Abstract
Introduction
Evidence regarding the development of pulmonary thromboembolism (PE) during hospitalization is unclear. We hypothesized that the incidence of PE could vary depending on clinical department and aimed to conduct a survey on the incidence of in-hospital PE.
Methods
We conducted a retrospective analysis using claims data of in-hospital patients in Japan. We collected background information regarding patients with and without PE occurrence during hospitalization. Further, we determined the incidence of PE and implemented prophylactic procedures in patients with and without surgery according to clinical department at admission. Finally, we examined the duration of hospital stay and in-hospital mortality rates in patients with and without PE.
Results
We found that 5007 (0.107%, 20.61 per 1000 person-years) patients developed PE during hospitalization and differed by clinical department at admission. Moreover, 2272 (0.095%, 19.3 per 1000 person-years) and 2735 (0.119%, 21.8 per 1000 person-years) patients with and without surgery, respectively, developed PE during hospitalization (P < 0.001). Further, 33.8% of inpatients underwent prophylactic procedures for PE; however, the implementation rate differed between patients with and without surgery (59.2% vs. 7.3%, P < 0.001). The median duration of hospital stay in patients with and without PE was 31.0 and 11.0 days, and the in-hospital mortality rates in patients with and without PE were 11.0% and 3.5%, respectively (P < 0.001).
Discussion
The incidence of in-hospital PE differed according to patient characteristics, clinical departments, and presence/absence of surgery. The onset of PE during hospitalization leads to prolonged hospital stay and in-hospital death.
Conclusion
It is important to conduct a proper risk assessment on admission as well as to implement proper prophylactic procedures to prevent the development of PE during hospitalization.
Similar content being viewed by others
Why carry out this study? | |
Acute pulmonary thromboembolism (PE) develops suddenly without specific early symptoms and can lead to death. | |
Long-term bed rest is among the risk factors for PE. | |
There has been an increase in hospital awareness and prevention efforts for PE; however, unexpected onset of in-hospital PE remains a significant safety issue. | |
Elucidating the characteristics of patients with in-hospital PE and their prognosis could inform preventive measures for patients at a high risk of developing PE. | |
What was learned from the study? | |
The incidence of in-hospital PE was 0.1%; moreover, it varied according to clinical department and presence/absence of surgery during hospitalization. | |
Furthermore, the implementation rate of prophylactic procedures for PE differed according to clinical department and the presence/absence of surgery. | |
Patients with in-hospital PE had a longer duration of hospital stay and a higher in-hospital mortality rate than patients without in-hospital PE. | |
It is important to appropriately evaluate the risk of PE in hospitalized patients to adopt informed preventative measures and implement appropriate prevention of in-hospital PE. |
Introduction
Pulmonary thromboembolism (PE) and deep vein thrombosis (DVT) are collectively termed as venous thromboembolism (VTE). PE is characterized by pulmonary artery occlusion by an embolus; moreover, the embolus source is usually a thrombus formed in a vein in the lower limbs or pelvis in 90% of cases [1] and can progress rapidly and lead to death. Virchow’s triad described three broad categories of factors that contribute to thrombosis: (1) blood flow stagnation, (2) vascular endothelial damage, and (3) hypercoagulability [2, 3].
Hospitalized patients are at a high risk for VTE due to long-term bed rest, disease backgrounds considered risk factors for VTE, and other hospitalization-related factors, including surgery [4]. Our previous study has suggested that half of patients with VTE had new diagnosis of VTE during hospitalization due to a disease other than VTE [5]. However, quantified evidence for VTE occurring during hospitalization is still lacking.
The in-hospital mortality rate attributable to PE has been reported to be 14% [6]; furthermore, 10–13.5% of PE-related deaths occur within 1 h of onset [7, 8]. In Europe and the USA, a scoring system and other methods have been proposed for assessing the risk of in-hospital PE to mitigate the in-hospital onset of VTE [9, 10]. Additionally, risk assessment is recommended in the Japanese Circulation Society guidelines [1]. Patients at high risk of PE, such as during the perioperative period, receive prophylactic drugs, including heparins and factor Xa inhibitors, as well as other prophylactic interventions, such as wearing compression stockings and intermittent air compression. However, unexpected onset of in-hospital PE remains a significant safety issue. Additionally, the epidemiology of in-hospital PE, including the in-hospital incidence, related background characteristics, and proper implementation of prophylactic interventions, remain unclear. PE is a life-threatening, serious disease and development of PE during hospitalization, which is one of the major risk factors, must be minimized as much as possible. However, there are differences in awareness of in-hospital PE among facilities, departments, and physicians, and it is believed that there are still sufficient differences in awareness and practice of prevention of PE during hospitalization. No nationwide survey on this belief exists.
