Abstract
A 30-year-old female was initially diagnosed with cardiac insufficiency and severe claudication. Additional imaging revealed a large iliac arteriovenous fistula, which was treated with an endovascular technique. A custom-made, self-expanding, polytetrafluorethylene-covered stent was implanted to restore the physiologic hemodynamic environment. The patient was asymptomatic at the 12-month clinical follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The etiology of iliac arteriovenous fistulas (AVF) may be categorized as spontaneous and traumatic. |
AVF formation may be related to penetrating injuries (gunshot and stab wounds) but may also occur after seat-belt trauma and laparoscopic appendectomy. |
Cardiac insufficiency may be caused by an undetected iliac AVF. |
Surgical repair is associated with high morbidity and mortality due to the complex anatomy and the unfavorable access site. |
Women of child-bearing age may benefit the most from endovascular repair using tapered, polytetrafluorethylene-covered stent grafts. |
Case Presentation
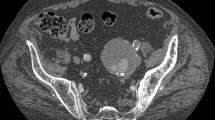
When the patient was 20 years old and in the third trimester of her pregnancy, she suffered a traumatic injury (stab wound) of the abdominal cavity. The patient had emergency surgery, which required laparotomy. The procedure was successful and saved both the patient and the fetus. She became pregnant again when she was 27 years old and, after giving birth to a second, healthy child, a rapidly progressing heart insufficiency was diagnosed. When she was 30 years old, the patient presented herself to her nearby hospital. She was not able to climb one flight of stairs, and her walking distance on a flat surface, prior to exhaustion, was only 10–20 m. The physical examination revealed, even without the use of a stethoscope, a murmur originating from the abdominal cavity. The murmur was audible from a distance. Angiographic tomography revealed a large arteriovenous fistula (AVF) with oval dimensions of 1.5 cm by 2.0 cm (Fig. 1), which communicated between the left internal iliac artery (IIA) and the internal iliac vein (IIV). Furthermore, an enlarged inferior vena cava (IVC), common iliac vein (CIV), and IIV were detected on the left side. The CIV and the proximal part of the IIA were also dilated (Figs. 2, 3). Chest X-ray imaging revealed cardiomegaly with enlarged ventricles and atria. There was no left ventricular hypertrophy.
Angiographic computed tomography (CT) of a 30-year-old female presenting with cardiac insufficiency and severe claudication. The patient was diagnosed with a large arteriovenous fistula. CT (GE Healthcare Revolution™ angio CT and 100 ml of Omnipaque 350) reveals a large, oval-shaped fistula approximately 1.5 cm × 2 cm in size between the left internal iliac artery (IIA) and the left internal iliac vein (IIV)
Due to adhesions after a previous laparotomy, an endovascular approach was chosen. Because of the limited availability of closure devices for routine interventions in the Polish health care system, a 2-cm incision was made in the right groin so that the right common femoral artery (CFA) was exposed. To better control bleeding, a slightly longer incision was necessary for vessel closure with a prepared suture line once the introducer had been removed. The vessel was punctured and a 7-French endovascular sheath was inserted to cross the bifurcation with a guidewire (Radifocus® Guidewire M Stiff type, Terumo Europe, Leuven, Belgium). A contralateral pelvic conventional angiogram confirmed the presence of the AVF. After reverting to a Meier stiff wire, the distal part of the IIA was cannulated. After the left CFA had been punctured, a 4-French introducer and a pigtail catheter were inserted. A custom-manufactured, polytetrafluorethylene (PTFE)-covered, tapered, self-expandable nitinol stent 9 mm × 14 mm × 60 mm in size (Balton Medical, Warsaw, Poland) was implanted (Fig. 4). Post-implantation control angiography (Fig. 5) confirmed a physiologic arterial flow pattern. Moreover, post-procedural computed tomography (CT) did not show any flow between the venous and arterial vessels (Fig. 6). Shortly after the intervention, the patient was able to walk and climb stairs 2 h after the procedure, and she was discharged after 2 days. At the 12-month follow-up, the patient was asymptomatic, and no procedure-related complications were observed. The patient gave her written permission to release her data for publication. The ethics committee at the Medical University of Warsaw was consulted and waived any approval process in light of the signed patient consent.
