Abstract
Introduction
Clinicians, payers, guideline committees, and policymakers support the use of high-intensity statins in patients at high risk for complications of cardiovascular disease (CVD). Guidelines and recommendations provide guidance on next steps for patients with inadequate low-density lipoprotein cholesterol (LDL-C) control on maximally tolerated statin or for those who are statin-intolerant. Ezetimibe and evolocumab improve CV outcomes when added to statins in high-CV-risk populations. The aim of the study was to compare evolocumab and ezetimibe for lipid-lowering efficacy and safety.
Methods
We summarized data from 1427 patients from three phase 3 evolocumab studies comparing double-blinded evolocumab vs. ezetimibe. These studies evaluated four distinct populations: those free of CVD receiving each agent as monotherapy, patients with CVD receiving add-on therapy to low- or high-intensity statin, and statin-intolerant patients. Lipid efficacy and safety were reported at week 12.
Results
Across the studies, evolocumab reduced LDL-C by a mean 55–61% from baseline to week 12; ezetimibe lowered LDL-C by 18–20% from baseline (mean difference = 38–43% favoring evolocumab; p < 0.0001). This corresponded to absolute reductions in LDL-C of 60–104 mg/dL with evolocumab vs. 17–35 mg/dL with ezetimibe. Evolocumab also significantly improved other lipids and led to a higher percentage of patients achieving LDL-C goals vs. ezetimibe. Adverse events and discontinuation rates (oral and parenteral therapy) were balanced across groups, suggesting good tolerance and acceptance of both treatments.
Conclusions
Evolocumab outperformed ezetimibe in efficacy and lipid goal attainment. Both products demonstrated good safety/tolerability. These data may help guide access decisions for high-risk patients with inadequate treatment response or intolerance to statin therapy.
Plain language summary
-
A statin is a type of medication that is used, with diet and exercise, to lower cholesterol levels and help prevent a heart attack or stroke caused by atherosclerosis. Atherosclerosis is the hardening and narrowing of blood vessels known as arteries from a buildup of plaque, usually made up of cholesterol and other fatty substances.
-
LDL cholesterol, also known as “bad” cholesterol, is one of the most important risk factors for having a heart attack, and can be lowered.
-
Patients with a high risk of heart disease may benefit from the additional lowering of LDL cholesterol beyond that achieved by statins alone.
-
The medication evolocumab reduced LDL cholesterol levels and improved other risk factors more than the medication ezetimibe in four studies. These studies included patients who were receiving statins and those who were not, including those who did not receive statins because of statin-related side effects. Both evolocumab and ezetimibe had good safety profiles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
High-risk patients may benefit from additional LDL-C lowering beyond that achieved by statins alone. |
Evolocumab outperformed ezetimibe in lipid goal attainment in four patient populations analyzed (patients receiving each agent as monotherapy, patients on low-intensity statin or high-intensity statin, and with statin intolerance). |
Both evolocumab and ezetimibe showed good safety and tolerability profiles. |
These data may guide management of high-risk patients with inadequate response or intolerance to initial lipid-lowering therapies. |
Introduction
For many years, clinicians worldwide have used low-density lipoprotein cholesterol (LDL-C) lowering as a cornerstone in their prevention strategy for patients with cardiovascular disease (CVD), with statins as the first-line agents [1,2,3,4,5,6,7,8,9,10,11,12,13].
Two classes of lipid-lowering therapies have shown clinical outcomes benefits when used in addition to statins: ezetimibe, an inhibitor of cholesterol absorption [14], and PCSK9 inhibitors, which include evolocumab, a monoclonal antibody directed against proprotein convertase subtilisin/kexin type 9 (PCSK9) [15].
Considering these outcomes studies, clinicians who treat CVD now have a choice of two prognostically proven drug classes for high-risk patients who may benefit from additional LDL-C lowering beyond that achievable with statins alone. To help understand the therapeutic and tolerability differences between these therapies in patients who require additional lipid-lowering therapy, we summarized data that compared the incremental lipid effects, safety, and tolerability of ezetimibe and evolocumab in controlled clinical trials from the PROFICIO development program for evolocumab.