We hypothesized that the incidence of PE could vary depending on clinical department. Therefore, we aimed to conduct a survey on the incidence of, and factors related to, in-hospital PE in Japan. Our findings may provide interventions that can help further prevent PE during hospitalization.
Methods
Study Design
In this non-interventional descriptive observational study, we extracted eligible patients’ data from the Medical Data Vision (Medical Data Vision Co., Ltd., Tokyo, Japan) administrative claims database.
Population
We included patients hospitalized for diseases other than DVT or PE. Since current guidelines recommend direct oral anticoagulants (DOACs) over warfarin, becoming the main treatment option in Japan since 2017, patients with new PE on and after January 1, 2017 were included in the analysis. The inclusion criteria were as follows: (1) record of hospitalization for treatment of any diseases other than DVT/PE between January 2017 and December 2021 and (2) age 20 years or more on the date of first hospitalization. The exclusion criteria were as follows: (1) a diagnosis of atrial fibrillation within 6 months before the admission date (baseline period); (2) having received anticoagulants, including parenteral anticoagulants, for more than 7 days during the baseline period; (3) having a duration of hospital stay of less than 3 days including the admission day; and (4) missing information regarding the clinical departments in which the patients were hospitalized. Eligible patients were divided into those with and without PE during hospitalization.
Definitions
In-hospital PE was defined as PE occurring during hospitalization for diseases other than DVT and/or PE. This included patients hospitalized for PE treatment who had also been hospitalized for a disease other than DVT and/or PE on the same day or the day before. The examination, diagnosis, treatment, and follow-up for PE were determined on the basis of a validated algorithm [11].
The diagnosis of PE was confirmed by the presence of ICD-10 codes specific for PE in the list of diseases that developed after hospitalization, or those for whom the first or second most medical resources utilized. We excluded patients with ICD-10 codes specific for PE or in the list of comorbidities at admission. PE examination was also confirmed using imaging protocols such as scintigram (E100-00) and computer tomography (E200, E2001, E2002, and E2003).
Treatment interventions for PE included more than 5000 units of heparin (unfractionated heparin), fondaparinux, oral anticoagulants, thrombolytic agents (urokinase, tissue plasminogen activator), or thromboembolectomy.
Follow-up examinations for PE included electrocardiography, chest radiography, ultrasonography, blood gas analysis to confirm the need for oxygen administration, and monitoring respiratory and heart rate within 7 days of treatment for PE.
The date of in-hospital PE development was defined as the date when treatment for PE was initiated. Moreover, for patients who developed PE during anticoagulant treatment for thrombosis prevention, the PE development date was determined as the date when the anticoagulant regimen or dosage was changed.
Surgery types were determined using “K codes”, which is a medical procedure coding system. Patients with and without surgery before in-hospital PE development were included in the “with surgery cohort” and “without surgery cohort”, respectively.
Prophylactic interventions were identified when a prophylactic procedure fee (N001-6) was claimed on health insurance. This fee can be claimed when elastic stockings or an intermittent air compression device is used to prevent PE but not when prophylactic drugs are administered.
Prophylactic anticoagulants for DVT and/or PE include edoxaban up to 30 mg/day, less than 5000 units of heparin, and enoxaparin. These anticoagulants were considered to be prophylactically used if they were administered after surgery and if the duration was 15 days or less.
Table S1 in the supplementary material describes the classification of clinical departments. Table S2 in the supplementary material presents the definitions of comorbidities.
Endpoint and Subgroup Analyses
We investigated the overall incidence of PE during hospitalization. Additionally, we investigated the incidence for in-hospital PE according to clinical departments and the presence/absence of surgery.
Furthermore, we investigated differences in patient background characteristics, including presence/absence of prophylactic and duration of hospital stay, between patients with and without in-hospital PE. Moreover, we investigated the mortality rate related to in-hospital PE and the timing of in-hospital PE development.
