Abstract
Introduction
During the transcatheter aortic valve replacement (TAVR) procedure, hemodynamic measurements can be used to evaluate transcatheter heart valve (THV) performance. We hypothesized that the occurrence of a significant decrease in invasive aortic pressure immediately after annular contact by a self-expanding THV indicates effective annular sealing. This phenomenon could thus be used as a marker for the occurrence of paravalvular leak (PVL).
Methods
Thirty-eight patients undergoing TAVR procedure with a self-expandable Evolut R or Evolut Pro (Medtronic) valve prosthesis were included in the study. Drop in aortic pressure during valve expansion was defined as a decrease in systolic pressure of 30 mmHg immediately after annular contact. The primary endpoint was the occurrence of more than mild PVL immediately after valve implantation.
Results
A pressure drop was seen in 60.5% (23/38) of patients. More than mild PVL requiring balloon post-dilatation (BPD) was significantly more frequent in patients who did not have a systolic pressure decrease > 30 mmHg during valve implantation (46.7% [7/15] vs. 13.0% [3/23], respectively; p = 0.03). Patients without a systolic pressure decrease > 30 mmHg also had a lower mean cover index on computed tomography analysis (16.2% vs. 13.3%; p = 0.016). The 30-day outcomes were similar between the two groups, and echocardiography at 30 days demonstrated more than none/trace PVL in 21.1% (8/38) of patients, with no difference between the two groups.
Conclusion
A decrease in aortic pressure after annular contact is associated with an increased probability of good hemodynamic outcome after self-expanding TAVR implantation. In addition to other methods, this parameter could be used as an additional marker for optimal valve positioning and hemodynamic outcome during the implantation procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
During the transcatheter aortic valve replacement (TAVR) procedure, hemodynamic measurements can be used to evaluate transcatheter heart valve (THV) performance. |
In this study a decrease in aortic pressure after annular contact was associated with an increased probability of good hemodynamic outcome after self-expanding TAVR implantation. |
What was learned from this study? |
For the intraprocedural evaluation of optimal hemodynamic outcome for self-expanding THV, a multimodal approach with the use of imaging modalities and hemodynamic measurements is essential. |
Introduction
Over the past decade, transcatheter aortic valve replacement (TAVR) has evolved into an effective treatment modality for patients with symptomatic severe aortic stenosis. Current guidelines of the European Society of Cardiology (ESC) recommend TAVR for older patients (≥ 75 years) or for those who are at high risk or deemed unsuitable for surgery [1]. As recent trials have shown favorable short-term outcomes for TAVR compared to surgical aortic valve replacement in low-risk patients, it is likely that indications for TAVR will be extended to younger, low-risk patient populations in the future [2, 3].
Many factors have contributed to the procedural and clinical success of TAVR, including appropriate patient selection, advances in preprocedural imaging and procedural techniques, and a continuous evolution in the design of transcatheter heart valves (THV) [1, 2]. These factors have played a pivotal role in reducing the rate of TAVR-related complications, such as paravalvular leak (PVL). Despite these advancements, PVL still remains a clinical challenge as even a mild paravalvular leak after TAVR is an independent risk factor for increased long-term mortality [4, 5]. As TAVR indications are moving into younger, low-risk patient populations, there is an even greater emphasis to achieve an optimal hemodynamic result during the implantation procedure.
Aortography and echocardiography are the cornerstones of intraoperative evaluation of PVL [6]. In addition, several hemodynamic markers, such as Aortic Regurgitant Index (AR index) [7], Dicrotic Notch Index (DNI) [8], and the pressure gradient between diastolic aortic pressure and left ventricular end-diastolic pressure (∆PDAP-LVEDP) [9] have been demonstrated to be useful in evaluating PVL. Limitations in the use of these markers include the need for more advanced calculations, left ventricular catheterization to measure the left ventricular end-diastolic pressure (LVEDP), and confounding factors, such as left ventricular stiffness that raises LVEDP. Pressure decrease during self-expanding THV implantation is a generally recognized phenomenon [10].
In the study reported here, we hypothesized that the occurrence of a significant decrease in invasive systolic aortic pressure after annular contact (when the THV is not yet functioning and the expanding THV frame occludes the aorta) by a self-expanding THV device indicates effective annular sealing. This parameter could be used as a simple and rapid marker to aid in the optimal positioning of the THV device during the implantation procedure.
Methods
This was investigator-initiated, academic, prospective, non-interventional trial of 41 patients undergoing the TAVR procedure due to symptomatic severe native aortic valve stenosis who were treated with a self-expandable valve prosthesis (Evolut R® or Evolut Pro®; Medtronic, Dublin, Ireland). All study subjects were treated at the Heart Center in Turku University Hospital, Finland during January 2018–May 2019. The study protocol was approved by the Medical Ethics Committee of The Hospital District of Southwest Finland. All patients enrolled in the study provided written informed consent for participation. The study conforms to the Declaration of Helsinki of 1964 and its later amendments.