Methods
Study Selection
The PROFICIO program involved 20 clinical trials conducted to evaluate and support the approval of evolocumab. The selection criteria for the inclusion of studies in this summary were as follows: (1) randomized allocation to at least one ezetimibe and one evolocumab treatment arm; (2) double-blinding of the ezetimibe and evolocumab treatment groups; and (3) a planned lipid assessment at week 12. Three previously published studies met these criteria [16,17,18]. These studies compared the two treatments in four different patient populations: patients on no background statin (as monotherapy), patients on low-intensity statin, patients on high-intensity statin, and statin-intolerant patients (Table 1).
Monotherapy patients included in this summary participated in the MENDEL-2 study (ClinicalTrials.gov, NCT01763827), a phase 3 study of evolocumab as monotherapy compared with placebo or ezetimibe in 615 patients with hypercholesterolemia [16]. This study randomized patients to daily oral placebo and biweekly subcutaneous (SC) placebo; daily oral placebo and monthly SC placebo; daily oral ezetimibe and biweekly SC placebo; daily oral ezetimibe and monthly SC placebo; daily oral placebo and biweekly SC evolocumab 140 mg; or daily oral placebo and monthly SC evolocumab 420 mg. MENDEL-2 contributed 460 patients to this summary from the ezetimibe and evolocumab treatment groups.
Patients on background statin therapy in this summary participated in the LAPLACE-2 study (ClinicalTrials.gov, NCT01763866), a phase 3 trial of evolocumab in combination with statin therapy [17]. The overall study included 1896 patients who were first randomized to one of five moderate- or high-intensity statin regimens (atorvastatin 10 or 80 mg, rosuvastatin 5 or 40 mg, or simvastatin 40 mg daily). Patients completed a lipid-stabilization period, after which they were randomized to evolocumab, ezetimibe (for patients receiving atorvastatin 10 or 80 mg only), or placebo. The current report includes patients from the atorvastatin 10 or 80 mg groups (660 patients in total) who received evolocumab or ezetimibe, because only these groups used ezetimibe as a comparator.
Statin-intolerant patients included in this summary participated in the GAUSS-2 study (ClinicalTrials.gov, NCT01763905), a phase 3 trial of evolocumab in patients unable to tolerate statin therapy [18]. The study randomized patients to receive daily oral ezetimibe and biweekly SC placebo; daily oral ezetimibe and monthly SC placebo; daily oral placebo and biweekly SC evolocumab; or daily oral placebo and monthly SC evolocumab. All patients from GAUSS-2 received either evolocumab or ezetimibe. The study contributed 307 patients to this report.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Efficacy and Safety Evaluations
Efficacy endpoints for the studies included here were the absolute value at week 12 and the percent change from baseline to week 12 for the following parameters: LDL-C, high-density lipoprotein cholesterol (HDL-C), non–HDL-C, very-low-density lipoprotein cholesterol (VLDL-C), lipoprotein(a) (Lp(a)), apolipoprotein B (ApoB), triglycerides, and PCSK9. This summary also reports the achievement of LDL-C levels < 70 and < 100 mg/dL at week 12, and the achievement of non–HDL-C levels < 100 and < 130 mg/dL at week 12 in each of the four patient populations examined. Safety endpoints included the incidence of any adverse event (AE), AEs occurring in > 1% of patients in any treatment group and in five or more evolocumab-treated patients in at least one study, potential muscle AEs, and study discontinuation due to AEs or potential muscle reasons.