Statistical Analysis
All analyses were conducted with SAS V.9.4 (SAS Institute Inc., Cary, NC, USA). Incidence and 1000 person-years were calculated for in-hospital PE by department and by presence or absence of surgery. Patients were followed up from the index date until discharge from the index hospitalization. The chi-square test was used to compare the incidence of in-hospital PE between patients with and without surgery within each medical department. Statistical significance was set at P < 0.05.
Clinical and demographic characteristics are presented as mean ± standard deviation (continuous variables) and patient percentage (%) (categorical variables). Because the value of N is large in most epidemiological studies, even a medically meaningless small difference would be significantly different when the difference is assessed using P values calculated by typical statistical tests (i.e., unpaired t test, chi-square test, etc.), we evaluated whether there was a difference in patient background variables between the two groups not by P values but by a standardized difference (std. diff.), intuitive indexes which measure the effect size between two groups and are independent of the sample size in contrast to P values. If the std. diff. was smaller than 0.1, the factor was not considered to be different between the two groups [12]. Comparisons regarding incidence, mortality, or percentage of procedures performed were conducted using the Wilcoxon rank-sum test or Fisher’s exact test to calculate P values because the number of incidence or percentage is not large and the difference between the two groups should be statistically compared.
The duration of hospital stay for patients with and without in-hospital PE is described in terms of the mean, median, and interquartile rage (IQR). The number of days until PE onset was defined as the duration from the hospitalization date to the date of PE onset in the “with surgery cohort” and “without surgery group”, or from the surgery date to the date of PE onset in the “with surgery cohort”.
This study is a retrospective database study that (1) is not related to the efficacy and safety of drugs or a disease and (2) uses data that do not contain complete personal information. Therefore, in accordance with the “ethical guidelines for medical and biological research involving human subjects”, which is the ethical guideline for clinical research in Japan, obtaining informed consent from patients and approval by the institutional review board or ethical committee are not required.
Results
Incidence of In-Hospital PE in Each Clinical Department
Overall, we extracted 7,800,521 patient records with a history of hospitalization from January 2017 to June 2021. Among them, 4,684,659 patients met the study criteria and 5007 (0.107%, 20.61 per 1000 person-years) developed PE during hospitalization (Figs. 1 and 2, Tables 1 and S3).
As demonstrated in Table 1, the proportion of women (52.2% vs. 58.9%, std. diff. = 0.1096) and mean age (66.8 ± 18.3 vs. 72.9 ± 14.3 years, std. diff. = 0.3709) are considered different between the non-PE and in-hospital PE cohorts because std. diff. values are higher than 0.1. Moreover, there was a higher incidence of stroke/transient ischemic attack, congestive heart disease, hypertension, history of VTE, fractures, and active cancer in the in-hospital PE cohort than in the non-PE cohort. Prescriptions for medications such as angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, calcium channel blockers, and chemotherapy were more common in the in-hospital PE cohort than in the non-PE cohort.
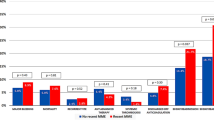
Regarding clinical department, the incidence of in-hospital PE was relatively higher in the respiratory surgery (53.9 per 1000 person-years), cardiovascular medicine (37.9 per 1000 person-years), and respiratory medicine (37.9 per 1000 person-years) departments (Fig. 2, Table S3 in the supplementary material).
In-hospital PE occurred in 2272 (0.095%, 19.3 per 1000 person-years) and 2735 (0.119%, 21.8 per 1000 person-years) patients with and without surgery, respectively (P < 0.001) (Fig. 2, Table S3).
Among the patients with in-hospital PE, the most common reasons for hospitalization were cancer (n = 913), fracture (n = 714), pneumonia (n = 236), heart failure (n = 208), and respiratory insufficiency (n = 202) (Tables 2, S4 in the supplementary material). Among the clinical departments with a high risk of PE development, the diseases that resulted in hospitalization were cancer (n = 60), pneumonia (n = 13), and pneumothorax (n = 11) in the respiratory surgery department; heart failure (n = 111), angina/myocardial infarction (n = 75), respiratory failure (n = 66), cardiac arrest (n = 38), and pneumonia (n = 23) in the cardiovascular department; and lung disease (n = 86), cancer (n = 78), pneumonia (n = 60), and respiratory failure (n = 60) in the respiratory medicine department.