Procedure
The TAVR procedure was performed using local anesthesia and the transfemoral approach in all cases. All patients were examined with contrast-enhanced multislice computed tomography (MSCT) to verify patient eligibility and to plan the procedure. All patients were paced at 110 beats per minute (bpm) during the expansion phase of the transcatheter valve as per standard protocol at our institution. Aortography and transthoracic echocardiography were used to evaluate intraprocedural PVL using the VARC2 criteria [6], and balloon post-dilatation (BPD) was performed when more than mild PVL was detected. Echocardiography was performed pre-operatively and at the 1-month follow-up visit at the outpatient clinic. The AR Index was measured after the THV implantation and possible BPD. The AR Index was calculated according to the following formula: [(diastolic blood pressure – left ventricular end-diastolic pressure)/systolic blood pressure] × 100.
Pressure Waveform Analysis
Continuous aortic pressure waveforms and electrocardiogram (ECG) were recorded throughout the procedure and analyzed afterwards. The start of the overdrive pacing at 110 bpm was used as a marker for the beginning of the valve expansion phase of the TAVR procedure.
Statistical Analysis
Study data were analyzed using the SPSS statistical software package (version 25; SPSS IBM, Armonk, NY, USA). For continuous variables, visual assessment was used to determine the normality of the variables. These variables are presented as the means and standard distribution (SD) if normally distributed and as the medians and interquartile range (25th–75th percentile) if not normally distributed. Student’s t-test and the Wilcoxon rank sum test were used for continuous variables and the Chi-square test or Fisher’s exact test was used for categorical variables. Levene’s test was used to assess the homogeneity of variances. Comparison between the two groups was performed using univariate analysis.
Results
Baseline Characteristics and Pressure Drop
Data on 38 patients were included in the final analysis. In three cases the pressure data was incomplete or not analyzable. All patients completed the 1-month follow-up. The mean (± SD) age of the study population was 80.8 ± 6.8 years, of which 63.2% were males. More than two thirds of the study population had New York Heart Association (NYHA) Class III dyspnea pre-operatively. The majority of patients were at low or intermediate surgical risk, with an average Society of Thoracic Surgery (STS) score of 2.7 (2.05–3.96) and European System of Cardiac Operative Risk Evaluation (EuroSCORE) II of 2.8 (1.8–4.1). Patient baseline characteristics are shown in Table 1.
Patients were classified into two groups, namely, those who experienced a pressure drop (PD) and those who did not (non-pressure drop [NPD] according to the aortic pressure changes after valve prosthesis achieved the annular contact. Only pressure changes occurring immediately after annular contact were analyzed. The cutoff value for decrease in aortic pressure during valve expansion was defined as a decrease of systolic pressure ≥ 30 mmHg after annular contact, calculated from a mean of ten consecutive cardiac cycles of prior pressure drop and five consecutive cycles at the lowest point. This cutoff value gave the best differentiation between the two groups. Examples of PD, and NPD waveforms and the hypothesized scheme of the mechanism of pressure decrease are shown in Fig. 1.
Hypothesized scheme of the mechanism of pressure decrease. Before valve expansion, the self-expandable valve is positioned across the aortic valve. At the opening phase of the valve, the transcatheter heart valve is not yet functioning, and the expanding valve contacts the annulus. a Effective annular contact is achieved during valve opening phase and pressure drop is seen in aortic pressure. b Effective annular contact is not achieved and aortic pressure remains stable
Procedural Characteristics
There was no difference in the annulus diameter nor area measured with MSCT between the groups. THV depth from the non-coronary cusp and left coronary cusp valve depth were also similar. However, the mean cover index of the study population was 15.7%, and it was significantly lower in the NPD group than in the PD group (13.3% vs. 16.2%, respectively; p = 0.016).
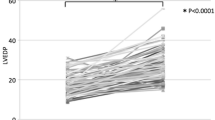
Mean (± SD) postoperative AR Index was 28.9 (± 8.8), and there was no difference between the two groups. More than mild PVL was detected in ten patients (26.3%), of whom three were in the PD group and seven were in the NPD group (13.0% vs. 46.7%, respectively; p = 0.03) (Fig. 2). All these PVLs were treated with BPD. Procedural characteristics are presented in Table 2.