Statistical Analyses
Data were analyzed by patient population: monotherapy (MENDEL-2 patients), combination therapy with a low-intensity statin (LAPLACE-2 patients randomized to atorvastatin 10 mg), combination therapy with a high-intensity statin (LAPLACE-2 patients randomized to atorvastatin 80 mg), and statin-intolerant patients (GAUSS-2). Within each patient population, biweekly and monthly evolocumab or placebo dose frequency groups were combined into a single treatment group, due to the known clinical equivalence of the two doses [19]. Efficacy and safety analyses were conducted in all randomized patients who received at least one dose of evolocumab or ezetimibe. A repeated-measures linear-effects model, which included the study’s stratification factors of treatment, visit, and treatment by visit terms, compared the ezetimibe and evolocumab groups for the percent change from baseline in the efficacy endpoints. Analyses did not impute missing data points, because the repeated-measures model accounts for the missing data. For achievement-based endpoints, a Cochran-Mantel–Haenszel test accounted for the stratification factor(s) to make treatment group comparisons and calculated percentages achieving such endpoints based on patients with an available week 12 measurement. All statistical analyses used SAS version 9.3 software (SAS Institute, Cary, NC, USA).
Results
Baseline Characteristics
The summary included 1427 patients: 477 treated with ezetimibe and 950 with evolocumab. Each study that contributed to this analysis randomized patients in a 1:2 ratio to ezetimibe and evolocumab—154 ezetimibe and 306 evolocumab in monotherapy patients, 111 ezetimibe and 220 evolocumab in low-intensity statin patients, 110 ezetimibe and 219 evolocumab in high-intensity statin patients, and 102 ezetimibe and 205 evolocumab in the statin-intolerant population (Table 1).
Within each patient population, baseline demographics (age, sex, and body mass index), CV risk factors (smoking, peripheral arterial disease, coronary artery disease, and others), and baseline lipids (LDL-C, non–HDL-C, HDL-C, VLDL-C, and others) were well matched between the ezetimibe and evolocumab treatment groups (Table 2). Overall, 53% of patients were female, and 90% were Caucasian. All patients in the low- and high-intensity statin populations received statins at baseline. One-third of the patients in the statin-intolerant population used lipid-lowering therapy at baseline. Of the latter, 18% received a low-dose statin [18].
Effects of Ezetimibe and Evolocumab on LDL-C and Non–HDL-C
Table 3 shows lipid efficacy results for evolocumab and ezetimibe. Across the four patient populations evaluated, evolocumab reduced LDL-C by a mean 55–61% from baseline to week 12, and ezetimibe lowered LDL-C by 18–20% from baseline, resulting in a mean difference of 38–43% favoring evolocumab compared with ezetimibe (p < 0.0001). These percentage reductions corresponded to lower achieved LDL-C levels at week 12 in evolocumab- vs. ezetimibe-treated patients in all groups studied (Fig. 1), with absolute reductions in LDL-C following evolocumab treatment of 59.9–104 mg/dL. These were greater than the absolute reductions in LDL-C seen with ezetimibe (16.5–35.3 mg/dL). Evolocumab also reduced non–HDL-C from baseline by a mean of 48–53% across the four populations, and ezetimibe reduced non–HDL-C from baseline by a mean of 16–17%, resulting in a mean difference between groups of 33–37% favoring evolocumab (p < 0.0001).
Baseline and week 12 achieved LDL-C. When calculated LDL-C was < 40 mg/dL or triglycerides were > 400 mg/dL, ultracentrifugation-determined LDL-C replaced calculated LDL-C from the same blood sample, if available. Diamond indicates mean; center line, median; top and bottom of box, 1st and 3rd quartiles; ends of whiskers, 5th and 95th percentiles. LDL-C low-density lipoprotein cholesterol
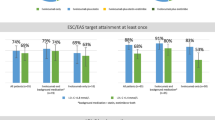
The greater reduction in LDL-C achieved with evolocumab vs. ezetimibe led to higher proportions of patients on evolocumab achieving LDL-C and non–HDL-C treatment goals (Table 3). In the monotherapy treatment groups, 69% of evolocumab- and 1% of ezetimibe-treated patients achieved an LDL-C level < 70 mg/dL. In patients on low-intensity background statin therapy, 85% (evolocumab) and 21% (ezetimibe) achieved an LDL-C level < 70 mg/dL. In patients on high-intensity background statin therapy, 92% (evolocumab) and 54% (ezetimibe) achieved an LDL-C level < 70 mg/dL. In the statin-intolerant patient population, 44% (evolocumab) and 1% (ezetimibe) achieved an LDL-C level < 70 mg/dL. All these comparisons favored evolocumab (p < 0.0001). Achievement of an LDL-C < 70 mg/dL goal was more likely to occur in patients with lower baseline LDL-C levels than in those with higher baseline LDL-C levels (Fig. 2). Similarly, for LDL-C < 100 mg/dL, evolocumab led to significantly higher proportions of patients achieving LDL-C goals across all patient populations, with the greatest treatment differences seen in the statin-intolerant (67% evolocumab vs. ezetimibe) and monotherapy groups (67% evolocumab vs. ezetimibe; p < 0.0001 for both).