Implementation Rate of Prophylactic Procedures for PE
We compared the implementation rate of prophylactic procedures for PE between patients with and without surgery (Fig. 3, Table S5 in the supplementary material). Prophylactic procedures for PE were performed in 33.8% of the inpatients; however, the implementation rate differed across the clinical departments. Prophylactic procedures for PE were more common in the oncology (68.6%), respiratory surgery (68.0%), obstetrics and gynecology (63.4%), orthopedics (62.7%), digestive surgery (62.6%), general surgery (60.4%), and urology (57.5%) departments. Furthermore, there was a significant difference in prophylactic procedures between patients with and without surgery (59.2% vs. 7.3%, P < 0.001). In each clinical department, the implementation rate of prophylactic procedures was higher in patients with surgery than in those without surgery.
PE Onset Date and Outcome
The median duration from admission to PE onset was 9.0 days (IQR 3–18, mean 13.3 days) in the without surgery cohort. In the surgery cohort, the median duration from admission and surgery to PE development was 14.0 days (IQR 6–27, mean 20.6 days) and 9.0 days (IQR 3–21, mean 16.0 days), respectively.
The median duration of hospital stay in the non-PE cohort and in-hospital PE cohorts was 11.0 days (IQR 7–22, mean 18.9 days) and 31.0 days (IQR 18–50, median 39.8 days), respectively (Fig. 4, Table S6 in the supplementary material).
The in-hospital mortality rates were 11.0% and 3.5% in the in-hospital PE and non-PE cohorts, respectively (P < 0.001) (Table S6).
Discussion
To the best of our knowledge, this is the first nationwide epidemiological study on in-hospital PE. First, the incidence of in-hospital PE differed according to clinical department and presence/absence of surgery. Second, the implementation rate of prophylactic procedures for PE was higher among patients with surgery than among patients without surgery. Third, the in-hospital PE cohort showed a longer duration of hospital stay and more in-hospital deaths than the non-PE cohort. Our findings demonstrate the need for prompt assessment of the risk of PE, preferably upon admission, followed by risk-appropriate implementation of prophylactic procedures.
The risk of PE in hospitalized patients was higher in this study than in general epidemiological studies. In 2006, there were 7864 (0.62 per 1000 person-years) PE patients in Japan [1, 13]. There is an increasing incidence of VTE in Japan; notwithstanding, the incidence of in-hospital PE in our study (20.6 per 1000 person-years) is quite high. This could be attributed to the incidence of PE being much higher in inpatients than in outpatients as a result of the multiple risk factors associated with hospitalization. Disease conditions requiring hospitalization, surgical procedures, and long-term bed rest are considered major risk factors for PE development [4].
Interestingly, the incidence of in-hospital PE differed across clinical departments. This could be attributed to between-department differences in patient characteristics, surgery frequency, and the implementation rate of prophylactic procedures for PE. Departments involving a high risk of PE development were the respiratory surgery, cardiovascular medicine, and respiratory medicine departments. The most common disease responsible for hospitalization in the respiratory surgery and respiratory medicine departments was cancer. The incidence of VTE in patients with cancer is 4–7 times higher than that in patients without cancer [14, 15]. The risk of VTE development differs according to cancer type, with lung cancer showing one of the highest risks among all cancer types [16], with 5–6% of patients with lung cancer having VTE in Japan [17, 18]. The high incidence of VTE in patients with cancer could be attributed to increased coagulation activities due to humoral factors produced by cancer cells and surrounding tissues, endothelial cell damage caused by chemotherapy and other anticancer treatments, surgical cancer treatment, decreased physical activity, and advanced age [19]. Although it is conceivable that PE is more likely to be detected in respiratory diseases at the time of admission, patients with ICD-10 codes specific for PE or in the list of comorbidities at admission were excluded. In other words, PE incidentally diagnosed at the time of hospitalization was not counted, and it is thought that there could be many cases of PE occurring during hospitalization in these clinical departments due to the background of the patients and medical practices. In the cardiovascular medicine and respiratory medicine departments, patients were also hospitalized for heart failure, chronic lung disease, pneumonia, and respiratory failure, which are risk factors for VTE because they are associated with decreased blood flow [1]. Moreover, heart failure could directly contribute to VTE through factors such as impaired hemodynamics [20]. The risk of developing PE may depend on the disease and the condition of the patient, indicating the need for a more detailed investigation of the risk of PE during hospitalization for each disease.