Control angiography after implantation of a PTFE-covered stent confirms a normalized hemodynamic flow pattern in the common iliac artery (CIA) and internal iliac artery (IIA) without any leak flow to the internal iliac vein. Left panel: flow patterns in the external iliac artery (EIA) are also normalized. Right panel: due to the high flow rate in the EIA, a separate still angiographic image was taken (imaged with a Siemens Artis zee; angiogram of the left common iliac artery; contrast injection in the left iliac trunk using 50 ml of Omnipaque 350, GE Healthcare)
Post-procedural CT with normalized hemodynamic flow patterns in the left common iliac artery (CIA), internal iliac artery (IIA), and external iliac artery (EIA). There is no leakage from the left internal iliac artery (IIA) into the internal iliac vein. Post-endovascular repair of the AVF did not reduce the size of the left CIA (imaged with a GE Healthcare Revolution™ and 100 ml of Omnipaque 350)
Discussion
We present a case that documents a minimally invasive endovascular repair of a very rare iatrogenic AVF between the internal iliac artery and the internal iliac vein.
Etiology and Demographics
Despite the low incidence rates of iliac AVF, the etiology of this vascular pathology (Table 1) may have several cardinal causes, which may be categorized as spontaneous and traumatic. Brewster et al. [1] investigated aortocaval and iliac AVF over a 30-year observation period. Their report of 20 abdominal AVFs revealed that the primary etiology was aneurysm erosion (70%), followed by iatrogenic causes (20%) and gunshot wounds (10%). Lumbar disk surgery was reported to be the most prominent iatrogenic cause of iliac AVF [2, 3]. However, Wang and coworkers [2] also pointed out that besides aneurysm formation (mycotic, syphilitic), neoplastic erosion affecting surrounding vasculatures and connective tissue pathologies (e.g., Marfan syndrome) may also be clinically significant causes of iliac AVF. Trauma may not always be related to penetrating injuries (gunshot and stab wounds); it may also occur after seat-belt trauma [4] and laparoscopic appendectomy [5].
Clinical and Imaging Findings
Depending on the etiology, the size, and the anatomic location of the iliac AVF, symptoms may be quite nonspecific, consisting of back pain [1], dyspnea commonly observed as pulmonary edema [2], or unilateral leg edema [1, 5, 6]. Due to the dramatically compromised hemodynamic environment, specific cardiovascular abnormalities may aid the decision regarding additional imaging diagnostics. The presence of a pulsatile abdominal mass with concomitant vascular bruit and thrill [6] has frequently been reported. Furthermore, intermittent claudication [2, 7], congestive heart failure [8, 9], pulmonary hypertension [3], T-wave abnormalities and renal failure [3, 10], and left ventricular hypertrophy [11] are described in the literature. Imaging findings based on exploratory ultrasound (US) are able to detect iliac AVFs with sufficient arterial leak flow rates into the venous system.
The cross-sectional area of the AVF in this case report was estimated at 2.2 cm2 (Aoval = ¼π × daxial × dlongitudinal = ¼π × 1.9 cm × 1.5 cm). Unfortunately, there is a paucity of data on actual cross-sectional areas of AVFs. However, Cronin et al. [12] reported a diameter of the left IIA of 24 mm, which corresponds well to the pathoanatomy in our case. They used a thoracic endograft that was longer (10 vs. 6 cm) and had a larger diameter (28 mm vs. 9/14 mm). We were able to use a shorter stent graft, since the iliac trunk was quite short in our patient. Ramacciotti and coworkers [13] conducted flow experiments in a canine AVF model and found that the flow through the fistula increases by a factor of 5 if the diameter of the AVF is 50% larger than the arterial diameter. This scenario from preclinical work appears to be similar to our case, without having measured actual flow rates.
Differential Diagnoses
All imaging modalities (Table 2) will show the focal widening caused by either a pseudoaneurysm or an aneurysm. These can be clearly differentiated from an AVF, which communicates between an artery and a vein. With duplex ultrasound, a pseudoaneurysm can have a characteristic yin–yang sign, which indicates the presence of bidirectional flow due to swirling of the blood within the lumen of the lesion. This swirling flow can also be seen in conventional angiograms. In contrast-enhanced CT angiography and MR angiography, this swirling flow can be detected from differences in the degree and shape of opacification of the contrast media within the lesion and/or in the vessels adjacent to the lesion. On duplex ultrasound, a true aneurysm of fusiform shape may show turbulent, disorganized flow, whereas a saccular aneurysm may show the yin–yang sign of swirling flow.
For the sake of discussion (Table 2), it can be stated that in CT or MRI, it may be difficult to differentiate a pseudoaneurysm from a true aneurysm. In a true aneurysm, there are usually no edema, blood products, and/or air in the tissues surrounding the aneurysm, unless the aneurysm has ruptured or is infected. If the pseudoaneurysm is chronic, the edema, blood products, and/or air in the tissues surrounding the pseudoaneurysm have usually dissolved. Consequently, exploratory ultrasound imaging is able to detect an iliac AVF with a sufficient arterial leak flow into the venous system [6], which may be in the range of 3 l/min [5]. Gadolinium-enhanced magnetic resonance (MR), computed tomography (CT), and angiography [7] are all suitable to detect even small-sized iliac AVFs with certainty. As concluded by Iijima et al. [6], any combination of imaging diagnostics are useful for detecting iliac AVF and deciding on the treatment modality.