Achievement of non–HDL-C < 100 mg/dL occurred significantly more often with evolocumab compared with ezetimibe across each population—80% and 6% in monotherapy patients, 87% and 34% in low-intensity statin patients, 93% and 63% in high-intensity statin patients, and 44% and 0% in statin-intolerant patients (all p < 0.0001). For the less stringent goal for non–HDL-C < 130 mg/dL, 72–97% of evolocumab-treated patients and 11%–81% of ezetimibe-treated patients achieved the goal (evolocumab vs. ezetimibe; p < 0.0001 for all patient populations).
Changes in Other Lipids Following Treatment with Ezetimibe or Evolocumab
Evolocumab compared with ezetimibe also had favorable effects on other atherogenic lipoproteins (Table 3). For example, patients treated with ezetimibe had a mean percent change from baseline in Lp(a) of 1.9% (monotherapy), 5.2% (low-intensity statin), 9.4% (high-intensity statin), and 2.0% (statin intolerance). In contrast, patients treated with evolocumab had a mean percent change from baseline in Lp(a) of −22% (monotherapy) and −24% (low-intensity statin, high-intensity statin, and statin intolerance), resulting in mean treatment differences vs. ezetimibe of −23%, −28%, −34%, and −26% in the monotherapy, low-intensity statin, high-intensity statin, and statin-intolerant populations, respectively (p < 0.0001 favoring evolocumab in all groups). Additionally, the mean treatment difference between groups in the reduction in ApoB (evolocumab vs. ezetimibe) was 33–36% across the patient populations (p < 0.0001 favoring evolocumab in all groups).
Ezetimibe treatment resulted in a mean percent change from baseline in triglycerides of −4.9 to 3.3% across patient populations. Evolocumab treatment resulted in a mean percent change from baseline in triglycerides of −3.4 to −8.3%. The treatment difference between ezetimibe and evolocumab was −0.3 to −11%, a difference that reached significance for the monotherapy and low-intensity statin populations (p < 0.05 in favor of evolocumab for both) but not for the high-intensity statin or statin-intolerant populations. Treatment with ezetimibe resulted in a mean percent change from baseline in HDL-C of −1.2 to 2.2%. In contrast, evolocumab treatment increased levels of HDL-C by a mean percent change from baseline of 4.8% in the monotherapy patient population, 7.4% in the low-intensity statin patient population, 8.5% in the high-intensity statin patient population, and 6.2% in the statin-intolerant patient population. Treatment differences for HDL-C for evolocumab vs. ezetimibe ranged from 4.3 to 8.6% across patient populations (p < 0.05 in all groups; Table 3).
Twelve weeks of evolocumab treatment significantly reduced PCSK9 levels from baseline by a mean of 54% in the monotherapy patient population, 45% in the low-intensity statin patient population, 32% in the high-intensity statin patient population, and 45% in the statin-intolerant patient population. In contrast, mean percent changes in PCSK9 levels in ezetimibe-treated patients were 16%, 13%, 17%, and 0.9% across the above populations, resulting in significant treatment differences of −64%, −57%, −49%, and −44% (p < 0.0001 favoring evolocumab in all groups), respectively.