Given that surgery is also reported as a risk factor for VTE [1, 21], we examined the risk of VTE by the presence or absence of surgery in each department. Tissue and vascular damage due to surgery might activate the coagulation system and cause thrombogenesis, which is why surgery is associated with a high risk of VTE development, specifically abdominal surgery or plastic surgery of the lower extremities involves an increased risk of developing DVT and subsequent PE [1, 21,22,23,24]. In practice, we observed a high risk of PE development among patients with surgery. However, compared with the “without surgery” cohort, the “with surgery” cohort showed a higher implementation rate of prophylactic procedures for PE (less than 10% vs. approx. 60%). Because the incidence of VTE is known to be high in the perioperative period and because prophylactic interventions are also recommended in the guidelines [1, 21], surgical patients often receive prophylactic interventions for PE, which may reduce the risk of PE development. Fractures were the second most common trigger for hospitalization in the PE group, but the implementation rate of PE prevention management in orthopedics was high, and the incidence of PE in orthopedics was comparatively low. Contrastingly, patients without surgery may not receive sufficient prophylactic interventions, which could result in the onset of preventable PE. It may be necessary to raise awareness about PE prevention for patients who do not undergo surgery during hospitalization.
Patients with in-hospital PE showed a longer duration of hospitalization than patients without (31.0 days vs. 11.0 days). Long-term hospitalization is considered to be a PE risk because of reduced blood flow due to decreased physical activity [4]. However, the time to PE onset was used to calculate the incidence using the person-year method in this study, and the risk of VTE during hospitalization was adjusted for duration of hospital stay. Moreover, considering that about half of PE develops within 9.0–14.0 days after admission or surgery, it is thought that additional hospitalization was necessary because PE developed and not that the risk of developing PE was high because of the long hospitalization period. Therefore, it is important to provide appropriate PE prevention not only for long-term hospitalization patients but also for hospitalized patients from the initial stage of hospitalization.
The mortality rates among patients with and without PE were 11.0% and 3.5%, respectively (P < 0.001). Similarly, a previous Japanese study reported that the mortality rate attributable to PE was 14% [6]. In about 25% of the patients with PE, the first sign/symptom of PE is death [25], and more than 10–13.5% of deaths occur within 1 h of onset of PE [7, 8]. Death in such a short time after onset may be difficult to avoid, but if the risk is properly assessed and if the onset of PE can be predicted, a prompt response may be possible. The results could be affected by clinical and demographic characteristics, including age and background diseases, but the present results were not adjusted for these potential confounding factors in this analysis. For this reason, comparisons between groups are not appropriate. Further study is needed after adjusting for patient background.
To prevent in-hospital PE, it is important to assess the risk of PE upon admission and implement risk-appropriate prophylactic procedures. DVT requires prompt diagnosis because early diagnosis and therapeutic intervention can improve the condition and prognosis. The scoring systems such as the Wells score are often used to estimate the preclinical establishment of the disease, and measurement of D-dimer is also useful for risk assessment [26, 27]. However, since it has been suggested that the Wells score is less useful in hospitalized patients [28], it is necessary to develop a more reliable score that can be used for assessment of developing PE risk in hospitalized patients. In addition, hospital manuals should help healthcare providers to understand the risk of in-hospital PE as well as the importance of early and appropriate prophylactic interventions. Furthermore, studies on what diseases are most likely to associated with development of in-hospital PE may be useful to predict the PE risk. Patients that developed in-hospital DVT should be aggressively treated with DOACs at defined doses to prevent disease progress to PE if the patients are estimated to be at higher risk of PE. Although prophylactic use of anticoagulants might be also useful for hospitalized patients at higher risk of DVT and/or PE, the current situation is that prophylactic use of DOACs can only be carry out under limited conditions. DOACs have been shown to be equivalent or superior to warfarin in terms of efficacy and safety [29,30,31]. Subgroup analyses in patients with various comorbidities and medical histories have also been performed, primarily in patients with atrial fibrillation (AF), and have consistently shown the benefit of DOACs. It has been suggested that DOACs are more useful than warfarin in patients with AF, even in patients with impaired renal function [28, 32], and the same is likely true in patients with VTE, but the renal excretion rate varies with the type of DOACs, and blood levels may be more affected by renal function in DOACs with high renal excretion rates. Therefore, DOACs with high renal excretion rates are likely to be more affected by renal function. The benefit of DOACs in patients with malignant tumors or other diseases with a high tendency to thrombus formation has not been adequately tested, and the use of DOACs in these patients remains an issue for the future.