Clinical Outcomes
There were no mortality, stroke, or major vascular events during the 30-day follow-up. Closure device failure rates were similar in both groups. Postoperative echocardiography measurements did not differ between the PD and the NPD groups. Eight (21.1%) patients had mild or more PVL (7 mild, 1 moderate) at the 30-day echocardiography of whom five were in the PD group and three in the NPD group (21.7% and 20%, respectively). Clinical outcomes are presented in Table 3.
Discussion
In this study we describe a clinically useful and easy-to-use intraprocedural hemodynamic marker to predict the hemodynamic outcome after TAVR with a self-expanding valve. The main finding of this study was that patients with a significant pressure decrease after annular contact had a higher probability of an optimal result as demonstrated by a lower risk for more than mild PVL. Aortic pressure can fluctuate during the TAVR procedure for various reasons, such as bleeding, ventricular pacing, and aortic regurgitation. To distinguish normal procedure-related pressure fluctuation from the pressure drop caused by annular contact, the cutoff value was set at a high value of 30 mmHg and only pressure changes occurring immediately after annular contact were analyzed. Here we describe the pressure drop as a phenomenon which can indicate proper valve skirt sealing to the aortic annulus.
Advances in surgical techniques and THV design have led to a significant decrease in PVL after TAVR. Despite this, accurate and rapid intraoperative quantification of PVL remains clinically important as even mild PVL increases long-term mortality [5,6,7]. Visual evaluation of PVL with aortography provides a qualitative method during the TAVR procedure, and it is often the first imaging modality used. However, visual grading of PVL is not easy and is dependent on the experience of the operators as well as on several technical and hemodynamic factors. In one study, compared to cardiovascular magnetic resonance imaging, aortography correlated only moderately with in the grading of PVL [11]. Echocardiography is the standard method for evaluating PVL, providing a semi-quantitative grading approach, but it can underestimate the degree of PVL [12]. Transesophageal echocardiography allows for quick intraprocedural assessment of PVL, but as most TAVR procedures are currently performed without general anesthesia, the usefulness of this imaging modality is limited.
As the visual appraisal of PVL is technically challenging and at best semi-quantitative, the evaluation of PVL should ideally be complemented with hemodynamic assessment. Several hemodynamic markers have been previously developed to assess THV performance, of which the AR Index is the most studied hemodynamic marker; it has been shown to predict more than mild PVL and mortality at 1 year after TAVR [7]. After the introduction of AR Index, several groups have developed refinements, such as the time-integrated AR Index (TIARI) [13] and the AR Index ratio [14], to increase the diagnostic accuracy of hemodynamic measurements. Although TIARI has a low sensitivity and specificity (0.75 and 0.65, respectively) for predicting the need for BPD, it may have additional value as well as aortographic and echocardiographic evaluation [13]. Recent studies suggest that hemodynamic markers like the DNI [8] and the ∆PDAP-LVEDP [9] could also be used to determine a significant PVL. Limitations in the use of these markers are the need for more time-consuming and advanced calculations, limiting the clinical usefulness of these parameters for intraprocedural assessment. In addition, they require catheterization of the left ventricle and are influenced by factors that lead to increased LVEDP, such as diastolic of systolic dysfunction, mitral valve regurgitation, or high systemic blood pressure [7].
The novel hemodynamic marker that is presented in this study has two distinct advantages. First, pressure changes can be evaluated during the procedure by following patient hemodynamics during valve expansion, and as the cutoff for significant pressure drop is set at 30 mmHg, a positive sign can easily and rapidly be detected without complicated calculations. Second, in contrast to previously developed markers, the marker presented here is assessed before the THV is in a final position and released from the delivery catheter, thus giving the operator the option to adjust THV position before final release.
Currently, there is a lack of consensus on the parameters to guide BPD after valve deployment. Intraprocedural management of significant PVL depends on the cause of the leak, which in most cases is incomplete circumferential apposition of the prosthesis to the aortic annulus. Although BPD can have a significant effect on reducing PVL, it should not be performed routinely, as it increases the risk of stroke [15], procedural time, and costs. In previous studies a lower cover index was associated with the higher rates of PVL and increased need for BPD [16]. In the current study, the cover index was significantly lower in patients in the NPD group, further supporting the hypothesis that the lack of a pressure drop is caused by insufficient annular contact of the THV.
The study has several limitations. This was a single-center study with a small sample size. The visual evaluation of more than minimal PVL with aortography and the subsequent need for BDP was determined by the primary operator and thus vulnerable for bias. Echocardiography was performed by several clinicians and subject to interobserver variability. During valve implantation, hemodynamics can vary for reasons other than annular contact by the THV, which can lead to misinterpretations, such as overdrive pacing. Baseline blood pressures before the TAVI procedure were not analyzed. As only one valve type (Medronic Evolut Pro or Evolut R) was included in this study, the findings cannot be generalized to other valve types.