Safety and Tolerability
Table 4 shows AE rates across the groups examined in this report. Overall, AEs appear balanced between evolocumab- and ezetimibe-treated patients. Statin-intolerant patients reported AEs more frequently than other populations. Serious AEs occurred in 0–3.9% of patients across the patient populations receiving ezetimibe, and in 1.3–2.9% across the patient populations receiving evolocumab. Calculations of the incidence of potential muscle events used Standardised MedDRA (Medical Dictionary for Regulatory Activities) Queries terms. These events occurred at rates of (ezetimibe vs. evolocumab) 3.2% vs. 2.6%, 4.5% vs. 0.9%, 2.7% vs. 1.8%, and 22.5% vs. 12.2% in the monotherapy, low-intensity statin, high-intensity statin, and statin-intolerant populations, respectively.
The decision to discontinue SC treatment by patients and/or physicians occurred at rates comparable to the discontinuation rates for oral treatment in the high-intensity statin and monotherapy treatment groups. In the statin-intolerant group, discontinuation occurred in 13.7% (ezetimibe) and 8.3% (evolocumab) of patients receiving blinded oral drug and 5.9% (ezetimibe) and 3.9% (evolocumab) of patients receiving blinded SC drug. In the low-intensity statin group, discontinuation occurred in 6.3% (ezetimibe) and 3.2% (evolocumab) of patients receiving oral drug and 8.1% (ezetimibe) and 4.1% (evolocumab) of patients receiving SC drug. Rates of drug discontinuation due to muscle events were < 1% in each group except for statin-intolerant patients. In the statin-intolerant population, 5.9% vs. 2.4% of patients in the ezetimibe- vs. evolocumab-treated groups stopped oral drug treatment due to muscle symptoms. In the same population, 2.9% vs. 0.5% of patients in the ezetimibe- vs. evolocumab-treated groups discontinued parenteral drug treatment due to muscle symptoms.
Discussion
Our summary of phase 3 patients in the PROFICIO program randomized to either ezetimibe or evolocumab provides a direct comparison of the lipid efficacy and safety effects of two therapies with proven incremental CV outcome benefits when used in addition to statins in CV outcomes trials. We demonstrate that evolocumab treatment led to a significantly greater reduction in LDL-C, non–HDL-C, and ApoB than ezetimibe, regardless of the background statin regimen, across four distinct patient populations. This greater efficacy occurred without an apparent safety or tolerability trade-off.
When choosing among lipid therapies, clinicians and patients with CVD may need to navigate trade-offs of efficacy, safety, tolerance, and access. Current cholesterol/dyslipidemia guidelines and recommendations advocate the use of high-intensity statin regimens for high-risk patients, which clinicians and payers strongly support. However, for patients with statin intolerance or for those with less than optimal atherogenic lipid levels while receiving maximally tolerated statin therapy and still at high cardiovascular risk, many factors might influence the choice of lipid treatment in these groups. These factors include the absolute CVD risk of the patients, LDL-C achieved on maximally tolerated statin therapy, magnitude of the lipid effects of the agent, safety and tolerability of the agent, and specific pathophysiology of the patients in need of treatment, such as familial hypercholesterolemia or Lp(a) elevation [20].
Ezetimibe inhibits cholesterol absorption by blocking uptake at the jejunal enterocyte brush border. Its primary site of action is the cholesterol transport protein Niemann-Pick C1-like 1 protein. Ezetimibe, an oral agent approved in the USA and in many other countries, lowers LDL-C modestly, with a good safety and tolerability record. Based on this record, ezetimibe has become the most commonly used drug for additional LDL-C lowering as an add-on to statins or alone in statin-intolerant patients.
Two monoclonal antibodies (alirocumab and evolocumab) that inhibit the effects of PCSK9 received regulatory approval in the USA in 2015 for the treatment of hypercholesterolemia. These drugs offer an alternative as second-line agents for LDL-C reduction. Evolocumab showed significant CV outcome benefits in high-risk patients with CVD on optimal statin therapy [15] and has received regulatory approval for the prevention of myocardial infarction, stroke, and coronary revascularization [21]. PCSK9 inhibitors appear safe and have high lipid efficacy.