Limitations
First, because we only included data from acute-phase hospitals using the Diagnosis Procedure Combination system, we could not include data from other hospitals, including psychiatric hospitals, clinics, and hospitals not using this system. Therefore, the risk of developing in-hospital PE could have been underestimated. Second, we investigated patients hospitalized for other diseases who unexpectedly developed PE in the hospital; however, some patients could have been admitted with undiagnosed PE. Third, some patients may have had a DVT at the time of admission. In this study, patients on VTE treatment were not included because anticoagulation for more than 7 days during the baseline period was an exclusion criterion. However, DVT could have been missed if no symptoms were present or no lower extremity ultrasonography was performed. Therefore, some PE cases could have resulted from a thrombus in the lower limbs at the time of admission. Fourth, PE can rapidly progress and cause death. Therefore, patients who died of PE without being diagnosed were not accounted for. Last, this study is a retrospective analysis using an administrative claims database. We used the algorithm that was validated in the previous study for identification of patients with in-hospital PE, but diagnosis of PE and its onset timing was not always accurate. Detailed information of treatment, medical procedures, and drug prescriptions are available, but as a result of the nature of retrospective analyses using the claims database, we do not know exactly for what purpose these were implemented.
Conclusion
The incidence of in-hospital PE differed according to the patient characteristics, clinical departments, and the presence/absence of surgery. Patients without surgery showed lower implementation of prophylactic procedures for PE than patients with surgery. Further, the onset of PE during hospitalization leads to prolonged hospital stay and in-hospital death. Therefore, it is important to conduct a proper risk assessment at admission and implement proper prophylactic interventions for PE as hospital medical safety.
References
Japanese Circulation Society. Guidelines for diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2017). https://www.j-circ.or.jp/cms/wp-content/uploads/2017/09/JCS2017_ito_h.pdf. 2017. Accessed 18 June 2022.
Hume M. Venous thrombosis: mechanisms and treatment. Adv Exp Med Biol. 1978;102:215–24.
Kushner A, West WP, Pillarisetty LS. Virchow triad. Treasure Island: StatPearls; 2021.
Yamada N, Hanzawa K, Ota S, et al. Occurrence of deep vein thrombosis among hospitalized non-surgical Japanese patients. Ann Vasc Dis. 2015;8:203–9.
Takahashi S, Imura M, Katada J. Epidemiology and treatment patterns of venous thromboembolism: an observational study of nationwide time-series trends in Japan. Cardiol Ther. 2022;11(4):589–609.
Nakamura M, Fujioka H, Yamada N, et al. Clinical characteristics of acute pulmonary thromboembolism in Japan: results of a multicenter registry in the Japanese Society of Pulmonary Embolism Research. Clin Cardiol. 2001;24:132–8.
Ota M, Nakamura M, Yamada N, et al. Prognostic significance of early diagnosis in acute pulmonary thromboembolism with circulatory failure. Heart Vessels. 2002;17:7–11.
Ouellettee, DR. Pulmonary embolism (PE). 2020. https://emedicine.medscape.com/article/300901.
Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e195S–e226S.
Nicolaides AN, Fareed J, Kakkar AK, et al. Prevention and treatment of venous thromboembolism–international consensus statement. Int Angiol. 2013;32:111–260.
Yamaguchi Y, Fuji T, Akagi M, et al. The epidemiological study of venous thromboembolism and bleeding events using a Japanese healthcare database—validation study. Jpn J Drug Inform. 2015;17:87–93.
Normand SLT, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following an acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–98.
Sakuma M, Nakamura M, Yamada N, et al. Venous thromboembolism: deep vein thrombosis with pulmonary embolism, deep vein thrombosis alone, and pulmonary embolism alone. Circ J. 2009;73:305–9.
Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–15.
Blom JW, Doggen CJM, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22.
Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27:4839–47.
Ohashi Y, Ikeda M, Kunitoh H, et al. Venous thromboembolism in cancer patients: report of baseline data from the multicentre, prospective Cancer-VTE Registry. Jpn J Clin Oncol. 2020;50:1246–53.
Tsubata Y, Hotta T, Hamai K, et al. Incidence of venous thromboembolism in advanced lung cancer and efficacy and safety of direct oral anticoagulants: a multicenter, prospective, observational study (Rising-VTE/NEJ037 study). Ther Adv Med Oncol. 2022;14:175883592211101.
