was reported for four and three patients (1.8% and 1.4%, respectively). “Other” cardiac events were documented for nine and four patients (4.3% and 1.8%, respectively) during or at the end of the observation period. These events mostly referred to a low HR which did, however, not meet the criteria of a bradycardia (45–60 BPM) or AV block other than 2nd or 3rd degree (i.e. AV-block 1st degree). None of these patients had any cardiovascular risks/history or took HR-lowering co-medication.
Discussion
Here, we analyze the cardiac side effects after the first dose of fingolimod in everyday practice and confirm the well-known favorable cardiac safety profile of this compound in a real-world setting. None of the few cardiac events in this study required medical attention. The positive cardiac safety profile also extended to blood pressure analyses with only mild changes in systolic and diastolic blood pressure and even to the 35.9% of patients in the study with any cardiac risk factor (mainly tobacco use, overweight, hypertension, or patients taking cardiovascular co-medications, see Table 1). The particular strength of this non-interventional study is that it encompasses patients with pre-existing cardiac risk factors, who are excluded from the majority of clinical studies investigating fingolimod. This cardiac safety profile, including the observed episodes of bradycardia and the few other cardiac events, is consistent with the data of the first-dose monitoring from pivotal phase III trials and from a phase IIb multi-center study as well as post-marketing experience from the ongoing START Study (ClinicalTrials.gov identifier, NCT01585298; a prospective, one-week, multi-center, open-label study enrolling up to 7000 patients with RRMS in more than 250 centers in Germany, which already analyzed data of 3.951 patients) [2, 3, 6–10]. The absence of an increased QTc period is also consistent with the previous findings from rat models [11].
Very encouraging is also the following result: from the 35.9% of patients who shared any cardiac risk profile (especially those having a history of hypertension or arrhythmia), none experienced bradycardia during the 6 h post-dose observation, including those who were under HR-lowering co-medication. Overall, only 1.8% of all patients in this small cohort displayed bradycardia (HR ≤45 BPM) during the 6 h post-dose observation. Analyses from other studies in “real-life” settings provide further evidence on fingolimod treatment initiation. Data of the first study (ClinicalTrials.gov identifier, NCT01497262), which also enrolled RRMS patients taking calcium-channel blockers and beta blockers (n = 2417), showed an increased frequency of bradycardia in patients receiving these cardiovascular co-medications [4]. This observation was not seen in our study, which may be limited by its lower number of participants. However, our results are in agreement with the data of other recent observations in real-life settings. For example, data of an Italian study assessing the safety and tolerability of fingolimod after first dose (n = 906) revealed that the concomitant use of drugs which may cause bradycardia or otherwise influence cardiac conduction was not associated with cardiac events during the first-dose observation [12]. Other studies in real-life settings also demonstrate the rare occurrence of cardiac events after the first dose of fingolimod and their self-limited character [2, 3, 12–17], thus corroborating the very favorable cardiac safety profile of fingolimod.
Another strength of this study refers to the ECG data collection obtained in real-world settings which prove that the recommended cardiovascular observation after the first administration of fingolimod in office-based settings is feasible and also adhered to.
Possible limitations of this study lie in the lower than expected number of recruited patients. It was planned to include approximately 2000 patients over a 3-year period. Only 217 patients were enrolled at 42 study centers possibly due to competing studies and the widespread use of fingolimod outside of study settings in everyday practice. This lower number of enrolled patients may affect the detection of possible rare AEs. Yet, few missing data and good compliance to the recommendations in the summary of product characteristics during this NIS indicate a good reliability of the results.
Conclusion
In summary, these data confirm: (1) the feasibility of ECG monitoring for the fingolimod initiation in real-life settings and (2) that the first-dose observation after the fingolimod initiation is usually uneventful (even in patients with pre-existing cardiovascular risk factors of this cohort). Rare cardiac events remained asymptomatic and self-limited.
References
Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747.
DiMarco JP, O’Connor P, Cohen JA, et al. First-dose effects of fingolimod: pooled safety data from three phase 3 studies. Mult Scler Relat Disord. 2014;3(5):629–38.
Gajofatto A, Turatti M, Monaco S, Benedetti MD. Clinical efficacy, safety, and tolerability of fingolimod for the treatment of relapsing-remitting multiple sclerosis. Drug Healthc Patient Saf. 2015;7:157–67.
Gold R, Comi G, Palace J, et al. Assessment of cardiac safety during fingolimod treatment initiation in a real-world relapsing multiple sclerosis population: a phase 3b, open-label study. J Neurol. 2014;261(2):267–76.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–52.
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–15.
Kappos L, Cohen J, Collins W, et al. Fingolimod in relapsing multiple sclerosis: an integrated analysis of safety findings. Mult Scler Relat Disord. 2014;3(4):494–504.
Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401.
Paolicelli D, Manni A, Direnzo V, et al. Long-term cardiac safety and tolerability of fingolimod in multiple sclerosis: a postmarketing study. J Clin Pharmacol. 2015;55(10):1131–6.
Limmroth V, Haverkamp W, Dechend R, et al. First dose effects of fingolimod: in depth electrocardiographic analysis confirms first dose observation is usually uneventful. In: EAN congress; 2016 (P12128).
Egom EE, Ke Y, Musa H, et al. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signaling. J Mol Cell Cardiol. 2010;48(2):406–14.
Laroni A, Brogi D, Morra VB, et al. Safety of the first dose of fingolimod for multiple sclerosis: results of an open-label clinical trial. BMC Neurol. 2014;14:65.
Ontaneda D, Hara-Cleaver C, Rudick RA, Cohen JA, Bermel RA. Early tolerability and safety of fingolimod in clinical practice. J Neurol Sci. 2012;323(1–2):167–72.
Ordonez-Boschetti L, Rey R, Cruz A, et al. Erratum to: safety and tolerability of fingolimod in latin american patients with relapsing-remitting multiple sclerosis: the open-label FIRST LATAM study. Adv Thery. 2015;32(7):636.
Ordonez-Boschetti L, Rey R, Cruz A, et al. Safety and tolerability of fingolimod in latin american patients with relapsing-remitting multiple sclerosis: the open-label FIRST LATAM study. Adv Thery. 2015;32(7):626–35.
Ramseier SP, Roth S, Czaplinski A. A Swiss real world best practice experience in three different clinical settings of the 6 hour fingolimod first dose observation procedure. BMC Pharmacol Toxicol. 2015;16:7.
Yamout BI, Zeineddine MM, Tamim H, Khoury SJ. Safety and efficacy of fingolimod in clinical practice: the experience of an academic center in the Middle East. J Neuroimmunol. 2015;289:93–7.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Novartis Pharma GmbH. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. We want to deeply thank all the physicians and patients who contributed to the results of this study.
Disclosures
Ralf A. Linker received travel grants or speaker honoraria from Bayer HealthCare Pharmaceuticals, Biogen Idec, Merck Serono, Novartis Pharma GmbH, Roche, and TEVA Pharmaceutical Industries LTD, as well as research support from Novartis Pharma GmbH, Biogen Idec, and Merck Serono. Guillaume Wendt is an employee of Novartis Pharma GmbH.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article, go to http://www.medengine.com/Redeem/A395F0605F6085FC.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Linker, R.A., Wendt, G. Cardiac Safety Profile of First Dose of Fingolimod for Relapsing-Remitting Multiple Sclerosis in Real-World Settings: Data from a German Prospective Multi-Center Observational Study. Neurol Ther 5, 193–201 (2016). https://doi.org/10.1007/s40120-016-0051-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-016-0051-7