Recent guidance from both US and European lipid and cardiology organizations regarding the use of non-statin therapies offers a number of factors to consider when deciding upon the next line of therapy on top of a maximally tolerated dose of statin [22,23,24]. These factors include the potential to benefit from added therapy based on patient risk and the additional lowering needed to reach a goal, such as an LDL-C/non–HDL-C level or a percentage reduction in LDL-C. Previously, in the 2017 Focused Update of the 2016 American College of Cardiology Expert Consensus Decision Pathway on the Role of Non-Statin Therapies, the authors suggested that when treating patients with clinical atherosclerotic cardiovascular disease (ASCVD) and comorbidities who require > 25% additional lowering of LDL-C, a PCSK9 inhibitor such as evolocumab may be preferred as the initial non-statin agent [22]. However, in patients who require < 25% additional lowering of LDL-C, the update suggests that one may consider ezetimibe as an initial choice [22]. The 2018 AHA/ACC Multisociety Guideline [27] on the Management of Blood Cholesterol and the 2019 ESC/EAS Guidelines for the management of dyslipidemias recommend additional therapy if the goals are not achieved [25]. In a real-world setting, in patients with clinical ASCVD or probable heterozygous familial hypercholesterolemia, adding ezetimibe therapy to statins resulted in a small percentage of patients achieving LDL-C goals [26]. This was also seen in our analysis, where there was a significantly higher proportion of patients on evolocumab achieving LDL-C and non–HDL-C treatment goals.
Our summary of the effects of evolocumab and ezetimibe across four specific patient populations provides specific and comparable data on the efficacy and safety of evolocumab vs. ezetimibe, which can inform clinical decisions. Our analysis also addresses various patient types, including those with and without established coronary heart disease and those on different background doses of statin therapy. These data also confirm previous observations that patients with hypercholesterolemia tolerate treatment with a parenteral agent well [27], including statin-intolerant patients who discontinued blinded oral medications at a higher rate than blinded parenteral treatments [28].
As a post hoc summary of data, this report has intrinsic limitations. The relatively short duration of the analysis represents a limitation, because hypercholesterolemia requires chronic treatment. We also did not include other agents that lower LDL-C that have not shown outcomes benefits in addition to statins, such as cholesterol resins or nicotinic acid. Nonetheless, these data confirm other, independent studies of the comparative effects of evolocumab and ezetimibe, including studies of much longer duration.
Conclusions
Evolocumab provided substantive atherogenic lipid improvements across a broad range of patient populations. Compared to ezetimibe, evolocumab treatment led to greater reductions in LDL-C and non–HDL-C levels and a higher likelihood of lipid goal attainment. Patients tolerated both therapies well. Consideration of these findings may help guide treatment decisions, particularly for statin-intolerant patients.
References
The lipid research clinics coronary primary prevention trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365–74.
Cholesterol Treatment Trialists Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81.
Cholesterol Treatment Trialists Collaborators, Mihaylova B, Emberson J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90.
Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–22.
Kinlay S, Timms T, Clark M, et al. Comparison of effect of intensive lipid lowering with atorvastatin to less intensive lowering with lovastatin on C-reactive protein in patients with stable angina pectoris and inducible myocardial ischemia. Am J Cardiol. 2002;89:1205–7.
LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35.
Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–45.
Robinson JG, Wang S, Smith BJ, Jacobson TA. Meta-analysis of the relationship between non-high-density lipoprotein cholesterol reduction and coronary heart disease risk. J Am Coll Cardiol. 2009;53:316–22.
Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–8.
Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58.
Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Drugs. 2004;64(Suppl 2):43–60.
Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7.
Wadhera RK, Steen DL, Khan I, Giugliano RP, Foody JM. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J Clin Lipidol. 2016;10:472–89.
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97.
Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22.
Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–40.
Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311:1870–82.
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–8.
Stroes E, Robinson JG, Raal FJ, et al. Clinical equivalence of evolocumab among patient subgroups in PROFICIO: a pooled analysis of 3146 patients from phase 3 studies. Presented at: European Society of Cardiology Congress; August 29–September 2, 2015; London, United Kingdom.
Robinson JG, Huijgen R, Ray K, Persons J, Kastelein JJ, Pencina MJ. Determining when to add nonstatin therapy: a quantitative approach. J Am Coll Cardiol. 2016;68:2412–21.