Treatment and Prognosis
Surgical repair in these scenarios is associated with high morbidity and mortality due to the complex anatomy and the unfavorable access site [6]. Taking into consideration post-laparotomy abdominal adhesions, surgical access to the pelvis would be very demanding, and is associated with a high procedural risk. Large incisions and significant blood loss would most likely be inevitable. Furthermore, vessel wall adhesion between the IIA and IIV could have led to vessel wall rupture or wall dissection during surgical repair, which is associated with fatal bleeding.
There is also a significant risk while obtaining vascular access. Perforation of the intestine or bladder could have occurred, which inevitably would have resulted in peritonitis. Such a situation would have made it impossible to use a surgical repair with vascular patches. The endovascular approach, however, requires only a puncture on the left side and a small incision on the right side exposing the right CIA. Since we were not routinely using closure systems, we used a slightly longer incision to create a vessel loop so that bleeding could be better controlled. We are also convinced that a decreased introducer diameter may have facilitated the puncture and may have even avoided an incision. The endovascular approach, however, became a valuable alternative when dealing with patients with an iliac AVF [11].
The in-hospital outcome of this endovascular approach with a short recovery time is noteworthy. The patient could walk 2 h after the procedure and was discharged after 2 days. A similar immediate improvement using surgical repair was also reported by Machado-Atías [3]. Thus, despite the potential higher cost of stenting in comparison to open surgery, the very short hospitalization made the procedure cost effective.
It is also of paramount importance to note the fact that the patient is in her procreative years. Surgical access to the pelvis might have led to complications in future pregnancies.
The alternative implantation of the covered stent in the corresponding vein in conjunction with coil implantation at the arterial lesion site was also reported in the literature [14]. However, we believe that it cannot be recommended due to difficult-to-detect microbleeding. The higher arterial pressure may exert enough forces on the stent and the vessel wall to provoke leakage at the lesion site.
Due to the low incidence rates of iliac AVF (less than 1% [6]) and the associated pathoanatomy, the manufacture of standard PTFE-covered stents is most likely not attractive for device manufacturers, despite their efficacy in this niche indication. In our case, we implanted a custom-made device whose dimensions were based on precise CT measurements obtained prior to our intervention. Another interesting solution, described by Cronin and coworkers, is the use of preexisting thoracic stents [12]. Nevertheless, despite the advantages of endovascular techniques, Kuehnle and coworkers [5] cautioned about the use of alloplastic material in patients who are not fully grown: even in very young patients, the open surgical repair option may still be associated with a higher benefit/risk ratio than endovascular repair with covered stents. Another potential risk factor for iliac AVF formation is the occurrence of thrombotic occlusions in the common iliac vein, as reported by Weyrich and Beck [15]. This rare complication may also necessitate an open surgical approach.
An important step, and the most technically demanding one, turned out to be the cannulation of the distal IIA. Despite the contralateral approach, the small size of the distal portion of the IIA and the significant blood flow through the fistula made it very cumbersome to cross the lesion with the guidewire and over-the-wire catheter. The other challenge for the operator was to cross the aortic bifurcation with the introducer. Significant device stiffness, the young age of the patient, and the fixation of the aorta due to the adhesions made the vessel much less movable. This increased the risk of potential dissections and/or wall perforations. The puncture of the left CFA, through which we introduced the pigtail catheter, greatly facilitated angiographic imaging. Furthermore, if the stent graft had migrated, the outlet of the external iliac artery would have been blocked. However, with the second guidewire in place, it would have enabled us to implant another “kissing” stent. Finally, both guidewires crossed each other, marking the beginning of the IIA.
It would have been preferable to use MRI imaging modalities to reduce radiation exposure to the patient. However, in our clinical setting, mandatory pregnancy tests permitted angiographic CT images, which were the design inputs for the custom-made device.
Conclusion
Despite the procedurally challenging implantation of a PTFE-covered, tapered, customized stent, the physiologic hemodynamic environment in a patient with a large iliac arteriovenous fistula was restored. In particular, women of childbearing age may benefit from endovascular techniques as compared to open surgical repair.
References
Brewster DC, Cambria RP, Moncure AC, Darling RC, LaMuraglia GM, Geller SC, Abbott WM. Aortocaval and iliac arteriovenous fistulas: recognition and treatment. J Vasc Surg. 1991;13(2):253–64 (discussion 264–5).