Connolly GC, Francis CW. Cancer-associated thrombosis. Hematol Am Soc Hematol Educ Program. 2013;2013:684–91.
Fanola CL, Norby FL, Shah AM, et al. Incident heart failure and long-term risk for venous thromboembolism. J Am Coll Cardiol. 2020;75:148–58.
Anderson DR, Morgano GP, Bennett C, et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019;3:3898–944.
Morrow M, Lynch-Smith D. Factor V Leiden: development of VTE in surgery and trauma patients: a systematic review. Dimens Crit Care Nurs. 2022;41:190–9.
Sakon M, Maehara Y, Yoshikawa H, et al. Incidence of venous thromboembolism following major abdominal surgery: a multi-center, prospective epidemiological study in Japan. J Thromb Haemost. 2006;4:581–6.
Fuji T, Akagi M, Abe Y, et al. Incidence of venous thromboembolism and bleeding events in patients with lower extremity orthopedic surgery: a retrospective analysis of a Japanese healthcare database. J Orthop Surg Res. 2017;12:55.
Aggarwal A, Rickles FR. Global public awareness of venous thromboembolism: comment. J Thromb Haemost. 2016;14:1110–1.
Tapson VF. Acute pulmonary embolism. N Eng J Med. 2008;358:1037–52.
Wells PS, Owen C, Doucette S, Fergusson D, Tran H. Does this patient have deep vein thrombosis? JAMA. 2006;295:199–207.
Scicchitano P, Tucci M, Bellino MC, et al. The impairment in kidney function in the oral anticoagulation era. A pathophysiological insight. Cardiovasc Drugs Ther. 2021;35:505–19.
Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Eng J Med. 2013;369:799–808.
Buller HR, Decousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Eng J Med. 2013;369:1406–15.
Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Eng J Med. 2010;363:2499–510.
Cortese F, Scicchitano P, Gesualdo M, et al. Apixaban: effective and safe in preventing thromboembolic events in patients with atrial fibrillation and renal failure. Curr Med Chem. 2017;24:3813–27.
Acknowledgements
The authors would like to thank the participants of the study.
Funding
This research was funded by Bristol-Myers Squibb K.K. and Pfizer Japan, Inc. The journal’s rapid service fee was provided by Bristol-Myers Squibb K.K. and Pfizer Japan, Inc.
Editorial Assistance
English language editing was provided by Editage, an English-editing service provided by Cactus Communications (Tokyo, Japan), which was funded by Bristol-Myers Squibb K.K. and Pfizer Japan, Inc.
Authorship
All named authors meet the criteria of the International Committee of Medical Journal Editors (ICMJE) and are responsible for the completeness of the entire work and authorize the publication of this version.
Author Contributions
M. Imura designed the study, managed the project, interpreted the data, critically reviewed the literature, and prepared and edited the final manuscript. T. Yamamoto designed the study, interpreted the data, critically reviewed the literature, and reviewed and edited the final manuscript. K. Hiasa interpreted the data, critically reviewed the literature, and reviewed and edited the final manuscript. All authors read and approved the final manuscript.
Disclosures
M. Imura is an employee of Pfizer Japan Inc. T. Yamamoto is an employee of Bristol-Myers Squibb K.K. K.H. received remuneration from Pfizer, Bristol-Myers Squibb, Daiichi Sankyo, Nippon Boehringer Ingelheim, Bayer, and Otsuka Pharmaceutical.
Compliance with Ethics Guidelines
This study is a retrospective database study that (1) is not related to the efficacy and safety of drugs or a disease and (2) uses data that does not contain complete personal information. Therefore, in accordance with the “ethical guidelines for medical and biological research involving human subjects”, which is the ethical guideline for clinical research in Japan, obtaining informed consent from patients and approval by the institutional review board or ethical committee are not required.
Data Availability
The datasets generated during and/or analyzed in the current study are not publicly available due to licensing agreements with Medical Data Vision Co., Ltd. Please contact Miki Imura, the corresponding author of this paper, for data availability.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Imura, M., Yamamoto, T. & Hiasa, KI. Pulmonary Thromboembolism Developed During Hospitalization: A Nationwide Retrospective Observational Study Using Claims Data. Cardiol Ther 12, 127–141 (2023). https://doi.org/10.1007/s40119-022-00290-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-022-00290-6