Conclusion
For the intraprocedural evaluation of optimal hemodynamic outcome for self-expanding THV, a multimodal approach with the use of imaging modalities and hemodynamic measurements is essential. In addition to other modalities to evaluate PVL and THV hemodynamic performance, the novel hemodynamic marker presented in this study could be used to guide decision-making during TAVR procedure.
References
Vahanian A, Beyersdorf F, Praz F, et al. ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. https://doi.org/10.1093/eurheartj/ehab395.
Mäkikallio T, Jalava MP, Husso A, et al. Ten-year experience with transcatheter and surgical aortic valve replacement in Finland. Ann Med. 2019;51(2–3):270–9.
Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706–15. https://doi.org/10.1056/NEJMoa1816885.
Jones BM, Tuzcu EM, Krishnaswamy A, et al. Prognostic significance of mild aortic regurgitation in predicting mortality after transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. 2016;152(3):783–90.
Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366(18):1686–95.
Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J. 2011;32(2):205–17.
Sinning J-M, Hammerstingl C, Vasa-Nicotera M, et al. Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;59(13):1134–41.
Mohananey D, Narayanswami J, Kumar A, et al. Association of a novel hemodynamic index with aortic regurgitation after TAVR with the Edwards SAPIEN valve. JACC Cardiovasc Interv. 2019;12(12):1194–5.
Patsalis PC, Konorza TFM, Al-Rashid F, et al. Incidence, outcome and correlates of residual paravalvular aortic regurgitation after transcatheter aortic valve implantation and importance of haemodynamic assessment. EuroIntervention. 2013;8(12):1398–406.
Iribarne A, Stefanescu Schmidt AC, Nguyen TC, editors. Transcatheter heart valve handbook: a surgeons’ and interventional council review. Washington, DC: American College of Cardiology; 2018.
Frick M, Meyer CG, Kirschfink A, et al. Evaluation of aortic regurgitation after transcatheter aortic valve implantation: aortic root angiography in comparison to cardiac magnetic resonance. EuroIntervention. 2016;11(12):1419–27.
Sherif MA, Abdel-Wahab M, Beurich H-W, et al. Haemodynamic evaluation of aortic regurgitation after transcatheter aortic valve implantation using cardiovascular magnetic resonance. EuroIntervention. 2011;7(1):57–63.
Kumar A, Sato K, Jobanputra Y, et al. Time-integrated aortic regurgitation index helps guide balloon postdilation during transcatheter aortic valve replacement and predicts survival. J Am Heart Assoc. 2019;8(14):1–10.
Sinning JM, Stundl A, Pingel S, et al. Pre-procedural hemodynamic status improves the discriminatory value of the aortic regurgitation index in patients undergoing transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2016;9(7):700–11.
Wang N, Lal S. Post-dilation in transcatheter aortic valve replacement: a systematic review and meta-analysis. J Interv Cardiol. 2017;30(3):204–11.
Nombela-Franco L, Rodés-Cabau J, Delarochellière R, et al. Predictive factors, efficacy, and safety of balloon post-dilation after transcatheter aortic valve implantation with a balloon-expandable valve. JACC Cardiovasc Interv. 2012;5(5):499–512.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
Concept and design: Mikko Savontaus. Statistical analysis: Jouni Pykäri. Data collection: Jouni Pykäri, Tuija Vasankari, Tuomas Paana, Pekka Porela, Antti Ylitalo, Sanna Laurila, Mikko Savontaus. Data analysis: Tero Koivisto and Juho Koskinen. Drafting of the manuscript: Jouni Pykäri, Mikko Savontaus and Tuija Vasankari. All authors have read and agreed the submitted version of the manuscript.
Disclosures
Mikko Savontaus is a proctor for Medtronic. Sanna Laurila’s affiliation changed from Satakunta Central Hospital to Turku University Hospital during the completion of the manuscript. Jouni Pykäri, Tuija Vasankari, Antti Ylitalo, Pekka Porela, Tuomas Paana, Markus Malmberg, Juho Koskinen, and Tero Koivisto have nothing to disclose.
Compliance with Ethics Guidelines
The study protocol was approved by the Medical Ethics Committee of The Hospital District of Southwest Finland. All patients enrolled in the study provided written informed consent for participation. The study conforms to the Declaration of Helsinki of 1964 and its later amendments.
Data Availability
Data underlying this article will be shared on reasonable request to the corresponding author
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Pykäri, J., Vasankari, T., Ylitalo, A. et al. Impact of Intraprocedural Pressure Changes on Hemodynamic Outcome During Self-Expanding TAVR. Cardiol Ther 12, 361–369 (2023). https://doi.org/10.1007/s40119-023-00307-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-023-00307-8