Wang EA, Lee MH, Wang MC, Lee HY. Iatrogenic left iliac-caval fistula: imaging and endovascular treatment. AJR Am J Roentgenol. 2004;183(4):1032–4. https://doi.org/10.2214/ajr.183.4.1831032.
Machado-Atías I, Fornés O, González-Bello R, Machado-Hernández I. Iliac arteriovenous fistula due to spinal disk surgery. Causes severe hemodynamic repercussion with pulmonary hypertension. Tex Heart Inst J. 1993;20(1):60–4 (discussion 65).
Auer AI, Sauer DC, Levin M. Iliac arterio-venous fistula from an aneurysm following seat belt trauma. Angiology. 1974;25(1):21–3.
Kuehnl A, Zimmermann A, Pongratz J, Eckstein HH. Young girl presenting with heart failure 5 years after laparoscopic appendectomy. Case report of an ilio-iliac AV fistula. Eur J Vasc Endovasc Surg. 2010;40(1):107–9. https://doi.org/10.1016/j.ejvs.2010.02.013.
Iijima M, Kawasaki M, Ishibashi Y. Successful surgical repair of an ilio-iliac arteriovenous fistula associated with a ruptured common iliac artery aneurysm. Int J Surg Case Rep. 2015;13:55–7.
Kotelis D, Klemm K, von Tengg-Kobligk H, Allenberg JR, Böckler D. Intermittent claudication secondary to a traumatic arteriovenous fistula. Vasa. 2007;36(4):285–7. https://doi.org/10.1024/0301-1526.36.4.285.
Bialy T, Gooch AS, Shahriari A. High-output congestive failure due to arteriovenous fistula resulting from lumbar disc surgery—a case report. Angiology. 1988;39(7 Pt 1):616–9. https://doi.org/10.1177/000331978803900709.
McAuley CE, Peitzman AB, deVries EJ, Silver MR, Steed DL, Webster MW. The syndrome of spontaneous iliac arteriovenous fistula: a distinct clinical and pathophysiologic entity. Surgery. 1986;99(3):373–7.
Sivakumaran Y, Khasram M, Haggart PC. An ilio-iliac arteriovenous fistula following spontaneous rupture of a right common iliac artery aneurysm. Vasc Endovasc Rev. 2018;1(1):30–2.
Dos Santos EP, Batista RR, Felici FA, Correia VE, Oliveira M, Alves R. Endovascular correction of a traumatic internal iliac arteriovenous fistula with a covered stent. J Vasc Bras. 2014;13(1):48–52.
Cronin B, Kane J, Lee W, Shriki J, Weaver FA. Repair of a high-flow iliac arteriovenous fistula using a thoracic endograft. J Vasc Surg. 2009;49(3):767–70. https://doi.org/10.1177/000331977402500104.
Ramacciotti E, Galego SJ, Gomes M, Goldenberg S, De Oliveira GP, Pinto OJ. Fistula size and hemodynamics: an experimental model in canine femoral arteriovenous fistulas. J Vasc Access. 2007;8(1):33–43.
Vandereyken F, Schwagten V, Hertoghs M, Beaucourt L, D’Archambeau O, Hendriks J. Spontaneous ilio-iliac arteriovenous fistula due to an iliac artery aneurysm: a case-report. Acta Chir Belg. 2012;112(2):164–6. https://doi.org/10.1080/00015458.2012.11680817.
Weyrich G, Beck A. Traumatic fistula between internal iliac artery and external iliac vein. Radiat Med. 1990;8(6):215–8.
Acknowledgements
We would like to sincerely thank Dr. Pablo A. Gamboa (Riverside Radiology and Interventional Associates, Inc., Columbus, Ohio, USA) for providing precious support during the publication process.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. RB, SM: concept and design; all authors: drafting the manuscript.
Disclosures
R. Proczka, M. Waliszewski, M. Proczka, and S. Mazur all have nothing to disclose.
Compliance with Ethics Guidelines
The patient gave her written permission to release her data for publication. Furthermore, the ethics committee at the Medical University of Warsaw was consulted and waived any approval process in light of the signed patient consent. No special authorization to publish this case was necessary within the relevant legislature.
Data Availability
The datasets obtained during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Proczka, R., Waliszewski, M., Proczka, M. et al. Endovascular Repair of an Iliac Arteriovenous Fistula in a 30-Year-Old Female Patient with Suspected Severe Cardiac Insufficiency: A Case Report. Cardiol Ther 11, 309–317 (2022). https://doi.org/10.1007/s40119-022-00264-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-022-00264-8