Repatha® (evolocumab) prescribing information, Amgen.
Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2017 focused update of the 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of cardiology task force on expert consensus decision Pathways. J Am Coll Cardiol. 2017;70:1785–822.
Orringer CE, Jacobson TA, Saseen JJ, et al. Update on the use of PCSK9 inhibitors in adults: recommendations from an Expert Panel of the National Lipid Association. J Clin Lipidol. 2017;11:880–90.
Landmesser U, Chapman MJ, Farnier M, et al. European Society of Cardiology/European Atherosclerosis Society Task Force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur Heart J. 2017;38:2245–55.
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Menzin J, Aggarwal J, Boatman B, et al. Ezetimibe use and LDL-C goal achievement: a retrospective database analysis of patients with clinical atherosclerotic cardiovascular disease or probable heterozygous familial hypercholesterolemia. J Manag Care Spec Pharm. 2017;23:1270–6.
Koren MJ, Sabatine MS, Giugliano RP, et al. Long-term low-density lipoprotein cholesterol-lowering efficacy, persistence, and safety of evolocumab in treatment of hypercholesterolemia: results up to 4 years from the open-label OSLER-1 extension study. JAMA Cardiol. 2017;2:598–607.
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315:1580–90.
Acknowledgements
Funding
Amgen Inc. (Thousand Oaks, CA, USA) funded this study and the journal’s Rapid Service Fee. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing, Editorial, and Other Assistance
The authors thank Annalise M. Nawrocki, PhD, of Amgen Inc., who provided medical writing and editorial support, and Elizabeth McIlvaine, PhD, of Amgen Inc., who provided statistical analysis support. Amgen Inc. (Thousand Oaks, CA, USA) funded the medical writing and editorial support.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Michael J. Koren, Thomas Hucko, J. Antonio G. Lopez, Alex N. Fleishman, and Ransi Somaratne conceived of the study analysis. Michael J. Koren, Thomas Hucko, J. Antonio G. Lopez and Alex N. Fleishman participated in the initial drafting of the manuscript.
Disclosures
Michael J. Koren is an employee of Jacksonville Center for Clinical Research, a company that has received research grants and consulting fees from Amgen Inc. and other companies developing therapies for hyperlipidemia. Peter H. Jones has been a consultant/scientific advisor to Amgen, Sanofi/Regeneron, Merck, and Aegerion, and also serves as Chief Science Officer for the National Lipid Association. Jennifer G. Robinson has received research grants to institution from Acasti, Amarin, Amgen, AstraZeneca, Eisai, Esperion, Merck, Novo Nordisk, Pfizer, Regeneron, Sanofi, and Takeda, and has been a consultant for Amgen, Eli Lilly, Merck, Novo Nordisk, Pfizer, Regeneron, and Sanofi. David Sullivan has received grants and personal fees from Abbott, Amgen, and Mylan; personal fees from AstraZeneca and Pfizer; and grants from Sanofi and Novartis, outside the submitted work. Leslie Cho has conducted research with Amgen and Esperion, has been a consultant for Amgen, and has been an unpaid consultant for Lilly. Thomas Hucko, J. Antonio G. Lopez, and Alex N. Fleishman are employees of Amgen Inc. and own Amgen stock/stock options. Ransi Somaratne is a former employee of Amgen, holds Amgen stock, and is the inventor on at least one pending patent application owned by Amgen Inc. relating to evolocumab. Ransi Somaratne was an employee of Amgen Inc. at the time of the study and is currently an employee of Epirium Bio Inc. Erik Stroes has received lecture fees for institution from Amgen, Sanofi-Regeneron, Novartis, and Ionis/Akcea.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://www.amgen.com/datasharing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12326483
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Koren, M.J., Jones, P.H., Robinson, J.G. et al. A Comparison of Ezetimibe and Evolocumab for Atherogenic Lipid Reduction in Four Patient Populations: A Pooled Efficacy and Safety Analysis of Three Phase 3 Studies. Cardiol Ther 9, 447–465 (2020). https://doi.org/10.1007/s40119-020-00181-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-020-00181-8